Abstract
Summary
Rehmanniae Radix Preparata (RRP) improves bone quality in OVX rats through the regulation of bone homeostasis via increasing osteoblastogenesis and decreasing osteoclastogenesis, suggesting it has a potential for the development of new anti-osteoporotic drugs.
Introduction
Determine the anti-osteoporotic effect of RRP in ovariectomized (OVX) rats and identify the signaling pathway involved in this process.
Methods
OVX rats were treated with RRP aqueous extract for 14 weeks. The serum levels of tartrate-resistant acid phosphatase (TRAP), receptor activator of nuclear factor kappa-Β ligand (RANKL), alkaline phosphatase (ALP), and osteoprotegerin (OPG) were determined by ELISA. Bone histopathological alterations were evaluated by H&E, Alizarin red S, and Safranin O staining. Bone mineral density (BMD) and bone microstructure in rat femurs and lumbar bones were determined by dual-energy X-ray absorptiometry and micro-computed tomography. Femoral bone strength was detected by a three-point bending assay. The expression of Phospho-glycogen synthase kinase 3 beta (p-GSK-3β), GSK-3β, Dickkopf-related protein 1 (DKK1), cathepsin K, OPG, RANKL, IGF-1, Runx2, β-catenin, and p-β-catenin was determined by western blot and/or immunohistochemical staining.
Results
Treatment of OVX rats with RRP aqueous extract rebuilt bone homeostasis demonstrated by increasing the levels of OPG as well as decreasing the levels of TRAP, RANKL, and ALP in serum. Furthermore, RRP treatment preserved BMD and mechanical strength by increasing cortical bone thickness and epiphyseal thickness as well as improving trabecular distribution in the femurs of OVX rats. In addition, RRP downregulated the expression of DKK1, sclerostin, RANKL, cathepsin K, and the ratio of p-β-catenin to β-catenin, along with upregulating the expression of IGF-1, β-catenin, and Runx2 and the ratio of p-GSK-3β to GSK-3β in the tibias and femurs of OVX rats. Echinacoside, jionoside A1/A2, acetoside, isoacetoside, jionoside B1, and jionoside B2 were identified in the RRP aqueous extract.
Conclusion
RRP attenuates bone loss and improves bone quality in OVX rats partly through its regulation of the canonical Wnt/β-catenin signaling pathway, suggesting that RRP has the potential to provide a new source of anti-osteoporotic drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rehmanniae Radix Preparata (RRP) is derived from the root of the perennial plant Rehmannia glutinosa (Gaertn.) DC., a well-known herb recorded in pharmacopeia of the People’s Republic of China (2015). In combination with other herbs, it has been used in the treatment of osteoporosis for more than 2000 years in China and other Asian countries [1]. RRP has been demonstrated to reduce lower back pain as well as increase bone mineral density (BMD) in osteoporotic patients [1]. Its efficacy is supported by in vivo and in vitro studies demonstrating the ability of attenuate BMD loss in the proximal tibial metaphysis and increase of the cortical bone thickness in ovariectomized (OVX) rats as well as promoting osteoblast formation and inhibiting osteoclast activity [2, 3]. In addition, a number of studies have revealed that prescriptions containing RRP, such as Liuwei Dihuang pills, have therapeutic effects on osteoporosis [4,5,6] through activation of the canonical Wnt/β-catenin signaling pathway in osteoblasts [7].
Osteoporosis, a progressive and silent bone disease, is characterized by reduction of bone mass and density, the deterioration of bone microstructures leading to enhanced skeletal fragility, and a consequent increased risk for hip and vertebral fractures [8]. Osteoporosis has become a major public health concern due to increased ageing of populations worldwide. This correlates with enormous health care costs [9]. Therefore, research efforts and novel treatment strategies with fewer side effects remain an unmet medical need [10]. Traditional Chinese herbs may fill this need because of their relative cost-effectiveness, low risk of adverse events, and potential combination therapy effects [11]. Among them, RRP has gained due to its bone-preserving properties and as an edible herb [1].
Previous studies have suggested that activation of the canonical Wnt/β-catenin signaling pathway contributes to osteoblast activation and bone formation. This occurs partly through the inhibition of GSK-3β, which then results in the nuclear translocation of β-catenin and in a change of the expression of the osteoblast differentiation transcription factor, Runx2 [12, 13]. Dickkopf-related protein 1 (DKK1) and sclerostin, mainly secreted by osteocytes, are the endogenous inhibitors of the Wnt/β-catenin signaling pathway with a consequence of reduced osteoblastogenesis and bone formation [14, 15]. The Wnt/β-catenin signaling pathway also suppresses osteoclast differentiation by stimulating osteoprotegerin (OPG) production and secretion, which then favors bone formation [16]. OPG could competitively bind to the receptor activator of nuclear factor kappa-Β ligand (RANKL), a cytokine required for osteoclastogenesis. This leads to the suppression of osteoclast formation including decreased expression of the collagenase, cathepsin K, and thus to reduced bone resorption [17, 18]. Although RRP was reported to exhibit an anti-osteoporotic capacity, the underlying mechanism remains unknown. In the current study, we hypothesized that RRP may attenuate bone loss and increase bone strength through the regulation of Wnt/β-catenin signaling in OVX rats.
Materials and methods
Chemicals and antibodies
Alizarin red S, Safranin O, and Fast Green were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies against insulin-like growth factor (IGF)-1 (sc-9013) were obtained from Santa Cruz Biotechnology (Dallas, TX, USA). Antibodies against β-catenin (9562S), phospho (p)-β-catenin (Ser33/37/Thr41) (9561), phospho-glycogen synthase kinase 3 beta (p-GSK-3β) (Ser9) (5B3) (9323S), and GSK-3β (D5C5Z) XP (12456S) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). The DKK1 antibody (21112-1-AP) was purchased from Proteintech Group, Inc. (Rosemont, USA). Antibodies against OPG (ab73400), sclerostin (ab63097), cathepsin K (ab19027), RANKL (ab62516), and Runx2 (ab76956) were obtained from Abcam (Cambridge, UK). All chemicals, except those specifically identified, were from Beijing Sino-pharm Chemical Reagents Co. Ltd. (Beijing, China).
Preparation of RRP aqueous extract
RRP was purchased from Beijing TongRenTang pharmacy (Beijing, China) and authenticated by Professor Zexin Ma (TCM museum at Beijing University of Traditional Chinese Medicine). For RRP aqueous extraction, 1200 g of raw RRP was ground into powder under liquid nitrogen and divided into four parts. Each part (300 g) was dissolved in distilled water (3 L) by continuous stirring for 72 h at 4 °C. Then, the aqueous extracts were collected by spinning (4000 rpm at 4 °C for 10 min). The supernatants were harvested and concentrated to an extractum by evaporating under reduced pressure (at 60 °C). Finally, the four parts of the extractums (561.6 g, 1 g contains 2.14 g of raw RRP) were combined and stored at 4 °C until use.
The RRP aqueous extract was analyzed by HPLC–DAD–ESI–MSn (SHIMADZU, Japan), equipped with a DAD detector (SPD-M10AVP, SHIMADZU) and IT–TOF–MS as previously described [19].
Determination of total polysaccharide content in RRP aqueous extract
The total polysaccharide content was determined using the phenol-sulfuric acid method according to previously published protocols with some modifications [20, 21]. Initially, RRP aqueous extract (0.1 mL, 0.00625 g raw RRP/mL) was pipetted into a test tube and then mixed with 5% phenol (1 mL). Subsequently, sulfuric acid (98%, 5 mL) was rapidly and evenly administrated into the test tube followed by heating in a boiling water bath for 20 min and then cooling in a cold water bath for 5 min. The absorbance of the solution was measured at 488 nm in a plate reader (FLUO star Omega, BMG LABTECH). The relative content of the total polysaccharide in RRP aqueous extract was determined using glucose concentration standard. The following glucose amounts were used: 0, 12.02, 24.04, 72.12, 96.16, and 120.02 μg/mL.
OVX rats model and RRP intervention
The protocol of the study was approved by the Ethics Committee of Beijing University of Chinese Medicine (BUCM). Forty female Sprague–Dawley rats (200 ± 20 g, 10 weeks of age) were purchased from SPF Laboratory Animal Technology Co. Ltd. [certification number SCXK (Jing) 2016-0002] and housed in an environmentally controlled room at a constant temperature (22 ± 1 °C) and humidity (55 ± 5%) on a 12-h light/dark cycle according to the national standard GB 14925–2010 (China) at BUCM [certification number SYXK (Jing) 2016-0038]. Animals were allowed free access to a standard diet and water.
After 1 week of acclimation, the rats were anesthetized (1% sodium pentobarbital, 0.4 mL/100 g, i.p.) and ovariectomized by removing the bilateral ovaries to establish osteoporotic models. Sham-operated rats were subjected to surgery exposure by removing the same volume of fat surrounding the ovaries. One week after surgery, ovariectomized rats were randomly divided into three groups with nine animals in each, namely in OVX, EV (estradiol valerate), and RRP groups. The rats in the EV and RRP group were treated daily, by gavage, with estradiol valerate (0.1 mg/1 mL/100 g) or the aqueous extract of Rehmanniae Radix Preparata (RRP, 2.5 g/1 mL/100 g), respectively. The rats in OVX group and sham-operated (Sham) group were treated daily, by gavage, with the same volume of distilled water. Body weight was measured every 2 weeks. After 14 weeks of treatment, rats were anesthetized (1% sodium pentobarbital, 0.4 mL/100 g, i.p.) and terminated by cervical dislocation. Serum was collected from abdominal aortic blood by centrifugation at 3500 rpm for 10 min, and the right femurs and tibias were dissected. All samples were stored either in 10% neutral buffered formalin at room temperature or at − 80 °C for further analyses.
ELISA
The serum levels of alkaline phosphatase (ALP), tartrate-resistant acid phosphatase (TRAP), RANKL, and OPG were determined by corresponding ELISA kits according to the manufacture’s instruction, which were purchased from Beijing Fangcheng Co. Ltd. (Beijing, China).
DXA and μCT scanning
The right femurs of the rats were cleaned of adhering soft tissues and analyzed with dual-energy X-ray absorptiometry (Discovery Wi; Hologic, Bedford, MA, USA) using a small animal protocol software program. BMD in the femur head and lumbar bone was determined. Briefly, the right femurs were further placed in the 48-mm specimen holder, and then subjected to X-ray micro-tomography using a micro-computed tomography (μCT) apparatus for small experimental animals (Model LaTheta LCT-200; Hitachi-Aloka, Tokyo, Japan), which was operated at 50 kV and 0.5 mA (radiation exposure remained below 40 mSv) for measurements [22]. First, an overview scan of the whole bone was performed allowing the selection of regions of interest for subsequent scans. Then, the areas between the proximal and distal end of femur were scanned to quantify cortical and spongy bones. For all scans, the same number of views was used, which correlates the number of data points collected during a single 360° rotation around the object. The parameters, including cancellous BMD, cortical bone thickness, cortical bone area ratio, and trabecular bone area ratio, were calculated automatically by the LaTheta software (version 3.20).
Bone biomechanics strength assay
After scanning, right femurs were submitted to three-point bending examination using an Electronic Universal Testing machine (Shenzhen Reger Instrument Co. Ltd., Model RGWF4005, China). The test was conducted according to the protocol previously described [23]. The load–displacement curves were obtained and used for the evaluation of the structural and material properties, including ultimate load, bending strength, and elastic modulus.
The hematoxylin/eosin (H&E), Alizarin red S, and Safranin O/Fast Green staining
The left femurs and uteri were immersed (10% neutral formalin for 72 h), decalcified (femurs only, 15% neutral EDTA buffer for 3 months), and embedded in paraffin. Sections of approximately 5-μm thickness were used for either H&E, Alizarin red S or Safranin O/Fast Green staining. H&E staining, Alizarin red S, and Safranin O/Fast Green staining were all conducted as described previously [23, 24]. After staining, the mounted slides were observed and photographed using an Olympus BX53 fluorescence microscope (Tokyo, Japan). The integrated optical density (IOD) values of Alizarin red S staining and Safranin O/Fast Green staining were quantified using the Image Pro Plus 6.0 software.
Immunohistochemical staining
Immunohistochemical (IHC) staining was done as previously described [23]. Briefly, 5-μm sections of demineralized and paraffin-embedded femurs were sequentially treated with xylene, descending graded ethanol (100–70%), antigen retrieval solution, and 3% hydrogen peroxide. Then, the slides were rinsed and incubated with the appropriate primary antibody [cathepsin K (1:200), IGF-1 (1:50), OPG (1:50), RANKL (1:100), sclerostin (1:50), or β-catenin (1:200)] overnight at 4 °C. For the negative controls, the primary antibody was replaced by nonimmunized goat serum. The next day, the slides were rinsed and incubated with the corresponding secondary antibodies (Beijing Biosynthesis Biotechnology Co. Ltd.; Beijing, China) for 30 min followed by DAB and hematoxylin staining, respectively. The slides were then examined and photographed using Olympus BX53 fluorescence microscope (Tokyo, Japan) and analyzed by using Image Pro Plus 6.0 software.
Western blot analysis
Protein expression levels in different groups were detected by western blot analysis [24]. Initially, the marrow in tibias and femurs was flushed followed by freezing and subsequent pulverizing in liquid nitrogen, and then the protein was extracted using a bone tissue protein extraction kit (Shanghai BestBio Science Biotechnology; Shanghai, China) and its concentration was determined using a bicinchoninic acid assay (Shanghai Beyotime Science Biotechnology; Shanghai, China). Subsequently, 80 μg of protein was loaded onto 10 or 12% polyacrylamide gels separated by SDS–PAGE and then transferred to a polyvinylidene difluoride (PVDF) membrane. The membranes were incubated with the appropriate primary antibody [β-catenin (1:1000), p-β-catenin (1:1000), p-GSK-3β (1:1000), GSK-3β (1:1000), Runx2(1:1000), DKK1(1:1000), cathepsin K (1:1000), OPG (1:1000), and RANKL(1:1000)] overnight at 4 °C. The membranes were then incubated with the corresponding horseradish peroxidase-labeled secondary antibodies for 1 h at room temperature. Immunopositive bands were visualized with high sensitivity enhanced chemiluminescence luminous liquid, and the images were captured with Azure Bio-imaging systems (California, USA). The same membrane was stripped and re-probed. The bands were quantified using Azurespot software and normalized with the corresponding β-actin band (1:5000).
Statistical analysis
All the data obtained from animal experiments of different groups were expressed as ‾x ± SD. When the data meets a normal distribution and variances are homogeneous, one-way analysis of variance (ANOVA) test was used (version 22.0, IBM SPSS Statistics, IBM Corp., Armonk, New York, NY, USA). When the data meets a normal distribution but the variances are not homogeneous, Dunnett’s T3 test was used. When the data do not meet a normal distribution, nonparametric analysis was used. Statistical significance was determined at p < 0.05, and p < 0.01.
Results
Characterization of the main constituents in RRP aqueous extract
Several ingredients in the RRP aqueous extract were identified by HPLC–DAD–ESI–MSn. As shown in supplement Fig. 1a, b as well as supplement Table 1, six compounds were distinguished: (1) echinacoside, (2) jionoside A1/A2, (3) acetoside, (4) isoacetoside, (5) jionoside B1, and (6) jionoside B2. In addition, the phenol-sulfuric acid assay revealed that the total polysaccharide content in the RRP aqueous extract was approximately 3.16 ± 0.01%.
RRP reduced the body weight gain in OVX rats
The body weight gains of the rats in each group are shown in Fig. 1a. Body weights significantly increased in all animal groups during the experiment. However, rat body weight increased more significantly from the 3rd week to the end of the experiment in the OVX control group than in Sham control group (p < 0.05). The ovariectomy induced body weight gain was effectively attenuated by the EV or RRP treatment starting from the 4th or 9th week until the end of experiment, respectively. And there was no significant difference in body weight gain between the RRP group and the Sham control group starting from the 11th week to the end of the intervention.
Rehmanniae Radix Preparata suppresses ovariectomy-induced body weight gain and alters serum biomarkers as well as does not show uterotrophic effect in rats. a The body weight changes of different groups of rats at 0, 4, 8, 12, and 14 weeks. b–e Serum levels of TRAP, RANKL, ALP, and OPG in the treatment and control groups of rats. Serum samples from nine rats per group were taken for each assay. f Light microscopy images of uteri from sham, OVX, EV, and RRP. Scale bar 500 μm. g Uterine weight/body weight. h Uterus size. i luminal area. j Luminal epithelial height. Size measurements were performed with ImageJ software. Data represent mean ± SD. #p < 0.05 compared with Sham group, *p < 0.05 compared with OVX group. n = 9
RRP increased bone formation and inhibited bone resorption in OVX rats
As shown in Fig. 1b to e, the serum levels of TRAP and RANKL were significantly increased in the rats of OVX control group as compared to those in the Sham controls. TRAP and RANKL were significantly decreased in the rats of the EV and RRP groups compared to those in the OVX controls (p < 0.05). Ovariectomy induced a high turnover in the rats as demonstrated by an increase in serum ALP activity in the rats of OVX group in comparison with those in the Sham controls (p < 0.05). Application of EV or RRP to OVX rats reduced bone turnover when compared to vehicle-treated OVX animals (p < 0.05). Furthermore, the observed serum OPG levels were improved in the EV and RRP groups as compared to those in the OVX control (p < 0.05). These results indicate that the aqueous RRP extract has the ability of rebuilding bone homeostasis in OVX rats.
RRP did not show uterotrophic effects in OVX rats
As shown in Fig. 1f to j, a significant reduction in relative uterine weight, uterine area, luminal area, and luminal epithelial cell height in vehicle-treated OVX rats was observed when compared to that of vehicle-treated Sham control rats (p < 0.05). The abovementioned parameters were significantly increased in EV-treated rats compared with those in vehicle-treated OVX controls. Interestingly, application of RRP to OVX rats did not induce a significant increase in the abovementioned parameters. Moreover, histological analysis of the uteri showed no difference between vehicle-treated OVX and RRP-treated OVX rats (Fig. 1f). These data suggest that RRP may not possess relevant estrogenic activity after 14 weeks of intervention.
RRP preserved the bone structures in OVX rats
As shown in Fig. 2a, H&E staining revealed a dense and regular meshwork of trabecular bone in rat femurs of the Sham control group. In contrast, the trabecular bone in rat femurs of the OVX control group became thinner and irregular, and the bone trabecular reticulate structure was destroyed. Interestingly, the trabecular bone became thicker and more regular in rat femurs of the EV or RRP group. These alterations are most evident in the rat femurs of the EV group, although a complete preservation of the normal bone structure was not achieved.
Rehmanniae Radix Preparata alleviates ovariectomy-induced changes in the bone microarchitecture and compromises bone strength in rat femurs. a The representative micro-images of H&E staining for the different treatments and control groups. b, c The representative micro-images and their analyses of Safranin O/Fast Green-stained tissue sections of the different groups. The red color in the b denotes glycosaminoglycans (GAGs). d, e The results of Alizarin Red S staining and their analyses in the different animal groups. Yellow star denotes calcified nodules. Data are presented as mean ± SD. #p < 0.05 compared with Sham group, *p < 0.05 compared with OVX group, n = 9
To further observe the effect of RRP on bones in the osteoporotic rats, Safranin O/Fast Green and Alizarin red S staining were used to examine the epiphyseal structure in femoral heads and osteoblastic differentiation. Glycosaminoglycans (GAGs) are naturally occurring carbohydrates found in cartilage that bind to hydroxyapatite and contribute to osteoblast differentiation [25]. As shown in Fig. 2b and c, the red color represents the staining of GAGs by Safranin O. The thickness of cartilage adjacent to the growth plate was significantly reduced in rat femoral heads of the OVX control group when compared to those in the Sham controls (p < 0.05), showing that an ovariectomy may impede osteoblast differentiation and bone formation. Supplementation with RRP or EV to OVX rats resulted in a significant improvement in the distribution and content of GAGs when compared to vehicle-treated OVX control rats (p < 0.05).
Alizarin red S reacts with calcium, which precipitates and forms calcified nodules during osteoblast differentiation and bone formation [26]. As shown in Fig. 2d and e, the distribution of femur calcified nodules was uneven and the area of calcified nodules (IOD value) was markedly reduced in the femurs of OVX control rats compared to that of Sham control rats (p < 0.05). Administration of RRP or EV to OVX rats for 14 weeks clearly preserved the distribution and increased the area of calcified nodules when compared to the rats in the OVX control group (p < 0.05). RRP treatment almost restored the distribution and content of GAGs and calcified nodules to the levels seen in the vehicle-treated Sham controls.
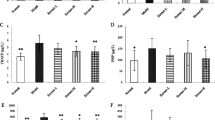
RRP increased BMD and cortical bone thickness in OVX rats
As shown in Fig. 3a to f, the rats in the OVX control group presented a notable reduction in the levels of femur head BMD, lumbar BMD, cancellous BMD, cortical bone thickness, cortical area ratio, and trabecular area ratio when compared to rats in the Sham controls, indicating that estrogen deprivation for 14 weeks induced a notable decrease in bone density and a thinning of the trabecular bone structure. In contrast, both treatment with EV or RRP led to a marked increase in the levels of femoral head BMD, lumbar head BMD, cancellous BMD, cortical bone thickness, and trabecular area ratio when compared with those of vehicle-treated OVX control group (p < 0.05). However, in comparison with the rats in the vehicle-treated OVX control group, neither RRP nor EV treatment increased the cortical area ratio in OVX rats. Furthermore, RRP treatment did increase lumbar BMD in OVX rats when compared to vehicle-treated OVX controls. But there are still statistical differences in the lumbar BMD between the Sham control and RRP group.
Rehmanniae Radix Preparata attenuates ovariectomy-induced decrease in BMD in rat femurs. a, b The BMD values of the femur head and lumbar bones in the treatment and control groups of rats as determined by dual-energy X-ray absorptiometry, respectively. c–f The values of cancellous BMD, cortical (Cort.) thickness, Cort. area ratio, and trabecular (Trab.) area ratio in different groups of rats determined by X-ray micro-computed tomography. g–i the results of three-point bending examination of ultimate load, elastic modulus, and bending strength in the respective animal groups. Data are presented as mean ± SD. #p < 0.05 compared with Sham group, *p < 0.05 compared with OVX group, n = 6
RRP preserved biomechanical properties in femurs of OVX rats
To investigate the effect of RRP treatment on the biomechanical properties of bone, rat femurs were subjected to a three-point bending assay. As shown in Fig. 3g to i, the ultimate load, bending strength, and elastic modulus were significantly reduced in the femurs of the rats in the OVX control group when compared with those in the Sham control group (p < 0.05). As expected from the bone morphogenic improvements reported above, treatment with either EV or RRP to OVX rats also maintained the ultimate load, bending strength, and elastic modulus (p < 0.05) in the femurs compared with untreated OVX controls. These results indicate that RRP treatment has the ability to increase the mechanical properties of the femurs in the OVX rats. However, RRP treatment did not preserve femoral ultimate load in OVX rats when compared to the vehicle-treated Sham control rats.
RRP regulated Wnt/β-catenin signaling in OVX rats
Wnt signaling promotes osteoblast differentiation and osteogenesis through increased expression of p-GSK-3β and its downstream factor β-catenin [27]. Since RRP treatment increased BMD and bone strength in OVX rats, we next studied whether these effects were mediated by the Wnt/β-catenin signaling. As shown in Fig. 4a, the relative ratio of p-GSK-3β to GSK-3β was decreased in rat tibias of the OVX control group as compared to those in the Sham control group (p < 0.05). Interestingly, the relative ratio of p-GSK-3β to GSK-3β was markedly increased in rat tibias of the EV group when compared to those in the OVX control group (p < 0.05) but did not reach the levels seen in the Sham controls (p < 0.05).
Treatment of OVX rats with Rehmanniae Radix Preparata improves WNT/β-catenin signaling in the rat tibias. The representative images of western blots and images analyses show the relative expression ratios of p-GSK-3β to GSK-3β (a), p-β-catenin to β-catenin (b), Runx2 (c), and DKK1 (f) in the tibias of treatment and control groups. The representative micro-images (d and g) and their analyses (e and h) of the immunohistochemical staining show β-catenin and sclerostin expressions in the tibias of the treatment and control groups of rats. The dark brown particles denote positive staining. IOD denotes integrated optical density of interested areas. Data are presented as mean ± SD. #p < 0.05 compared with Sham group, *p < 0.05 compared with OVX group, n = 9
Furthermore, as shown in Fig. 4b, d, e, the relative ratio of p-β-catenin to β-catenin was significantly increased, and the expression of β-catenin was decreased in the rat tibias of the OVX control group in comparison with that in the Sham control group (p < 0.05). As expected, RRP or EV treatment significantly reversed the trend of the expression of β-catenin and the relative ratio of p-β-catenin to β-catenin in the tibias of OVX rats as compared to that of OVX control rats (p < 0.05) but again did not reach the level seen in the Sham controls (p < 0.05).
Runx2 is one of the major transcription factors for inducing osteogenic differentiation [28]. As shown in Fig. 4c, Runx2 expression was decreased in rat tibias of the OVX control group as compared to that in the Sham control group (p < 0.05). Interestingly, the Runx2 expression was markedly enhanced in the rat tibias of the EV and RRP groups as compared to those of the OVX control group (p < 0.05).
As is well known, DKK1 and sclerostin can induce an inhibition of Wnt signaling and subsequent bone formation [27, 29]. Accordingly, as shown in Fig. 4f to h, the expression of DKK-1 and sclerostin was significantly increased in the rat tibias of OVX group when compared with the Sham control group (p < 0.05). After 14 weeks of treatment, the expression of DKK-1 and sclerostin was significantly attenuated in rat tibias of the RRP or EV group compared to those of the OVX control group (p < 0.05). Moreover, RRP intervention reversed the expression of DKK-1 and sclerostin in OVX rats compared to the expression measured in the Sham controls. These results indicate that RRP treatment may promote bone formation through activation of the Wnt/β-catenin signaling pathway.
RRP increased the expression of OPG but decreased the expression of RANKL and cathepsin K in OVX rats
OPG is mainly secreted by stromal cells and osteoblasts, which bind to RANKL and further inhibit the interaction between RANKL and RANK, thereby preventing subsequent osteoclastogenesis and bone resorption [30]. Cathepsin K is mainly secreted by osteoclasts and is considered key factor of osteoclast activity and bone resorption [18]. Therefore, we examined the effect of RRP on the expression of OPG, RANKL, and cathepsin K in OVX rats. IHC staining results (Fig. 5a–f) indicated that the expression of RANKL and cathepsin K was significantly enhanced and OPG expression was markedly decreased in the proximal femurs of OVX control rats, when compared to those in the Sham control group (p < 0.05). On the other hand, RRP or EV treatment reversed the expression of OPG, RANKL, and cathepsin K in the femurs of OVX rats compared with those in OVX controls (p < 0.05), but less than that in the Sham controls (p < 0.05). These results were also corroborated by western blot analysis (Fig. 5g, h).
Treatment of OVX rats with Rehmanniae Radix Preparata improves OPG/RANKL/cathepsin K signaling in the tibial and femoral bones. The representative micro-images of immunohistochemical staining (sections were counterstained with hematoxylin; original magnification, × 200 and × 400) show the expression of OPG (a, b), RANKL (c, d), and cathepsin K (e, f) in the rat femurs of treatment and control groups. The representative images and their analyses of western blot assay show that the relative expression of OPG to RANKL (g) and that of cathepsin K (h) in the rat tibias of the treatment and control groups. Data are presented as mean ± SD. The dark brown particles denote positive staining. IOD denotes integrated optical density of interested areas. #p < 0.05 compared with Sham group, *p < 0.05 compared with OVX group. n = 9
RRP increased IGF-1 expression in OVX rats
IGF-1 promotes osteoblasts differentiation and bone mineralization [24]. Therefore, we determined the effect of RRP on the expression of IGF-1 in the femurs of OVX rats using IHC staining. As shown in Fig. 6a, b, the expression of IGF-1 in rat femurs of the OVX control group was markedly decreased in comparison with expression in the Sham controls (p < 0.05). In contrast, treatment of OVX rats with RRP and EV for 14 weeks significantly promoted IGF-1 expression when compared with the expression in vehicle-treated OVX controls (p < 0.05).
Rehmanniae Radix Preparata (RRP) improves IGF-1 expression in the tibias and potential mechanism of RRP prevents the development of osteoporosis in OVX rats. The representative micro-images (a) and their analyses (b) of immunohistochemical staining (sections were counterstained with hematoxylin; original magnification, × 200 and × 400) show the IGF-1 expression in the rat femurs of the treatment and control groups. Data are presented as mean ± SD. The dark brown particles denote positive staining. IOD denotes integrated optical density of interested areas. #p < 0.05 compared with Sham group, *p < 0.05 compared with OVX group. n = 9. (c) The potential mechanism of Rehmanniae Radix Preparata (RRP) against osteoporosis in OVX rats. RRP may downregulate overexpression of DKK1 and sclerostin as well as increase IGF-1 expression, which further activates the Wnt/β-catenin signaling pathway and contributes to subsequent bone formation. RRP may also increase OPG expression and secretion, which leads to an inhibition of the RANKL/cathepsin K-mediated bone resorption. In addition, the increased expression of β-catenin further activates OPG/RANKL sginaling. The sign (↓) means promoting. The sign (┴) means inhibiting. CTSK cathepsin K, DKK1 Dickkopf-related protein-1, GSK-3β glycogen synthase kinase 3β, IGF-1 insulin-like growth factor-1, OPG osteoprotegerin, RANKL receptor activator for nuclear factor-κB ligand
Discussion
In the present study, we (i) demonstrated that administration with RRP to OVX rats decreased serum levels of TRAP, RANKL, and ALP and increased serum OPG level and (ii) showed that RRP aqueous extract also preserved BMD, bone strength, and the bone microstructure in OVX rats. Moreover, we found that RRP reduced the expressions of DKK1 and sclerostin and the ratio of p-β-catenin to β-catenin in OVX rats. This was in line with increased expression of OPG, IGF-1, Runx2, and β-catenin, which was also reflected increased ratios of p-GSK-3β to GSK-3β and OPG to RANKL. The analysis of long bones (femurs and tibias) also revealed suppressed expression of cathepsin K and RANKL in OVX rats.
Previous clinical studies showed that RRP, in combination with other herbs, was able to increase BMD and improve bone quality as well as alleviate lower back pain in osteoporotic patients [1, 31]. In addition, Liuwei Dihuang pills, in which RRP is one of the main ingredients, have been shown to increase BMD in femurs and to improve the biomechanical stability of vertebrae bones in OVX rats [32]. Furthermore, Lim et al. [33] demonstrated that an 8-week treatment with the dried root of Rehmannia glutinosa significantly preserved BMD in the femurs and lumbar vertebrates of OVX rats. Moreover, RRP also increased the trabecular area in the tibias and the modulus of rigidity in the lumbar vertebrae in rats with glucocorticoid-induced osteoporosis [34]. Our current findings in OVX rats are fully in line with these previous studies.
In the current study, we demonstrate that OVX induced a significant body weight gain in rats. This is in line with the previous observation that OVX-induced hyperphagia led to adiposity and subsequent body weight gains in the lean and fat mass in rats [35]. Indeed, OVX rats consumed more chow than the Sham controlled littermates. Moreover, OVX-induced hyperphagia and subsequent body weight gain do not affect BMD or bone biomechanical strength at 14th week after surgery. Therefore, pair-feeding may not be essential when the study is aimed to observe the effect on bone quality. In addition, ovariectomy did not lead to the alteration of total level of fuel utilization in rats [36]. And daily leptin injections to OVX rats initially increased the feeding in the first 2 weeks and then remained at the stable level for the duration of the study. Furthermore, Rehmannia glutinosa is demonstrated to have the ability of improving glucolipid metabolism and redox hemostasis [37, 38], which may contribute to inhibit body weight gain in OVX rats. However, further studies are still needed to figure out the potential mechanism of RRP on the body weight gain in OVX rat.
Our current findings demonstrated that RRP did not show uterotrophic effect in OVX rats. The results are consistent with the previous observation that the administration of RRP to OVX rats for 3 months did not increase serum estrogen levels [39]. However, one study performed by Zhang et al. claimed that Rehmanniae Radix treatment for 20 weeks and 40 weeks did upregulate serum estrogen level as well as inhibiting endometrial atrophy and loss in the management of OVX rats [2]. Thus, long-term administration of RRP to OVX rats may still have estrogen-like effects.
Our current results revealed that RRP suppressed the expressions of DKK1 and sclerostin and reduced the ratio of p-β-catenin to β-catenin in OVX rats. It has been demonstrated that overexpression of DKK1and sclerostin reduces osteoblast activity and further diminishes bone formation [29, 40]. Interestingly, Liuwei Dihuang pills containing RRP have been demonstrated to activate Wnt/β-catenin signaling through increased expression of Lrp-5, β-catenin, Runx2, and osterix in osteoporotic rats [32]. Here, we demonstrated that β-catenin accumulation upregulated Runx2 expression in the rats of the RRP group, which contributes to osteoblasts differentiation and proliferation [7]. Moreover, catalpol and acteoside, two major components in RRP, have been previously shown to inhibit the expression of GSK-3 and increase the accumulation of β-catenin in the nucleus [41]. The activated Wnt/β-catenin also suppresses high bone turnover, evidenced by decreasing ALP levels in OVX rats [1]. Together, these findings may indicate that the anti-osteoporotic effect of RRP in OVX rats is signaled via improving the Wnt/β-catenin signaling, which results in enhanced bone formation and bone homeostasis.
The present study showed that RRP increased OPG expression and secretion in OVX rats, which competitively binds to RANKL and subsequently prevents the binding of RANKL to RANK and thus osteoclast activation and bone resorption. In addition, the activation of Wnt/β-catenin may further stimulated OPG expression [42] in response to RRP treatment. Indeed, we observed reduced RANKL secretion and expression as well as consolidated BMD and bone strength in RRP-treated OVX rats. The anti-bone loss effect of RRP in OVX rats was also reflected by decreased TRAP activity and reduced cathepsin K expression. Interestingly, the aqueous extract of RRP has been reported to increase expression of collagen and OPG in primary and MG63 osteoblastic cells, as well as decrease the numbers of TRAP positive bone marrow mononuclear cells and to reduce resorption by primary osteoclasts [3]. In addition, echinacoside, one of the main ingredients in the aqueous RRP extract, has been demonstrated to increase the relative ratio of OPG to RANKL in MC3T3-E1 osteoblastic cells [43]. Therefore, it is reasonable to conclude that RRP exhibited anti-bone resorptive activity through regulation of the OPG/RANKL/cathepsin K signaling pathway.
IGF-1 is highly expressed in osteoblasts and chondrocytes [44] and exhibits a bone anabolic effect through promoting osteoblast differentiation and mineralization [45]. Clinically, decreased serum IGF-1 is positively associated with vertebral fractures in postmenopausal women with type-2 diabetes [46]. IGF-1 may improve bone remodeling and bone strength through inhibiting GSK-3β and enhancing the stability of β-catenin [23]. Ovariectomy increases the RANKL to OPG ratio and thus accelerates trabecular bone loss by downregulating IGF-1 [47]. However, whether the increased IGF-1 levels will reduce the RANKL to OPG ratio remains to be clarified. The results from the present study demonstrate that RRP increases IGF-1 expression in the femurs of OVX rats, which may further stimulate Wnt/β-catenin signaling and subsequently inhibits bone loss and increase bone strength.
In the present study, six compounds were identified in the aqueous RRP extract, which included echinacoside, jionoside A1/A2, acetoside, isoacetoside, jionoside B1, and jionoside B2. Echinacoside was reported to increase BMD and improve femur microstructure as well as bone strength in OVX rats [48]. In addition, echinacoside [43], acteoside, and isoacteoside [49] were shown to promote the proliferation, differentiation, and mineralization of osteoblastic MC3T3-E1 cells. Furthermore, Rehmannia glutinosa polysaccharides, the main ingredients in the aqueous extract of RRP, may also contribute to bone improvement through their effects on the intestinal microbiota [50, 51]. Therefore, the abovementioned identified compounds of RRP may in large part account for the anti-osteoporotic activity of herbal extract in OVX rats.
However, there are some limitations in the current study. First, a daily single dose of RRP (2.5 g/mL/100 g) administered to OVX rats for the duration of the treatment in the current study may not provide strong evidence to show the efficacy of this herb. Indeed, Radix Rehmanniae and RRP have repeatedly been demonstrated to inhibit ovariectomy-induced bone loss in rats [1] and the current study was intended to investigate the potential mechanism of RRP on bone improvement in OVX rats. Second, the female rats used were around 10 weeks of age in the current study, which may resemble adolescence in human life spans [52]. Therefore, the effects of OVX may be compensated because of the growing skeleton. In fact, RRP was exposed to OVX rats for 14 weeks at 2nd week after surgery. It can therefore be suggested that the bones during this treatment period resemble that of bones in peri-menopausal women. Furthermore, the bone loss lasts from 4 weeks to 6 months. Therefore, the current study was conducted mainly to demonstrate the preventive effect on bone quality in OVX by RRP and would thus constitute a prophylactic anti-osteoporotic therapy. Further studies are needed to study the curative effect of RRP on osteoporotic rats. Clinically, accumulating evidence also suggests that RRP may be effective for preventing the development of primary and secondary osteoporosis [1].
In conclusion, RRP attenuates the decrease in bone mass and strength along with the deterioration of the bone microstructure in OVX rats. The underlying mechanism may be partly attributed to the regulation of the Wnt/β-catenin signaling pathway (Fig. 6c). Although the active substances remain to be ultimately determined, our study has the potential to develop new anti-osteoporotic drugs from this herb in the future.
Change history
18 June 2019
There was a mistake in the part of <Emphasis Type="Italic">OVX rats model and RRP intervention</Emphasis> in the original publication.
Abbreviations
- ALP:
-
Alkaline phosphatase
- ANOVA:
-
One-way analysis of variance
- BMD:
-
Bone mineral density
- CT:
-
Computed micro-tomography
- DKK1:
-
Dickkopf-related protein 1
- ELISA:
-
Enzyme-linked immunosorbent assay
- EV:
-
Estradiol valerate
- GAGs:
-
Glycosaminoglycans
- GSK-3β:
-
Glycogen synthase kinase 3 beta
- H&E:
-
Hematoxylin/eosin
- IGF-1:
-
Insulin-like growth factor 1
- IOD:
-
Integrated optical density
- OPG:
-
Osteoprotegerin
- OVX:
-
Ovariectomized
- p-GSK-3β:
-
Phospho-glycogen synthase kinase 3 beta
- RANK:
-
Receptor activator of nuclear factor kappa-Β
- RANKL:
-
Receptor activator of nuclear factor kappa-Β ligand
- RRP:
-
Rehmanniae Radix Preparata
- Runx2:
-
Runt-related transcription factor 2
- TCM:
-
Traditional Chinese medicine
- TRAP:
-
Tartrate-resistant acid phosphatase
References
Liu C, Ma R, Wang L, Zhu R, Liu H, Guo Y, Zhao B, Zhao S, Tang J, Li Y, Fu M, Zhang D, Gao S (2017) Rehmanniae Radix in osteoporosis: A review of traditional Chinese medicinal uses, phytochemistry, pharmacokinetics and pharmacology. J Ethnopharmacol 198:351–362
Zhang X (2014) Experimental study of Selaginella and Rehmannia glutinosa libosch extract on rats with osteoporosis intervention of the ovary. Science of Chinese materia medica. Henan University of Chinese Medicine, p 73
Oha K-O, Kim S-W, Kim J-Y, Ko S-Y, Kim H-M, Baek J-H, Hyun-Mo Ryoo KJ-K (2003) Effect of Rehmannia glutinosa Libosch extracts on bone metabolism. Clin Chim Acta 334:185–195
Chen Y, Qu C, Zhong H, Xue Y, Zhou C, Li W, Cheng X (1994) Effects of Liuwei Dihuang wan and some other TCM drugs on bone biomechanics and serum 25 (OH) D3 content in rats. J Tradit Chin Med 14:298–302
Sun H, Zhang N, Li LJ, Wang XJ (2006) Promoting effect of constituents in plasma after oral administration of Liuwei Dihuang wan on proliferation of rat osteoblast. China J Chin Mater Med 33:2161–2164
Peng SF (2007) Study of added decoction of six drugs containing Rehmannia root on osteoporosis after menopause. Mod J Integr Tradit Chin West Med 16:4592–4593
Xia B, Xu B, Sun Y, Xiao L, Pan J, Jin H, Tong P (2014) The effects of Liuwei Dihuang on canonical Wnt/β-catenin signaling pathway in osteoporosis. J Ethnopharmacol 153:133–143
Wang L, Li Y, Guo Y, Ma R, Fu M, Niu J, Gao S, Zhang D (2016) Herba epimedii: an ancient Chinese herbal medicine in the prevention and treatment of osteoporosis. Curr Pharm Des 22:328–349
Lin X, Xiong D, Peng YQ, Sheng ZF, Wu XY, Wu XP, Wu F, Yuan LQ, Liao EY (2015) Epidemiology and management of osteoporosis in the People’s Republic of China: current perspectives. Clin Interv Aging 10:1017–1033
Tabatabaei-Malazy O, Salari P, Khashayar P, Larijani B (2017) New horizons in treatment of osteoporosis. Daru 25:2
Guo Y, Li Y, Xue L, Severino RP, Gao S, Niu J, Qin LP, Zhang D, Bromme D (2014) Salvia miltiorrhiza: an ancient Chinese herbal medicine as a source for anti-osteoporotic drugs. J Ethnopharmacol 155:1401–1416
Hu J, Mao Z, He S, Zhan Y, Ning R, Liu W, Yan B, Yang J (2017) Icariin protects against glucocorticoid induced osteoporosis, increases the expression of the bone enhancer DEC1 and modulates the PI3K/Akt/GSK3beta/beta-catenin integrated signaling pathway. Biochem Pharmacol 136:109–121
Sheng H, Li W, Sheng C, Chen X, Su B, Li H, Yu Y, Xu G, Zhang G, Wu G, Qin L, Qu S (2012) Experimental study of GSK-3β for the development of anti-diabetic molecular target drug for bone protection. The Proceedings of the Eleventh Endocrine Academic Conference 2
Tsentidis C, Gourgiotis D, Kossiva L, Marmarinos A, Doulgeraki A, Karavanaki K (2017) Increased levels of Dickkopf-1 are indicative of Wnt/beta-catenin downregulation and lower osteoblast signaling in children and adolescents with type 1 diabetes mellitus, contributing to lower bone mineral density. Osteoporosis Int 28:945–953
Appelman-Dijkstra NM, Papapoulos SE (2016) Sclerostin inhibition in the management of osteoporosis. Calcif Tissue Int 98:370–380
Boyce BF, Xing L (2007) The RANKL/RANK/OPG pathway. Curr Osteoporos Rep 5:98–104
Fassio A, Rossini M, Viapiana O, Idolazzi L, Vantaggiato E, Benini C, Gatti D (2017) New Strategies for the Prevention and Treatment of Systemic and Local Bone Loss: from Pathophysiology to Clinical Application. Curr Pharm Des 23:6241–6250
Brömme D, Panwar P, Turan S (2016) Cathepsin K osteoporosis trials, pycnodysostosis and mouse deficiency models: commonalities and differences. Expert Opin Drug Discovery 11:457–472
Wang L, Ma R, Guo Y, Sun J, Liu H, Zhu R, Liu C, Li J, Li L, Chen B, Sun L, Tang J, Zhao D, Mo F, Niu J, Jiang G, Fu M, Brömme D, Zhang D, Gao S (2017) Antioxidant effect of fructus ligustri lucidi aqueous extract in ovariectomized rats is mediated through Nox4-ROS-NF-kappaB pathway. Front Pharmacol 8:266
Nielsen SS (2010) Phenol-sulfuric acid method for total carbohydrates. In: Nielsen SS (ed) Food analysis laboratory manual. Springer US, Boston, pp 47–53
Jain VM, Karibasappa GN, Dodamani AS, Mali GV (2017) Estimating the carbohydrate content of various forms of tobacco by phenol-sulfuric acid method. J Educ Health Promot 6(90):90
Ueyama T, Yamamoto Y, Ueda K, Yajima A, Maeda Y, Yamashita Y, Ito T, Tsuruo Y, Ichinose M (2013) Is gastrectomy-induced high turnover of bone with hyperosteoidosis and increase of mineralization a typical osteomalacia. PLoS One 8:e65685
Ma R, Wang L, Zhao B, Liu C, Liu H, Zhu R, Chen B, Li L, Zhao D, Mo F, Li Y, Niu J, Jiang G, Fu M, Dieter B, Zhang D, Gao S. (2017) Diabetes Perturbs Bone Microarchitecture and Bone Strength through Regulation of Sema3A/IGF-1/β-Catenin in Rats. Cell Physiol Biochem 41:55–66
Guo Y, Wang L, Ma R, Mu Q, Yu N, Zhang Y, Tang Y, Li Y, Jiang G, Zhao D, Mo F, Gao S, Yang M, Kan F, Fu M, Zhang D (2016) JiangTang XiaoKe granule attenuates cathepsin K expression and improves IGF-1 expression in the bone of high fat diet induced KK-Ay diabetic mice. Life Sci 148:24–30.
Mathews S, Mathew SA, Gupta PK, Bhonde R, Totey S (2014) Glycosaminoglycans enhance osteoblast differentiation of bone marrow derived human mesenchymal stem cells. J Tissue Eng Regen Med 8:143–152
Song L, Zhao J, Zhang X, Li H, Zhou Y (2013) Icariin induces osteoblast proliferation, differentiation and mineralization through estrogen receptor-mediated ERK and JNK signal activation. Eur J Pharmacol 714:15–22
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19:179–192
Jiang Z, Wang H, Yu K, Feng Y, Wang Y, Huang T, Lai K, Xi Y, Yang G (2017) Light-Controlled BMSC Sheet-implant Complexes with Improved Osteogenesis via an LRP5/β-Catenin/Runx2 Regulatory Loop. ACS Appl Mater Interfaces 9:34674–34686
Shu R, Ai D, Bai D, Song J, Zhao M, Han X (2017) The effects of SOST on implant osseointegration in ovariectomy osteoporotic mice. Arch Oral Biol 74:82–91
Zhang C, Liao Q, Ming J, Hu G, Chen Q, Liu S, Li Y (2017) The effects of chitosan oligosaccharides on OPG and RANKL expression in a rat osteoarthritis model. Acta Cir Bras 32:418–428
Ge J, Xie L, Chen J, Li S, Xu H, Lai Y, Qiu L, Ni C (2016) Liuwei Dihuang pill treats postmenopausal osteoporosis with Shen (kidney) yin deficiency via Janus kinase/signal transducer and activator of transcription signal pathway by up-regulating cardiotrophin-like cytokine factor 1 expression. Chin J Integr Med 24:415–422
Xia B, Xu B, Sun Y, Xiao L, Pan J, Jin H, Tong P (2014) The effects of Liuwei Dihuang on canonical Wnt/beta-catenin signaling pathway in osteoporosis. J Ethnopharmacol 153:133–141
Lim DW, Kim YT (2013) Dried root of Rehmannia glutinosa prevents bone loss in ovariectomized rats. Molecules 18:5804–5813
Wu T, Zou L, He H, Wu KW (2015) Prevention of Radix Rehmanniae Preparata on glucocorticoid-induced osteoporosis in rats. Chem Eng Trans 46:67–72
Jiang JM, Sacco SM, Ward WE (2008) Ovariectomy-induced hyperphagia does not modulate bone mineral density or bone strength in rats. J Nutr 138:2106–2110
Chen Y, Heiman ML (2001) Increased weight gain after ovariectomy is not a consequence of leptin resistance. Am J Phys Endocrinol Metab 280:E315–E322
Wu P, Wu S, Tsai Y, Lin Y, Chao J (2011) Hot water extracted Lycium barbarum and Rehmannia glutinosa inhibit liver inflammation and fibrosis in rats. Am J Chin Med 39:1173–1191
Zhou J, Xu G, Yan J, Li K, Bai Z, Cheng W, Huang K (2015) Rehmannia glutinosa (Gaertn.) DC. polysaccharide ameliorates hyperglycemia, hyperlipemia and vascular inflammation in streptozotocin-induced diabetic mice. J Ethnopharmacol 164:229–238
Sheng L, Xing GS, Wang Y, Tan ZL, Lou JS (2006) The effect of Rehmanniae Radix Preparata on biochemical markers of bone metabolism and the bone mineral density in ovariectomized rats. Chin J Osteoporos 12:496–498
Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W, Kostenuik P, Lacey DL, Simonet WS, Bolon B, Qian X, Shalhoub V, Ominsky MS, Zhu Ke H, Li X, Richards WG. (2006) Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone 39:754–766
Zhang N (2016) Studies on chemical constituents and mechanism of Rehmanniae Radix Preparata for diabetic osteoporosis based on molecular docking strategy. Chinese Medicinal Resources. Second Military Medical University, Nanjing, p 97
Amirhosseini M, Madsen RV, Escott KJ, Bostrom MP, Ross FP, Fahlgren A (2018) GSK-3beta inhibition suppresses instability-induced osteolysis by a dual action on osteoblast and osteoclast differentiation. J Cell Physiol 233:2398–2408
Li F, Yang Y, Zhu P, Chen W, Qi D, Shi X, Zhang C, Yang Z, Li P (2012) Echinacoside promotes bone regeneration by increasing OPG/RANKL ratio in MC3T3-E1 cells. Fitoterapia 83:1443–1450
Masanobu Kawai RCJ (2012) The insulin-like growth factor system in bone. Endocrinol Metab Clin 41:323–333
Sheng MH, Lau KH, Baylink DJ (2014) Role of osteocyte-derived insulin-like growth factor I in developmental growth, modeling, remodeling, and regeneration of the bone. J Bone Miner Metab 21:41–54
Ardawi MS, Akhbar DH, Alshaikh A, Ahmed MM, Qari MH, Rouzi AA, Ali AY, Abdulrafee AA, Saeda MY (2013) Increased serum sclerostin and decreased serum IGF-1 are associated with vertebral fractures among postmenopausal women with type-2 diabetes. Bone 56:355–362
Lindberg MK, Svensson J, Venken K, Chavoshi T, Andersson N, Moverare Skrtic S, Isaksson O, Vanderschueren D, Carlsten H, Ohlsson C (2006) Liver-derived IGF-I is permissive for ovariectomy-induced trabecular bone loss. Bone 38:85–92
Li F, Yang X, Yang Y, Guo C, Zhang C, Yang Z, Li P (2013) Antiosteoporotic activity of echinacoside in ovariectomized rats. Phytomedicine 20:549–557
Van Kiem P, Quang TH, Huong TT, le TH N, Cuong NX, Van Minh C, Choi EM, Kim YH (2008) Chemical constituents of Acanthus ilicifolius L. and effect on osteoblastic MC3T3E1 cells. Arch Pharm Res 31:823–829
Yan H, Lu J, Wang Y, Gu W, Yang X, Yu J (2017) Intake of total saponins and polysaccharides from Polygonatum kingianum affects the gut microbiota in diabetic rats. Phytomedicine 26:45–54
Xu X, Jia X, Mo L, Liu C, Zheng L, Yuan Q, Zhou X (2017) Intestinal microbiota: a potential target for the treatment of postmenopausal osteoporosis. Bone Res 5:17046
Andreollo NA, Santos EF, Araujo MR, Lopes LR (2012) Rat’s age versus human’s age: what is the relationship? Arq Bras Cir Dig 25:49–51
Acknowledgments
The authors deeply thank Dr. Dahong Ju at the China Academy Sciences of Traditional Chinese Medicine for his great contribution to the current study. We also thank Angela Tether for the thorough English editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The protocol of the study was approved by the Ethics Committee of Beijing University of Chinese Medicine (BUCM).
Conflicts of interest
Dr. Dongwei Zhang received grants to conduct this study from the Beijing Municipal Natural Science Foundation (7172126) and the National Natural Science Foundation of China (NSFC81273995). Dr. Sihua Gao received grants from the National Natural Science Foundation of China (NSFC81274041) and the key drug development program of MOST (20122X09103201-005). Dr. Jianzhao Niu received a grant from the 111 project of MOE (B07007). Dr. Dieter Brӧmme received a grant from the Canadian Institute of Health Research (CIHR-MOP). All authors declare that the results presented in this manuscript are the true expression of findings, and there has been no interference in respondents’ free communication and dissemination.
Electronic supplementary material
Figure S1.
The potential compounds identified in the Rehmanniae Radix Preparata (RRP) aqueous extract. (a) HPLC chromatogram of RRP (λ = 334nm). The peaks in chromatogram were identified by LC-MSn as followings: (1) Echinacoside, (2) Jionoside A1/A2, (3) Acetoside, (4) Isoacetoside, (5) Jionoside B1, (6) Jionoside B2. (b) HPLC chromatogram of standard acetoside (λ = 334nm). (c) Chemical structures of six compounds in RRP aqueous extract. (PNG 214 kb)
Supplementary table 1
(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Liu, C., Wang, L., Zhu, R. et al. Rehmanniae Radix Preparata suppresses bone loss and increases bone strength through interfering with canonical Wnt/β-catenin signaling pathway in OVX rats. Osteoporos Int 30, 491–505 (2019). https://doi.org/10.1007/s00198-018-4670-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-018-4670-y