Abstract
Summary
Osteoporotic hip fractures can be life changing and can increase mortality. Treatment of osteoporosis following hip fracture is often delayed. We began offering osteoporosis medication during hospitalization for hip fracture, dramatically increasing the number of patients meeting standard of care.
Introduction
Osteoporotic hip fracture is a debilitating condition with major morbidity and mortality implications. Osteoporosis medication given within 90 days of hip fracture improves mortality and reduces risk of future fractures. The aim of this project was to improve rates of timely osteoporosis treatment following fragility hip fracture.
Methods
This was a two-step intervention utilizing the Plan-Do-Study-Act cycle, beginning with resident-focused education in cycle 1. In cycle 2, we offered osteoporosis medication to inpatients for hip fracture with help from a new electronic order set.
Results
Prior to this intervention, 32% of patients received osteoporosis medication within 90 days of fragility hip fracture; this improved to 81% after intervention.
Conclusions
Resident education and an electronic order set dramatically improved the percentage of patients meeting standard of care with osteoporosis pharmacotherapy following fragility fracture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteoporotic hip fractures are an unfortunate but common source of morbidity and mortality for the aging population [1, 2]. Timely medical therapy after hip fracture can mitigate this suffering. Studies show that zoledronate within 90 days of hip fracture reduces all-cause mortality by 28% and provides a 35% risk reduction in new fractures without slowing the surgical healing process [3,4,5]. Despite this clear benefit, rates for osteoporotic pharmacotherapy after fragility fractures are abysmally low [1, 6]. As of 2013, bisphosphonate therapy following fragility hip fracture decreased from 15 to 3% [7]. Only 23% of older women received either a bone mineral density scan or osteoporotic medication within 6 months of fragility fracture [8]. Inpatient teams have tried educating patients about osteoporosis and encouraging outpatient medical management with little success [6]. However, osteoporotic pharmacotherapy during hospitalizations for fragility fracture is gaining popularity [4, 9]. The aim of this project was to provide osteoporosis medication to patients with fragility hip fractures before hospital discharge. We hypothesized that this intervention would improve the rate of patients receiving osteoporosis medication within 90 days of injury, which is when they can gain the greatest benefit.
Methods
Upon review of this performance improvement project, Brooke Army Medical Center’s Exemption Determination Officer concluded that it did not require an institutional review board (IRB).
To establish a baseline understanding of our institutional practices, we reviewed 3 months of data on elderly patients admitted for osteoporotic hip fracture to the “Hip Service,” an internal medicine co-management service with orthopedic surgery. The setting was a Level 1 Trauma Center at a military hospital. Patients were a mixture of Department of Defense (DoD) beneficiaries who typically receive medical care in our system and civilian trauma patients who do not otherwise receive care in our hospital system. We annotated extenuating circumstances and comorbidities, like end-stage renal disease on hemodialysis, which could preclude osteoporotic therapies. We reviewed admission laboratory data and deemed patients eligible for osteoporosis pharmacotherapy if albumin-corrected calcium was ≥ 8 mg/dL, 25-hydroxy vitamin D ≥ 20 ng/mL, and glomerular filtration rate (GFR) ≥ 35 for zoledronate. We deemed patients with lower GFR’s as eligible for denosumab if they did not require dialysis. We then reviewed outpatient records of these same patients for 90 days following their hospital discharge to assess how many received osteoporotic therapy in that time period. Patients who did not present for outpatient care in our system during this time because they were not DoD beneficiaries or were otherwise lost to follow-up were classified as having an unknown outcome.
We then implemented a tool for health care quality improvement, the Plan-Do-Study-Act (PDSA) method [10]. This method provides structure and is a supportive mechanism for quality improvement in complex health care systems. Our first intervention cycle (PDSA cycle 1) consisted of presenting new literature during academic conferences about feasibility and safety of early pharmacotherapy for osteoporotic hip fracture. This didactic lecture was given in two settings: the internal medicine residency and the orthopedic surgery residency. For a 4-week period (corresponding to the duration of an internal medicine resident rotation on the Hip Service), we reminded internal medicine residents and staff to prescribe zoledronate 5 mg intravenously or denosumab 60 mg subcutaneously to patients with fragility hip fracture on the day of discharge. In keeping with the principles of the PDSA method, after a reasonable 4-week period of study, we recognized that a prime barrier to adherence with treatment was that our Hip Service team was simply forgetting to order the laboratory evaluations or medications in time for discharge. We felt that a structured and standardized approach via an electronic order set would help to overcome this barrier.
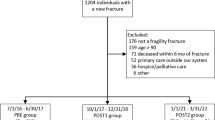
The second intervention cycle (PDSA cycle 2) was developed in conjunction with several services: Endocrinology, Internal Medicine, Pharmacy, Orthopedic Surgery, and Information Management. We created an electronic admission order set for hip fractures that includes standard admission orders with the key additions of 25-hydroxy vitamin D serum analysis and orders for zoledronate administration on the day of discharge. Upon completion, it became standard of practice to use the order set for all hip fracture admissions. We followed patients admitted for fragility hip fracture over the next 4 months, tracking how many were eligible for therapy at time of discharge, how many actually received therapy during hospitalization or outpatient within 90 days of fracture, and reasons why patients did not receive therapy (Fig. 1). Of note, we observed 4 months of data to ensure we had the opportunity to see several rotations of residents’ cycle through the Hip Service and assess for sustainability of our efforts. After 4 months, we felt that the paradigm shift in practice of treating prior to discharge was effectively becoming enculturated.
Results
Pre-intervention retrospective review demonstrated 40 patients admitted for hip fractures from June 6 to August 29, 2016. Three of these patients were excluded from analysis due to other factors including transfer to another hospital before surgery, multiple additional fractures, and transition to hospice for a separate terminal condition. Of the 37 remaining patients, 28 were DoD beneficiaries and 9 were not. Of these, 24 were eligible for osteoporotic pharmacotherapy before hospital discharge based on satisfactory laboratory values. However, none of these patients (0/24) received zoledronate or denosumab prior to discharge. Chart review over the next 90 days demonstrated that only 22 of the original 37 patients had known outcomes. Of these, only 7 of 22 (32%) received osteoporotic pharmacotherapy: 6 with a bisphosphonate and 1 with denosumab.
Eight patients were admitted for hip fracture during PDSA cycle 1, which lasted from November 21 to December 19, 2016. Five were eligible for pharmacotherapy during hospitalization but only 2 received zoledronate prior to discharge. The other 3 patients had borderline-low vitamin D levels so the inpatient team was hesitant to treat them. One patient received pharmacotherapy 4 months after injury and 2 had unknown outcomes.
There were 57 admissions for hip fracture during PDSA cycle 2, which lasted from December 19, 2016, to April 9, 2017 (Fig. 2). Two patients were excluded due to multiple fractures, leaving a total of 55 patients for evaluation of fragility hip fracture. Of these, 27 were DoD beneficiaries and 28 were not. Eleven patients were ineligible for inpatient pharmacotherapy due to low vitamin D levels. Of the 44 eligible patients, 25 received pharmacotherapy prior to discharge (25/44 = 57%); most received zoledronate and 4 received denosumab. Two eligible patients were offered but ultimately declined osteoporosis therapy prior to hospital discharge. The inpatient team was uncomfortable with borderline calcium, vitamin D, or GFR levels in the remaining 17 eligible patients who did not receive pharmacotherapy prior to discharge. Two eligible patients who did not receive therapy prior to discharge eventually received it in the outpatient setting. Two ineligible patients, due to low vitamin D levels, received vitamin D therapy and then outpatient osteoporosis pharmacotherapy once vitamin D levels had normalized. Thus, there is documentation for a total of 29 patients receiving osteoporosis pharmacotherapy within 90 days of injury. However, 13 of the eligible patients and 6 of the initially ineligible patients had unknown outcomes after hospital discharge. If we consider only patients with known outcomes, 81% (29/36) received osteoporosis pharmacotherapy within 90 days of injury (Fig. 3). Importantly, there were no readmissions for acute kidney injury or hypocalcemia following osteoporosis therapy.
Comparison osteoporosis pharmacotherapy for fragility hip fracture. Bar graph demonstrating rates of patients receiving osteoporosis pharmacotherapy after hip fracture. Graphic reflects upper limit of nationwide estimate. At baseline, 32% of patients in this project received osteoporosis medications within 90 days of injury. This rose dramatically to 81% with the project’s intervention (if only accounting for patients with known outcomes). BAMC, Brooke Army Medical Center
Discussion
There are many barriers to care that contribute to the abysmally low rates of osteoporotic pharmacotherapy after fractures; they include patient concern, provider discomfort, and logistics. Concerns for nonunion fractures with anti-resorptive therapy stem from conflicting animal and retrospective studies. An example is a retrospective study of humerus fractures that suggested higher rates of nonunion fractures with bisphosphonate therapy [11]. However, baseline characteristics between groups were not equal and rates of nonunion fractures were very low regardless of group (0.4 or 0.8%). The study conclusion was that clinicians could consider delaying bisphosphonate therapy as long as it does not interfere with ultimately initiating it.
It is fortunate that prospective, randomized studies of early bisphosphonate therapy have shown fracture healing to be non-inferior to delayed bisphosphonate therapy. One study randomized patients to zoledronate or placebo at various times within 3 months of hip fracture [3]. Of these, 46 patients received zoledronate within 2 weeks of hip fracture; their healing times and rates of nonunion fracture were non-inferior to placebo [12]. Another study randomized 80 patients suffering distal radius fracture to placebo or bisphosphonate therapy on postoperative day 1; there was no difference in fracture healing times [13]. An additional study randomized 90 patients after hip fracture to bisphosphonate therapy starting 7 days, 1 month, or 3 months after surgery; there was no difference in fracture healing times [14]. A further study randomized 82 patients after vertebral fracture to placebo or zoledronate on postoperative day 3; there was no difference in nonunion fractures [15].
There are other benefits of early bisphosphonate therapy. One study randomized 20 patients to placebo or bisphosphonate treatment for hip or knee replacements. All patients received hardware and allographic bone transplant. Operating room nurses soaked the allograph in either saline or bisphosphonate solution prior to implantation. Patients who received bisphosphonate-treated bone had better long-term joint alignment than placebo [16].
Many institutions employ fracture liaison services (FLS) to overcome barriers to care. They can identify patients with fragility fractures, refer for necessary assessment of bone health, and initiate appropriate treatment. There are four FLS types: Type A service identifies, evaluates, and initiates treatment; Type B service identifies and evaluates the patient but refers back to primary provider for treatment; Type C service identifies patients but notifies patient and primary provider to initiate evaluation and treatment as indicated; Type D service identifies patients at risk but no other communication is made with a provider for evaluation or treatment. FLS models can improve bone mineral density assessment and treatment for patients with fragility fractures, but require intensive resources. The International Osteoporosis Foundation described the optimal structure for FLS models in the Best Practice Framework [17]. Our project is analogous to an FLS model as represented by the orthopedic service identifying those with hip fragility fractures and the internal medicine hip service completing evaluation and treatment. One limitation to our system is the lack of outpatient follow-up for further evaluation and bone mineral density assessment that some FLS models include. This limitation is partly due to an institutional lack of resources to implement an operational follow-up system, frequent turnover in military primary care providers, and limited follow-up of civilian trauma patients.
Current guidelines recommend denosumab, rather than zoledronate, for use in patients with GFR < 35, and recommend against anti-resorptive therapy in those with chronic kidney disease (CKD) on hemodialysis [18]. Guidelines from Kidney Disease: Improving Global Outcomes (KDIGO) in 2017 no longer recommend bone biopsy for patients with CKD prior to anti-resorptive therapy [19]. There is speculation that anti-resorptive therapy, which includes denosumab, confers less benefit in patients with CKD and adynamic bone disease. This would lead to a less-efficacious treatment with denosumab, but may still offer some fracture protection, with minimal adverse effects.
Many patients do not understand that the risk/benefit ratio of osteoporosis medication is analogous to wearing a seatbelt [20]. By default, our patients received zoledronate prior to discharge unless medical providers specifically canceled the order. The opt-out rather than opt-in nature of osteoporosis medication administration demonstrated significant increase in rates of therapy provided prior to discharge. Patients continue to have the right to refuse medication, but offering it to them as normal practice can help them understand that this is standard of care. Making osteoporosis medication administration standard hospital practice prior to discharge can alleviate both patient and provider discomfort.
Logistical barriers to care are laboratory analysis and nursing support. Our laboratory performs vitamin D and electrolyte analysis; results are typically available within 24 h. The majority of our patients with fragility fractures are hospitalized for about 3 days before transferring to a physical rehabilitation facility. This gives sufficient time to assess if they meet safety criteria for pharmacotherapy without prolonging hospitalization as long as the laboratory is ordered and drawn early in the admission.
Many patients would benefit from injectable osteoporosis medication; however, this requires nursing support, which is typically more limited in the outpatient setting. After hip fracture, many patients have mobility limitations and require prolonged rehabilitation. This complicates outpatient management as patients often need significant transportation assistance to medical treatment facilities capable of providing these injections. The administrative burden of arranging transportation is a barrier to timely outpatient care. Additionally, inpatient teams have a difficult time predicting when patients will be ready to see their outpatient provider pending patient performance at the rehabilitation centers. As a result, many patients leave the hospital without outpatient follow-up appointments. Outpatient providers are then juggling new anticoagulation requirements, pain medications, rehabilitation needs, and complications from immobility during brief follow-up appointments; this greatly increases the chances of neglecting to address osteoporosis therapy within the standard of care timeframe. Long-acting, injectable osteoporosis medication prior to hospital discharge gives outpatient providers 6 to 12 months before needing to readdress this issue.
Standardized order sets can improve quality of care and patient outcomes for numerous conditions [21]. Other hospital systems have developed electronic order sets specifically for osteoporosis and have had improvement in osteoporosis management [22]. Multiple methods for designing strategies and measurement of improvement have been utilized in the past, with the PDSA method as the gold standard and specifically utilized to improve osteoporosis treatment with the implementation of an EMR-based order set [21, 23]. The PDSA method involves multiple cycles of process alteration and data analysis, reviewing lessons learned from prior cycles to improve subsequent ones. As the duration of each cycle is determined by the team assessing for improvement, each cycle does not need to be equal in length and should continue for as long as it takes to suggest sustainability or to develop a modification to the process to further improve the metrics in question. In our study, 4 weeks of evaluation was enough in cycle 1 to determine that mere education alone was not enough to significantly and sustainably change our practice. In contrast, 4 months use of the order set in cycle 2 suggested a definite and sustainable effect after adopting a more concrete means of ensuring considerations for treatment were made and orders were placed appropriately. The combination of efforts in these 2 cycles led to a 49% (81–32 = 49) improvement in osteoporosis treatment within 90 days of osteoporotic hip fracture, which places our institution well ahead of national efforts.
Limitations to this quality improvement project include its setting and design. It was a single-centered project in an urban environment over a short duration with a predominately military population; this may limit extrapolation to rural, suburban, and non-military settings. Some patients did not receive osteoporosis medication while inpatient because of vitamin D deficiency and many did not have evidence of receiving osteoporosis medication at later dates. This quality improvement project had a narrow scope of focus and did not seek to prove either efficacy or safety of this early treatment protocol, as that would have constituted a true research endeavor. While we are encouraged and confident that data already exists to suggest early intervention is safe, we recognize that the universal adoption of this practice is contingent on robust data and likely the adoption of this practice by a large society or organization in future guidelines for care of the osteoporotic hip fracture.
Our process improvement project lends itself to numerous future studies and projects of both a research and a quality improvement nature. For example, one could focus on tracking the treatment of vitamin D deficiency and ensuring implementation of osteoporosis therapy following normalization of these values in the outpatient setting following discharge. Possible options include giving high-dose vitamin D inpatient and proceeding with inpatient osteoporosis medication or facilitating outpatient fracture care through a coordinated effort with the Departments of Orthopedic Surgery and Medicine. Further areas of interest include long-term follow-up assessments for repeat fractures, mortality, or prolonged and sustained osteoporosis pharmacotherapy as these areas were beyond the scope of this project. Other notable projects might include evaluation for secondary causes of osteoporosis and how that might alter treatment plans or routine performance of dual-energy X-ray absorptiometry (DXA) scans after admission to assess for improvement in bone mineral density and compliance with other metrics of standard of care evaluation and therapy. Lastly, outcome data is partially affected by a military-specific limitation in that there is a higher rate of unknown patient outcomes due to the fact that many patients were not DoD beneficiaries and therefore had no follow-up in our system. Looking specifically at this vulnerable population may demonstrate added importance of treatment before disposition to the outpatient setting when these individuals are at especially heightened risk due to lack of ongoing care and may benefit from a longer-duration therapy that can bridge them until they have established insurance and follow-up.
In order to ensure sustainability of our project, we have taken measures to ingrain these practices into our institutional culture. The Hip Admission Order Set is available for all physicians to utilize at admission. This has since been coupled with a companion orthopedic post-operation order set to better facilitate care upon discharge from the operating room. A new Standard of Practice has been drafted between the Departments of Medicine and Orthopedic Surgery to facilitate best practices of care for patients co-managed on the Hip Service. Within this, the use of the order set as well as expectations for therapy for all eligible patients prior to discharge is clearly articulated. Furthermore, the documentation of treatment and with which modality is now clearly listed in all orthopedic follow-up notes after discharge. Lastly, we removed barriers to referral for outpatient injections of anti-resorptive therapy in the Medicine and Endocrine clinics if the orthopedic team identified them during follow-up care.
Summary/Conclusions
This project demonstrates feasible interventions that resulted in dramatic improvements in management of osteoporosis therapy following hip fractures. Pharmacotherapy during hospitalization for hip fracture tremendously improved the rate of patients receiving standard of care treatment for osteoporosis following fragility fracture. With the electronic order set, there was little additional burden on inpatient teams, and it did not increase hospital length of stay or readmission rates. A few providers appropriately changed the zoledronate order to denosumab for poor renal function. Importantly, no patients inappropriately received therapy when calcium, vitamin D, or renal function should have precluded it. Our pharmacy reported that pricing for osteoporosis medication did not increase when given inpatient, and actually saved money in our system by avoiding costs associated with future outpatient visits specifically for the purpose of medication administration.
The ultimate goal is to reduce the incidence of recurrent fractures and improve mortality. Future studies could assess the long-term safety and costs of this practice. While we recognize our specific approach may not be generalized to other practice settings and reimbursement structures, we hope that our successes might serve as a nidus for introspection and innovation at other institutions.
References
Cosman F, de Beur SJ, LeBoff M, Lewiecki EM, Tanner B, Randall S, Lindsay R, National Osteoporosis Foundation (2014) Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 25(10):2359–2381
Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C (2009) Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 20(10):1633–1650
Lyles KW, Colón-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, Hyldstrup L, Recknor C, Nordsletten L, Moore KA, Lavecchia C, Zhang J, Mesenbrink P, Hodgson PK, Abrams K, Orloff JJ, Horowitz Z, Eriksen EF, Boonen S, HORIZON Recurrent Fracture Trial (2007) Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357(18):1799–1809
Li YT, Cai HF, Zhang ZL (2015) Timing of the initiation of bisphosphonates after surgery for fracture healing: a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int 26(2):431–441
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR, HORIZON Pivotal Fracture Trial (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356(18):1809–1822
Gardner MJ, Brophy RH, Demetrakopoulos D, Koob J, Hong R, Rana A, Lin JT, Lane JM (2005) Interventions to improve osteoporosis treatment following hip fracture. A prospective, randomized trial J Bone Joint Surg Am 87(1):3–7
DHHS, Bone health and osteoporosis: a report of the Surgeon General. http://www.surgeongeneral.gov/library/bonehealth/content.html, 2004
Proposed changes to existing measure for HEDIS®1 2015: osteoporosis management in women who had a fracture (OMW). Available from: www.ncqa.org/HEDISQualityMeasurement.aspx
Drew S et al (2014) Describing variation in the delivery of secondary fracture prevention after hip fracture: an overview of 11 hospitals within one regional area in England. Osteoporos Int 25(10):2427–2433
Taylor MJ, McNicholas C, Nicolay C, Darzi A, Bell D, Reed JE (2014) Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf 23(4):290–298
Solomon DH, Hochberg MC, Mogun H, Schneeweiss S (2009) The relation between bisphosphonate use and non-union of fractures of the humerus in older adults. Osteoporos Int 20(6):895–901
Colón-Emeric C et al (2011) Association between timing of zoledronic acid infusion and hip fracture healing. Osteoporos Int 22(8):2329–2336
Uchiyama S, Itsubo T, Nakamura K, Fujinaga Y, Sato N, Imaeda T, Kadoya M, Kato H (2013) Effect of early administration of alendronate after surgery for distal radial fragility fracture on radiological fracture healing time. The Bone Joint J 95-B:1544–1550
Kim T-Y, Ha YC, Kang BJ, Lee YK, Koo KH (2012) Does early administration of bisphosphonate affect fracture healing in patients with intertrochanteric fractures? J Bone Joint Surg 94-B(7):956–960
Li C, Wang HR, Li XL, Zhou XG, Dong J (2012) The relation between zoledronic acid infusion and interbody fusion in patients undergoing transforaminal lumbar interbody fusion surgery. Acta Neurochir 154(4):731–738
Zampelis V et al (2017) Decreased migration with locally administered bisphosphonate in cemented cup revisions using impaction bone grafting technique. Acta Orthop
Walters S, Khan T, Ong T, Sahota O (2017) Fracture liaison services: improving outcomes for patients with osteoporosis. Clin Interv Aging 12:117–127
Camacho P et al (2016) American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract 22:1–42
Isakova T, Nickolas TL, Denburg M, Yarlagadda S, Weiner DE, Gutiérrez OM, Bansal V, Rosas SE, Nigwekar S, Yee J, Kramer H (2017) KDOQI US Commentary on the 2017 KDIGO clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Am J Kidney Dis 70(6):737–751
Qaseem A, Forciea MA, McLean RM, Denberg TD, for the Clinical Guidelines Committee of the American College of Physicians (2017) Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med 166(11):818–839
Senay A, Delisle J, Giroux M, Laflamme GY, Leduc S, Malo M, Nguyen H, Ranger P, Fernandes JC (2016) The impact of a standardized order set for the management of non-hip fragility fractures in a fracture liaison service. Osteoporos Int 27(12):3439–3447
Edwards BJ, Bunta AD, Anderson J, Bobb A, Hahr A, O’Leary KJ, Agulnek A, Andruszyn L, Cameron KA, May M, Kazmers NH, Dillon N, Baker DW, Williams MV (2012) Development of an electronic medical record based intervention to improve medical care of osteoporosis. Osteoporos Int 23(10):2489–2498
Harrington JT, Barash HL, Day S, Lease J (2005) Redesigning the care of fragility fracture patients to improve osteoporosis management: a health care improvement project. Arthritis Rheum 53(2):198–204
Acknowledgements
We appreciate the contributions of the Brooke Army Medical Center nursing staff, as well as the Departments of Internal Medicine, Endocrinology, Orthopedic Surgery, and Informational Management in their support of this quality improvement project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The views expressed herein are those of the authors and do not reflect the official policy or position of Brooke Army Medical Center, the US Army Medical Department, the US Army Office of the Surgeon General, the Department of the Army, the Department of the Air Force, the Department of Defense, or the US Government.
Rights and permissions
About this article
Cite this article
Kuiper, B.W., Graybill, S., Tate, J.M. et al. After the fall: improving osteoporosis treatment following hip fracture. Osteoporos Int 29, 1295–1301 (2018). https://doi.org/10.1007/s00198-018-4416-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-018-4416-x