Abstract
Introduction
Case reports of women sustaining multiple vertebral fractures (VF) soon afterdenosumab discontinuation are accumulating.
Methods
We report a woman with five new vertebral fractures in ~8 months following discontinuation of long-term odanacatib (ODN), an experimental cathepsin K inhibitor.
Results
DXA examination demonstrated an ~12% decline in bone mineral density (BMD) and ~9% decline in trabecular bone score (TBS) since ODN discontinuation. Laboratory evaluation did not reveal a secondary cause of bone loss.
Conclusions
This case mimics observations following denosumab discontinuation, but, to our knowledge, is the first reported with ODN and the first documenting substantial decline in TBS. While not directly clinically relevant as ODN is no longer being developed, this case raises the possibility that a syndrome of multiple vertebral fractures could follow discontinuation of various potent osteoporosis therapies that produce major BMD increases but do not have persisting bone effects (i.e., all non-bisphosphonates). Use of antiresorptive therapies to prevent rapid bone loss following discontinuation of potent bone active agents seems appropriate. Identification of those patients who could be at risk for the multiple VF syndrome is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Case series of women sustaining multiple vertebral fractures shortly after discontinuing denosumab have recently been reported. [1,2,3,4,5] This phenomenon has been described as “rebound-associated” vertebral fractures [3]; however, a recent post hoc analysis of the pivotal denosumab clinical trial (FREEDOM) found fracture risk returns to the level prior to treatment initiation, rather than rebounding to a higher level. [6] The mechanism(s) to explain this fracture syndrome, and the individuals at risk for its occurrence, remain to be defined; however, it could be speculated that this syndrome results from elevated bone remodeling. It is known that denosumab discontinuation leads to rapid elevation of bone remodeling markers to values substantially above pre-treatment levels with associated rapid decline in bone mineral density (BMD). [7,8,9] Elevated bone remodeling following resolution of anti-resorptive effect could potentially be mediated via the mechanostat or driven by repair of prior microcrack accumulation. [10, 11] It could be postulated that major remodeling increases could lead to trabecular perforation with resultant microarchitectural deterioration. If so, it is plausible that a similar phenomenon could occur when other bone-active agents that produce substantial BMD increases but do not have prolonged skeletal residence are discontinued. Here we report, to our knowledge, the first such case following discontinuation of odanacatib (ODN), a selective and rapidly reversible cathepsin K inhibitor.
Case history
A 67-year-old white woman entered an ODN phase III clinical trial in 2009. Details of the phase III study design and methods have been published previously. [12] At study baseline, she related no history of osteoporosis, prior clinical fracture, or use of bone-active medications. She was taking hydrochlorothiazide for hypertension and using calcium + vitamin D supplements. She had no history of clinical fragility fracture and no parental history of hip fracture. At study initiation, her L1–L4, total femur, and femoral neck BMD values in grams/cm2 were 1.072, 0.823, and 0.820 yielding T-scores of − 0.9, − 1.5, and − 1.6 respectively. Her L1–L4 TBS was 1.308 and her vertebral fracture assessment (VFA) demonstrated mild T8, T9, and T11 vertebral fractures (Fig. 1a) that were subsequently confirmed by spine radiographs (not shown). Her FRAX-estimated 10-year risk of major osteoporosis-related fractures was 16% and for a hip fracture 2.1% at that time. At study baseline, laboratory evaluation including calcium, creatinine, 25(OH)D, and liver function tests were normal.
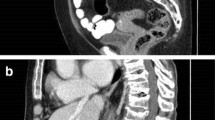
Vertebral fracture status in this individual over time. At study baseline, mild vertebral fractures were present on DXA vertebral fracture assessment at T7, T8, and T9 (1a; white arrows). Spine radiographs were routinely obtained as part of the study and validated these three fractures at study initiation (not shown). Radiographs at the time of study conclusion in September 2016 (1b; prevalent fractures noted by white arrows) confirmed that no new vertebral fractures occurred while on odanacatib. Note that for presentation here, the thoracic and lumbar radiographs were superimposed to make a “VFA-like” image. The five new vertebral fractures since odanacatib discontinuation are noted in 1c (black arrows with white outline)
She received blinded study drug as part of a placebo-controlled phase III fracture trial for ODN, an experimental cathepsin K inhibitor in development for postmenopausal osteoporosis. In addition, she received supplemental calcium and 5600 IU/week of vitamin D3 for the study duration. Upon study termination and unblinding of all subjects, it was determined that she received ODN 50 mg once weekly from September 2009 to September 2016 when the clinical trial was discontinued. While receiving ODN, her L1–L4, FN, and TF BMD increased by 13.3, 8.9, and 10.7% respectively (Table 1). Over the course of the study, she sustained no clinical fragility fractures. Additonally, spine radiographs at the time of study conclusion in September 2016 demonstrated no change in her vertebral fracture status (Fig. 1b). Retrospective lumbar spine trabecular bone score (TBS) analysis was performed and demonstrated a 10.0% increase over the course of the study (Table 1). At the time of study termination, her L1–L4, TF, and FN T-score were + 0.3, − 0.7, and − 0.7. No osteoporosis treatment was initiated following ODN discontinuation.
In January 2017 (approximately 4 months after discontinuation of ODN), she developed bronchitis; treatment included an 11-day tapering schedule of prednisone (maximum dose 40 mg/day). With this illness, she developed back pain following that required narcotics following coughing that ultimately led to spine MRI in February 2017 demonstrating acute vertebral fractures at T6, T7, T10, and L1. These fractures were treated initially with a Jewett brace; bedrest was not required. Laboratory evaluation in March 2017 found normal PTH, TSH, 25(OH)D, serum protein electrophoresis, salivary cortisol, creatinine, calcium, and alkaline phosphatase. Teriparatide, 20 mcg subcutaneously once daily, was initiated on April 20, 2017. BMD and VFA were performed on the research DXA instrument used for the ODN study in June 2017. This VFA demonstrated her known vertebral fractures at T6 through 11, L1, and a new vertebral fracture at L3 (Fig. 1c). In ~8 months since following ODN discontinuation, her L2, L4 BMD, and TBS (L1 and L3 were excluded from analysis as the vertebral fractures at those levels elevate BMD results) declined by 11.9 and 9.4% respectively (Table 1).
Discussion
We report a 74-year-old woman whose lumbar spine BMD and TBS declined by ~ 12 and ~ 9%, respectively, and who sustained five vertebral fractures in from ~4 to ~8 months following ODN discontinuation. This mimics reports of women with multiple spontaneous vertebral fractures seen promptly following denosumab discontinuation, [1, 3] but, to our knowledge, is the first case reported with ODN. This is perhaps not surprising as the BMD increases reported with ODN, and rapid loss following treatment discontinuation, are similar to those observed with denosumab. [13] Similarly, an overshoot of bone remodeling markers has been reported when ODN is discontinued. [14]
This is the first case documenting rapid decline in TBS associated with the multiple vertebral fracture syndrome. The possibility that rapid microarchitectural deterioration could contribute to a syndrome of multiple vertebral fractures has previously been challenged. [15] However, the rapid acceleration of bone resorption, with concomitant rapid bone mass loss, could hardly be expected not to be associated with rapid loss of bone strength. Given the much greater remodeling surface in cancellous versus cortical bone, it could be expected that the major structural consequences would occur in the vertebrae. Recognizing that TBS is not a direct assessment of bone microarchitecture but rather an assessment of bone texture, it is nonetheless widely considered a surrogate for bone microarchitecture. [16, 17] The rapid and substantial decrease in TBS following ODN discontinuation directly supports the hypothesis that this syndrome occurs, at least in part, due to microarchitectural deterioration. It seems likely that a similar phenomenon occurs following denosumab discontinuation; further evaluation of architectural changes following discontinuation of bone-active therapies that do not have a persisting effect is indicated.
While the occurrence of multiple vertebral fractures following ODN discontinuation is not directly relevant as this medication is no longer being developed for clinical use, this case raises the possibility that a syndrome of multiple vertebral fractures could promptly follow discontinuation of various potent osteoporosis therapies that produce major BMD increases but do not have persisting bone effects (i.e., all non-bisphosphonates) in a subgroup of individuals. Given the imperfect long-term adherence with osteoporosis medications, discontinuation is not rare. [18, 19] Further study to facilitate identification of those at risk for multiple vertebral fractures is needed. Indeed, a recent systematic review attempted to identify clinical and imaging findings associated with increased vertebral fracture risk after denosumab discontinuation. [3] In that review, one-third of patients had prior vertebral fractures, as did our patient reported here. Other potential risk factors include absence of prior osteoporosis treatment and aromatase inhibitor therapy. [3] It is apparent that further work is need to define the phenotype of at risk individuals; if this can be clarified, initiation of bisphosphonate or other anti-resorptive therapy at the time of osteoporosis treatment discontinuation to prevent/blunt the increase in bone remodeling seems reasonable.
An apparent limitation of this case report is the absence of bone remodeling marker measurements. Unfortunately, this individual is not within the healthcare system of the research team; as such, bone turnover markers could not be obtained. Additionally, it should be noted that she did receive a brief course of prednisone therapy; however, given the relatively short duration, it is unclear if/how this contributed to the development of her multiple vertebral fractures.
In conclusion, we report a woman who sustained five vertebral fractures within 8 months of ODN discontinuation during which time lumbar spine BMD and TBS dramatically declined. We infer that the rapid increase in bone remodeling following discontinuation of ODN resulted in microarchitectural deterioration contributing to her multiple subsequent fractures. That this vertebral fracture syndrome occurs with two classes of bone-active anti-resorptive medications raises the possibility that it could occur with other osteoporosis therapies. As this multiple vertebral fracture syndrome is likely uncommon, clinicians are advised to be aware of the possibility of this syndrome and report cases when observed. The clinical profile increasing risk for this syndrome needs to be clarified. Until the phenotype of those at risk for multiple vertebral fractures is determined, use of anti-resorptive therapies to prevent rapid bone loss following discontinuation of potent bone-active agents without long-term skeletal residence, with close monitoring to document preservation of BMD, seems appropriate.
References
Lamy O, Gonzalez-Rodriguez E, Stoll D, Hans D, Aubry-Rozier B (2017) Severe rebound-associated vertebral fractures after denosumab discontinuation: 9 clinical cases report. J Clin Endocrinol Metab 102:354–358
Popp AW, Zysset PK, Lippuner K (2016) Rebound-associated vertebral fractures after discontinuation of denosumab—from clinic and biomechanics. Osteoporos Int 27:1917–1921
Anastasilakis AD, Polyzos SA, Makras P, Aubry-Rozier B, Kaouri S, Lamy O (2017) Clinical features of 24 patients with rebound-associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J Bone Miner Res 32:1291–1296
Aubry-Rozier B, Gonzalez-Rodriguez E, Stoll D, Lamy O (2016) Severe spontaneous vertebral fractures after denosumab discontinuation: three case reports. Osteoporos Int 27:1923–1925
Polyzos SA, Terpos E (2016) Clinical vertebral fractures following denosumab discontinuation. Endocrine 54:271–272
Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JEB, McClung M, Roux C, Törring O, Valter I, Wang AT, Brown JP (2017) Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res. https://doi.org/10.1002/jbmr.3337
Miller PD, Bolognese MA, Lewiecki EM, McClung MR, Ding B, Austin M, Liu Y, San Martin J (2008) Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 43:222–229
Miller PD, Wagman RB, Peacock M, Lewiecki EM, Bolognese MA, Weinstein RL, Ding B, San Martin J, McClung MR (2011) Effect of denosumab on bone mineral density and biochemical markers of bone turnover: six-year results of a phase 2 clinical trial. J Clin Endocrinol Metab 96:394–402
Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, Grazette L, San Martin J, Gallagher JC (2011) Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 96:972–980
Frost HM (2003) Bone’s mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol 275:1081–1101
Ma S, Goh EL, Jin A, Bhattacharya R, Boughton OR, Patel B, Karunaratne A, Vo NT, Atwood R, Cobb JP, Hansen U, Abel RL (2017) Long-term effects of bisphosphonate therapy: perforations, microcracks and mechanical properties. Sci Rep 7:43399
Bone HG, Dempster DW, Eisman JA, Greenspan SL, McClung MR, Nakamura T, Papapoulos S, Shih WJ, Rybak-Feiglin A, Santora AC, Verbruggen N, Leung AT, Lombardi A (2015) Odanacatib for the treatment of postmenopausal osteoporosis: development history and design and participant characteristics of LOFT, the long-term odanacatib fracture trial. Osteoporos Int 26:699–712
Langdahl B, Binkley N, Bone H, Gilchrist N, Resch H, Rodriguez Portales J, Denker A, Lombardi A, le Bailly de Tilleghem C, DaSilva C, Rosenberg E, Leung A (2012) Odanacatib in the treatment of postmenopausal women with low bone mineral density: five years of continued therapy in a phase 2 study. J Bone Miner Res 27:2251–2258
Eisman JA, Bone HG, Hosking DJ, McClung MR, Reid IR, Rizzoli R, Resch H, Verbruggen N, Hustad CM, DaSilva C, Petrovic R, Santora AC, Ince BA, Lombardi A (2011) Odanacatib in the treatment of postmenopausal women with low bone mineral density: three-year continued therapy and resolution of effect. J Bone Miner Res 26:242–251
Rao SD, Qiu S, Dhaliwal R, Bhadada SK (2017) Letter to the editor: severe rebound-associated vertebral fractures after denosumab discontinuation. J Clin Endocrinol Metab 102:2111
Silva BC, Broy SB, Boutroy S, Schousboe JT, Shepherd JA, Leslie WD (2015) Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD official positions part 2: trabecular bone score. J Clin Densitom 18:309–330
Muschitz C, Kocijan R, Haschka J, Pahr D, Kaider A, Pietschmann P, Hans D, Muschitz GK, Fahrleitner-Pammer A, Resch H (2015) TBS reflects trabecular microarchitecture in premenopausal women and men with idiopathic osteoporosis and low-traumatic fractures. Bone 79:259–266
van der Zwaard BC, van Hout W, Hugtenburg JG, van der Horst HE, Elders PJ (2017) Adherence and persistence of patients using oral bone sparing drugs in primary care. Fam Pract 34:525–531
Fahrleitner-Pammer A, Papaioannou N, Gielen E, Feudjo Tepie M, Toffis C, Frieling I, Geusens P, Makras P, Boschitsch E, Callens J, Anastasilakis AD, Niedhart C, Resch H, Kalouche-Khalil L, Hadji P (2017) Factors associated with high 24-month persistence with denosumab: results of a real-world, non-interventional study of women with postmenopausal osteoporosis in Germany, Austria, Greece, and Belgium. Arch Osteoporos 12:58
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Binkley, N., Krueger, D. & de Papp, A.E. Multiple vertebral fractures following osteoporosis treatment discontinuation: a case-report after long-term Odanacatib. Osteoporos Int 29, 999–1002 (2018). https://doi.org/10.1007/s00198-018-4385-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-018-4385-0