Abstract
Summary
In this large registry-based study, women with diabetes had marginally greater bone mineral density (BMD) loss at the femoral neck but not at other measurement sites, whereas obesity was not associated with greater BMD loss. Our data do not support the hypothesis that rapid BMD loss explains the increased fracture risk associated with type 2 diabetes and obesity observed in prior studies.
Introduction
Type 2 diabetes and obesity are associated with higher bone mineral density (BMD) which may be less protective against fracture than previously assumed. Inconsistent data suggest that rapid BMD loss may be a contributing factor.
Methods
We examined the rate of BMD loss in women with diabetes and/or obesity in a population-based BMD registry for Manitoba, Canada. We identified 4960 women aged ≥ 40 years undergoing baseline and follow-up BMD assessments (mean interval 4.3 years) without confounding medication use or large weight fluctuation. We calculated annualized rate of BMD change for the lumbar spine, total hip, and femoral neck in relation to diagnosed diabetes and body mass index (BMI) category.
Results
Baseline age-adjusted BMD was greater in women with diabetes and for increasing BMI category (all P < 0.001). In women with diabetes, unadjusted BMD loss was less at the lumbar spine (P = 0.017), non-significantly greater at the femoral neck (P = 0.085), and similar at the total hip (P = 0.488). When adjusted for age and BMI, diabetes was associated with slightly greater femoral neck BMD loss (− 0.0018 g/cm2/year, P = 0.012) but not at the lumbar spine or total hip. There was a strong linear effect of increasing BMI on attenuated BMI loss at the lumbar spine with negligible effects on hip BMD.
Conclusions
Diabetes was associated with slightly greater BMD loss at the femoral neck but not at other measurement sites. BMD loss at the lumbar spine was reduced in overweight and obese women but BMI did not significantly affect hip BMD loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite being associated with higher bone mineral density (BMD), type 2 diabetes is a risk factor for osteoporotic fracture independent of FRAX probability [1, 2]. Obesity, also associated with higher BMD, may be less protective against fracture than previously assumed [3, 4] and may be associated with increased fracture hazard when adjusted for higher BMD [5].
Mechanisms responsible for these observations are unclear but are likely to be multifactorial and include impaired muscle strength and quality, falls, greater skeletal impact forces related to a fall, and alterations in bone strength [5,6,7,8,9,10,11,12,13]. Inconsistent data also suggest that more rapid BMD loss in obese individuals or those with type 2 diabetes may be a contributing factor [14,15,16]. In support of the latter, there are data suggesting that serum parathyroid hormone is positively correlated with body fat, with this relationship being independent of vitamin D status [17].
To test the hypothesis that there is more rapid BMD loss in women with diabetes and/or obesity contributing to their higher fracture risk, we examined the rate of BMD loss in a large clinical cohort of women with longitudinal BMD measurements, clinical diagnosis of diabetes, and repeated measures of weight.
Methods
Study population
We included all women age 40 years and older registered for health coverage in the province of Manitoba (Canada) who underwent baseline and follow-up bone density measurements of the lumbar spine and proximal femur with a single fan-beam scanner configuration (Prodigy or iDXA, GE Healthcare) between 1996 and 2013. We excluded women with significant exposure to osteoporosis medications, systemic estrogens, glucocorticoids or aromatase inhibitors, or women with measured weight change > 10% due to possible confounding. In Manitoba, health services are provided to virtually all residents and recorded through a single public healthcare system. Bone density testing with DXA has been managed as an integrated program since 1997 and uses targeted case-finding prior to age 65 years, with screening of women after age 65 years [18]. The program maintains a database of all DXA results which can be linked with other population-based computerized health databases through an anonymous personal identifier [19]. The DXA database has been previously described with completeness and accuracy in excess of 99%. The study was approved by the University of Manitoba Health Research Ethics Board.
Bone density measurements
DXA scans were performed and analyzed in accordance with manufacturer recommendations. Hip T-scores and Z-scores were calculated using NHANES III white female reference values [20]; manufacturer reference data for white females were used to compute lumbar spine T-scores and Z-scores. The DXA instruments used in the province were cross-calibrated using anthropomorphic phantoms and no clinically significant differences were identified (T-score differences < 0.1). Therefore, all analyses are based upon the unadjusted numerical results provided by the instrument. Rate of BMD change (g/cm2/year) for the lumbar spine (L1-L4), total hip, and femoral neck was studied in relation to previously diagnosed diabetes and body mass index (BMI). Densitometers showed stable long-term performance (coefficient of variation [CV] < 0.5%) and satisfactory in vivo precision (CV 1.1% for total hip to 2.3% for femoral neck).
Obesity and diabetes
Weight and height were obtained at the time of each DXA examination using a wall-mounted stadiometer and floor scale, respectively. Diabetes diagnosed prior to the baseline DXA was ascertained from the presence of at least two physician billing claims with a diabetes diagnosis within 2 years or at least one hospitalization with a diabetes diagnosis [21, 22]. Hospital discharge abstracts (diagnoses and procedures) were coded using the International Classification of Diseases (ICD), 9th revision, Clinical Modification [i.e., ICD-9-CM] prior to 2004 and the 10th revision of ICD, Canadian version [i.e., ICD-10-CA] thereafter. Physician billing claims were coded using ICD-9-CM as previously described [23, 24]. Medication use was obtained from the provincial pharmacy system [25].
Statistical analysis
Rate of BMD change (g/cm2/year) approximated a normal distribution (confirmed graphically and with the Kolmogorov-Smirnov test). Independent sample t-tests and analysis of variance (ANOVA) were used to compare baseline measures. Generalized additive models were fit to the annual rate of BMD change (cubic splines with four degrees of freedom [df] [26]) with covariates: BMI (coded as WHO category or continuous); age (coded as continuous); and a diagnosis of diabetes (coded as categorical). Statistical analyses were performed with Statistica (Version 10.0, StatSoft Inc., Tulsa, OK).
Results
The final study population included 4960 women (mean age 62.1, SD 9.8 years). The mean BMD testing interval was 4.3 years. Average rates of BMD loss varied from 0.4% per year for the lumbar spine to 0.7% per year for the femoral neck. Baseline BMD T-scores and age-adjusted BMD Z-scores were greater at all sites for women with versus without diabetes (Table 1, all P < 0.001) and for each category of increasing BMI (Table 2, all P trend < 0.001).
In women with diabetes (N = 346) versus without diabetes (N = 4614), unadjusted BMD loss was less at the lumbar spine (− 0.0026 vs − 0.0050 g/cm2/year, P = 0.017), not significantly greater at the femoral neck (− 0.0068 vs − 0.0056 g/cm2/year, P = 0.085), and similar at the total hip (− 0.0082 vs − 0.0068 g/cm2/year, P = 0.488). Adjusted for age and BMI category, diabetes was associated with slightly greater femoral neck BMD loss (− 0.0018 g/cm2/year, P = 0.012) but no statistically significant effect of diabetes was seen on the lumbar spine or total hip (Table 3). Further adjustment for weight change between the scans did not alter the results (data not shown). There was no evidence of differential age effect on BMD loss in those with diabetes for any of the BMD measurement sites (all ANOVA P ≥ 0.2).
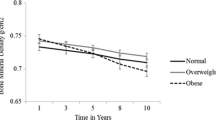
Unadjusted BMD loss was less with increasing BMI category (Table 2). Compared with normal BMI, overweight and obesity categories were associated with reduced BMD loss at the lumbar spine (adjusted for age and diabetes status) but BMI category did not affect BMD loss at the total hip or femoral neck (Table 3). When modeled as a continuous variable, there was a strong linear effect of greater BMI to attenuate BMD loss at the lumbar spine while there were negligible effects of BMI on hip BMD loss (Fig. 1). There was no evidence of a threshold above which BMI was associated with more rapid BMD loss.
Partial residual plot of the estimated association between body mass index (BMI) on and rate of BMD loss (dashed lines = 95% CIs; Y-axis negative = greater BMD loss; positive = attenuated BMD loss; zero = average BMD loss). No threshold was seen above which BMI was associated with more rapid BMD loss. Results are from generalized additive models adjusted for the effects of age and diabetes. Upper panel = lumbar spine; middle panel = total hip; lower panel = femoral neck
Discussion
Diabetes was associated with slightly greater unadjusted and covariate-adjusted BMD loss at the femoral neck but not at other measurement sites. The clinical significance of this finding is questionable, however, as baseline BMD was significantly greater in women with diabetes and this higher rate of BMD loss would need to be sustained for over 20 years before femoral neck BMD would even become average for age. Therefore, our data do not support the hypothesis that rapid BMD loss explains the increased fracture risk that has been associated with type 2 diabetes [1, 2]. BMD loss at the lumbar spine was reduced in overweight and obese women, while BMI did not significantly affect hip BMD loss. This would suggest that other factors are more important in explaining higher fracture risk in diabetes and obesity and likely include skeletal and non-skeletal mechanisms [5,6,7,8,9,10,11,12,13].
Previous studies that have examined the effect of diabetes on BMD loss have shown conflicting results. Schwartz et al. [14] examined change in hip BMD over 4 years in 480 participants with diabetes, 439 with impaired glucose metabolism, and 1172 with normal glucose metabolism from the Health ABC study of 70–79 year-old well-functioning white and black men and women. Diabetes was associated with higher baseline hip BMD; among white women with diabetes (but not among men or black women), there was greater loss in femoral neck BMD (− 0.33%/year) while total hip BMD loss was similar. More recently, Schwartz et al. [27] reported annualized BMD change in the Study of Osteoporotic Fractures (SOF) cohort, which included 409 older women with diabetes at baseline. Baseline BMD was higher in women with diabetes but there was greater BMD loss at the femoral neck, total hip, calcaneus, and spine (but not the distal radius). Greater total hip BMD loss has also reported among older men from the MrOS cohort [15]. The Study of Women’s Health Across the Nation (SWAN), which included 117 women classified as having diabetes at baseline, also noted more rapid hip BMD loss (but slower spine BMD loss) in women with diabetes during menopausal transition (median follow-up was 3.1 years) [28]. In part, this may have been related to earlier menopause in women with diabetes, and the authors noted a significant interaction between menopause status and diabetes for both the total hip and lumbar spine. Greater total hip BMD loss (with a similar change in lumbar spine BMD) was seen in the placebo arm of the Fracture Intervention Trial (FIT) for diabetic versus non-diabetic women [29]. In contrast, a small cohort with 12-year follow-up found that radial bone loss was slower than expected in patients with type 2 diabetes [30]. This is consistent with the observation that bone turnover is generally reduced in those with type 2 diabetes [31], possibly mediated through increased levels of sclerostin or the accumulation of advanced glycation end products [12, 32].
Lloyd et al. [33] reported that BMD change evaluated up to 10 years according to baseline BMI (excluding underweight) in 2570 men and women of black and white race/ethnicity (age 70–79 years at enrollment). Change in total hip and whole body BMD did not differ according to BMI group, but among obese older adults, there was 0.003 g/cm2/year greater femoral neck BMD loss compared with normal weight older adults (P < 0.001). Mean BMD values were not statistically different from the normal weight group at year 10, however, and the analysis did not account for possible change in BMI over time. In contrast, SWAN reported that greater BMI was related to slower loss rates [34].
It is difficult to reconcile these conflicting findings. Differences in population demographics and characteristics likely contribute to variations in the results. Technical factors in assessing BMD change further complicate interpretation. The well-known problem of age-related degenerative changes in the lumbar spine likely contributes to inconsistent results and the tendency of spine BMD to increase over time, particularly among obese individuals [29, 35]. Hip BMD measurements are also confounded by obesity, overlapping fat panniculus, and weight change [36, 37]. Femur neck BMD is more sensitive to the effects of fat panniculus than the total hip [37], while the latter provides a more reproducible site for detecting BMD change and may be more accurate for assessing changes in those with diabetes. We attempted to minimize these technical problems by excluding individuals with a large degree of weight change.
Strengths of our study include the large number of subjects, including the number with diabetes and broad representation across the spectrum of BMI. Limitations are also recognized, including reliance on a clinical registry with potential for referral bias, particularly in women less than age 65 years (confounding by indication). Linkage to population-based data made it possible to exclude individuals with confounding medication use. It is uncertain whether differential DXA utilization according to diabetes and BMI status would confound results, although we also note that neither diabetes nor obesity is an approved reason that community-based physicians order DXA tests irrespective of other indications. However, observed rates of BMD loss are similar to what would be expected in postmenopausal women not receiving anti-osteoporosis therapy [38]. Although type 1 and type 2 diabetes cannot be definitively distinguished in administrative data, our case definition is dominated by type 2 diabetes (over 97% based upon use of oral anti-diabetes agents and/or non-use of insulin). Menopausal status is an important determinant of BMD loss in women. Unfortunately, we did not have information on menopausal status, though the vast majority of women in this cohort is expected to be postmenopausal. The testing interval was slightly shorter in those with versus without diabetes (3.9 vs 4.3 years), but since BMD change was annualized to account for varying testing intervals, this should not affect the results. Finally, we were unable to assess diabetes complications or other diabetes-specific risk factors as modifiers of BMD loss, and this might be an important direction for future research.
In summary, our findings suggest that diabetes is associated with a slightly greater rate of BMD loss at the femoral neck but this small difference is unlikely to be clinically significant or explain the increased fracture risk that has been associated with type 2 diabetes. No significant effect of diabetes was seen on BMD loss at the total hip or lumbar spine. BMD loss at the lumbar spine was lower in overweight and obese women but BMI did not significantly affect hip BMD loss.
References
Schwartz AV, Vittinghoff E, Bauer DC, Hillier TA, Strotmeyer ES, Ensrud KE et al (2011) Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA 305(21):2184–2192
Giangregorio LM, Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E et al (2012) FRAX underestimates fracture risk in patients with diabetes. J Bone Miner Res 27(2):301–308
Compston JE, Watts NB, Chapurlat R, Cooper C, Boonen S, Greenspan S et al (2011) Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med 124(11):1043–1050
Prieto-Alhambra D, Premaor MO, Fina AF, Hermosilla E, Martinez-Laguna D, Carbonell-Abella C et al (2012) The association between fracture and obesity is site-dependent: a population-based study in postmenopausal women. J Bone Miner Res 27(2):294–300
Ishii S, Cauley JA, Greendale GA, Nielsen C, Karvonen-Gutierrez C, Ruppert K et al (2014) Pleiotropic effects of obesity on fracture risk: the Study of Women’s Health Across the Nation. J Bone Miner Res 29(12):2561–2570
Park SW, Goodpaster BH, Strotmeyer ES, de Rekeneire N, Harris TB, Schwartz AV et al (2006) Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes 55(6):1813–1818
Barrett-Connor E, Weiss TW, McHorney CA, Miller PD, Siris ES (2009) Predictors of falls among postmenopausal women: results from the National Osteoporosis Risk Assessment (NORA). Osteoporos Int 20(5):715–722
Schwartz AV, Hillier TA, Sellmeyer DE, Resnick HE, Gregg E, Ensrud KE et al (2002) Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 25(10):1749–1754
Beck TJ, Petit MA, Wu G, Leboff MS, Cauley JA, Chen Z (2009) Does obesity really make the femur stronger? BMD, geometry, and fracture incidence in the Women’s Health Initiative Observational Study. J Bone Miner Res 24(8):1369–1379
Scott D, Seibel M, Cumming R, Naganathan V, Blyth F, Le Couteur DG et al (2017) Sarcopenic obesity and its temporal associations with changes in bone mineral density, incident falls, and fractures in older men: the Concord Health and Ageing in Men Project. J Bone Miner Res 32(3):575–583
Compston J (2013) Obesity and fractures. Joint Bone Spine 80(1):8–10
Saito M, Kida Y, Kato S, Marumo K (2014) Diabetes, collagen, and bone quality. Curr Osteoporos Rep 12(2):181–188
Burghardt AJ, Issever AS, Schwartz AV, Davis KA, Masharani U, Majumdar S et al (2010) High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 95(11):5045–5055
Schwartz AV, Sellmeyer DE, Strotmeyer ES, Tylavsky FA, Feingold KR, Resnick HE et al (2005) Diabetes and bone loss at the hip in older black and white adults. J Bone Miner Res 20(4):596–603
Strotmeyer ES, Boudreau RM, Marshall LM, Schwartz AV, Bauer DC, Barrett-Connor E et al (2008) Higher bone mineral density loss in older men with diabetes: the Osteoporotic Fractures in Men Study. J Bone Miner Res 23(Suppl 1):S59
Gilbert MP, Pratley RE (2015) The impact of diabetes and diabetes medications on bone health. Endocr Rev 36(2):194–213
Bolland MJ, Grey AB, Ames RW, Horne AM, Gamble GD, Reid IR (2005) Fat mass is an important predictor of parathyroid hormone levels in postmenopausal women. Bone 38(3):317–321
Leslie WD, Metge C (2003) Establishing a regional bone density program: lessons from the Manitoba experience. J Clin Densitom 6(3):275–282
Leslie WD, Caetano PA, Macwilliam LR, Finlayson GS (2005) Construction and validation of a population-based bone densitometry database. J Clin Densitom 8(1):25–30
Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP et al (1998) Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int 8(5):468–489
Blanchard JF, Ludwig S, Wajda A, Dean H, Anderson K, Kendall O et al (1996) Incidence and prevalence of diabetes in Manitoba, 1986-1991. Diabetes Care 19(8):807–811
Lix L, Yogendran M, Shaw S, Burchill C, Metge C, Bond R (2008) Population-based data sources for chronic disease surveillance. Chronic Dis Can 29(1):31–38
Lix LM, Azimaee M, Osman BA, Caetano P, Morin S, Metge C et al (2012) Osteoporosis-related fracture case definitions for population-based administrative data. BMC Public Health 12:301
O’Donnell S, Canadian Chronic Disease Surveillance System Osteoporosis Working Group (2013) Use of administrative data for national surveillance of osteoporosis and related fractures in Canada: results from a feasibility study. Arch Osteoporos 8:143
Kozyrskyj AL, Mustard CA (1998) Validation of an electronic, population-based prescription database. Ann Pharmacother 32(11):1152–1157
Hastie TJ, Tibshirani RJ 1990 Generalized additive models. Chapman & Hall/CRC Monographs on Statistics & Applied Probability, Boca Raton
Schwartz AV, Ewing SK, Porzig AM, McCulloch CE, Resnick HE, Hillier TA et al (2013) Diabetes and change in bone mineral density at the hip, calcaneus, spine, and radius in older women. Front Endocrinol (Lausanne) 4:62
Khalil N, Sutton-Tyrrell K, Strotmeyer ES, Greendale GA, Vuga M, Selzer F et al (2011) Menopausal bone changes and incident fractures in diabetic women: a cohort study. Osteoporos Int 22(5):1367–1376
Keegan TH, Schwartz AV, Bauer DC, Sellmeyer DE, Kelsey JL (2004) Fracture intervention t. Effect of alendronate on bone mineral density and biochemical markers of bone turnover in type 2 diabetic women: the fracture intervention trial. Diabetes Care 27(7):1547–1553
Krakauer JC, McKenna MJ, Buderer NF, Rao DS, Whitehouse FW, Parfitt AM (1995) Bone loss and bone turnover in diabetes. Diabetes 44(7):775–782
Hygum K, Starup-Linde J, Harslof T, Vestergaard P, Langdahl BL (2017) Diabetes mellitus, a state of low bone turnover—a systematic review and meta-analysis. Eur J Endocrinol 176(3):R137–RR57
Garcia-Martin A, Rozas-Moreno P, Reyes-Garcia R, Morales-Santana S, Garcia-Fontana B, Garcia-Salcedo JA et al (2012) Circulating levels of sclerostin are increased in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 97(1):234–241
Lloyd JT, Alley DE, Hochberg MC, Waldstein SR, Harris TB, Kritchevsky SB et al (2016) Changes in bone mineral density over time by body mass index in the health ABC study. Osteoporos Int 27(6):2109–2116
Greendale GA, Sowers M, Han W, Huang MH, Finkelstein JS, Crandall CJ et al (2012) Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the Study of Women’s Health Across the Nation (SWAN). J Bone Miner Res 27(1):111–118
Jones G, Nguyen T, Sambrook PN, Kelly PJ, Eisman JA (1995) A longitudinal study of the effect of spinal degenerative disease on bone density in the elderly. J Rheumatol 22(5):932–936
Yu EW, Bouxsein ML, Roy AE, Baldwin C, Cange A, Neer RM et al (2014) Bone loss after bariatric surgery: discordant results between DXA and QCT bone density. J Bone Miner Res 29(3):542–550
Binkley N, Krueger D, Vallarta-Ast N (2003) An overlying fat panniculus affects femur bone mass measurement. J Clin Densitom 6(3):199–204
Berger C, Langsetmo L, Joseph L, Hanley DA, Davison KS, Josse R et al (2008) Change in bone mineral density as a function of age in women and men and association with the use of antiresorptive agents. CMAJ 178(13):1660–1668
Acknowledgements
This article has been reviewed and approved by the members of the Manitoba Bone Density Program Committee. The authors acknowledge the Manitoba Centre for Health Policy for the use of data contained in the Manitoba Population Research Data Repository (HIPC File No. 2011/2012-31). The results and conclusions are those of the authors and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, or other data providers is intended or should be inferred. The data used in this study are from the Manitoba Population Research Data Repository housed at the Manitoba Centre for Health Policy, University of Manitoba, and were derived from data provided by Manitoba Health.
Funding
No funding support was received for this research project. SNM is chercheur-clinicienne boursier des Fonds de Recherche du Québec en Santé. LML is supported by Manitoba Health Research Chair. SRM holds the Endowed Chair in Patient Health Management supported by the Faculties of Medicine and Dentistry and Pharmacy and Pharmaceutical Sciences at the University of Alberta.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Suzanne Morin: Consultant to: Amgen; Research Grants: Amgen, Merck.
William Leslie, Sumit Majumdar, Lisa Lix: None.
Rights and permissions
About this article
Cite this article
Leslie, W.D., Morin, S.N., Majumdar, S.R. et al. Effects of obesity and diabetes on rate of bone density loss. Osteoporos Int 29, 61–67 (2018). https://doi.org/10.1007/s00198-017-4223-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4223-9