Abstract
Summary
Bone marrow adipose tissue has not been studied in patients with inactive inflammatory bowel disease. We found that these patients have preserved marrow adiposity even with low bone mass. Factors involved in bone loss in active disease may have long-lasting effects but do not seem to affect bone marrow adiposity.
Introduction
Reduced bone mass is known to occur at varying prevalence in patients with inflammatory bowel diseases (IBD) because of inflammation, malnutrition, and steroid therapy. Osteoporosis may develop in these patients as the result of an imbalanced relationship between osteoblasts and adipocytes in bone marrow. This study aimed to evaluate for the first time bone mass and bone marrow adipose tissue (BMAT) in a particular subgroup of IBD patients characterized by long-term, steroid-free remission.
Methods
Patients with Crohn’s disease (CD; N = 21) and ulcerative colitis (UC; N = 15) and controls (C; N = 65) underwent dual X-ray energy absorptiometry and nuclear magnetic resonance spectroscopy of the L3 lumbar vertebra for BMAT assessment.
Results
Both the CD and UC subgroups showed significantly higher proportions of patients than controls with Z-score ≤−2.0 at L1–L4 (C 1.54%; CD 19.05%; UC 20%; p = 0.02), but not at other sites. The proportions of CD patients with a T-score ˂−1.0 at the femoral neck (C 18.46%; CD 47.62%; p = 0.02) and total hip (C 16.92%; CD 42.86%; p = 0.03) were significantly higher than among controls. There were no statistically significant differences between IBD patients and controls regarding BMAT at L3 (C 28.62 ± 8.15%; CD 29.81 ± 6.90%; UC 27.35 ± 9.80%; p = 0.67).
Conclusions
IBD patients in long-term, steroid-free remission may have a low bone mass in spite of preserved BMAT. These findings confirm the heterogeneity of bone disorders in IBD and may indicate that factors involved in bone loss in active disease may have long-lasting effects on these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone disorders may be an important complication of inflammatory bowel diseases (IBD), a group of chronic intestinal conditions mainly consisting of Crohn’s disease (CD) and ulcerative colitis (UC). The complexity of bone disorders in IBD derives from their multifactorial pathogenesis, which includes nutritional deficiency and weight loss, as well as flares of inflammatory activity and use of steroids [1].
Low bone mass may already be present in newly diagnosed, untreated CD patients, suggesting that inflammatory mediators may negatively affect the skeleton [2]. Activated T cells produce receptor activator of nuclear factor kappa-B ligand (RANKL), which binds to its receptor (RANK) and causes osteoclastogenesis [3]. On the other hand, osteoprotegerin produced by monocytes, macrophages, and dendritic cells competes with RANK, blocking bone resorption activity. Imbalances in these mechanisms are thought to hamper the balance of bone remodeling in IBD [3]. Other inflammatory mediators such as tumor necrosis factor (TNF), interleukin (IL) 1-beta, IL-6, and gamma interferon may also contribute to bone loss in IBD [3].
The therapeutic use of glucocorticoids, although reducing inflammation, does increase the negative balance of bone remodeling in IBD, and there is extensive evidence that steroids decrease bone mineral density (BMD) in both CD and UC [4–6] through a number of different mechanisms. However, these detrimental effects seem to be reversible following steroid withdrawal [1].
The reported prevalence of bone disorders in IBD is highly variable, not only because different patient samples may carry distinct pathogenic factors, but also in association with the use of different measures of BMD, such as T-score or Z-score [7]. In two studies evaluating large numbers of predominantly young CD and UC patients, the prevalence of cases with Z-score ≤−2.0 ranged from 2 to 15%, but the relative bone mass loss at the lumbar spine was small (4.1–7.3%) [4, 6]. Higher prevalence figures (22 to 30%) were observed in other smaller studies also using as cutoff Z-score ≤−2.0 [8, 9]. Taking these and other studies into account, a recent literature review has suggested that CD is responsible for a small effect on BMD after adjusting for other risk factors, whereas UC does not appear to be an independent risk [10].
Bone metabolism may also be assessed using markers such as osteocalcin and cross-linking carboxyl-terminal telopeptide of type I collagen (CTX), which seems to reflect bone remodeling activity [11, 12]. A Spanish study reported an association between increased markers of bone resorption and clinically active disease in CD patients but failed to show any correlation with BMD [13]. Nevertheless, the relationship between biochemical markers of bone remodeling and BMD in IBD still is to be better investigated.
In recent years, there has been growing interest in the role of cellular components of the bone microenvironment, such as bone marrow adipose tissue (BMAT), in bone remodeling [14]. Adipocytes and osteoblasts share a common origin from the same bone marrow stem cell [15], and the expansion of one cell type may be associated with reduction of the other. Thus, the increase of BMAT in adults may limit bone formation [16]. An inverse relationship between bone mass and BMAT has been reported in bone disorders associated with aging or eating disorders [17–19]. Nevertheless, BMAT has not been assessed so far in patients with IBD and data on the relationship between bone mass and BMAT in chronic diseases are scarce, particularly in patients in remission.
The objective of the present study was to assess BMD, BMAT, and bone metabolism markers in IBD patients in long-term, steroid-free remission. We sought to address a number of clinically relevant questions in this particular group of IBD patients such as what is their bone health status? Is BMAT increased and is there any correlation between BMAT and BMD? What is the role of biochemical markers of bone metabolism?
Methods
This was a cross-sectional study including 21 patients with CD, 15 patients with UC, and 65 control subjects. Patients were regularly followed at the University Hospital outpatient clinics. The control group included apparently healthy hospital workers (N = 45) and patients with minor digestive diseases (N = 20) without any relevant inflammatory component. This subgroup included patients with gastroesophageal reflux disease, functional dyspepsia, functional abdominal pain, peptic ulcer disease, idiopathic achalasia, noncardiac chest pain, esophageal papillomatosis, and esophageal retention cyst. Fourteen patients in this subgroup were taking omeprazole. The demographic and anthropometric characteristics of the three groups are shown in Table 1. There were no statistically significant differences between groups regarding gender, age, weight, height, or body mass index (BMI), except for male’s BMI that was significantly higher in CD than among controls (C 27.3 ± 4.5 kg/m2; CD 23.7 ± 4.3 kg/m2; UC 24.3 ± 3.1 kg/m2; p = 0.02). Among the 54 men from the three groups, 12 (22.22%) were older than 50 years. Age ranges in the control group (20–57 years) and in the CD (21–59 years) and UC (23–60 years) subgroups were similar. All women in the study were premenopausal.
Inclusion criteria
The CD and UC groups comprised patients older than 18 years, with well-documented IBD in clinical remission, currently not using steroids, and without current or previous evidence of metabolic bone disease. All patients and control subjects were in good nutritional conditions and specifically denied having experienced any acute disorder within the last 30 days.
Exclusion criteria
Individuals reporting current smoking or alcohol drinking habits were excluded, as were those with previous gastrointestinal tract cancer or current clinical and laboratory evidence of autoimmune, liver, kidney, thyroid, and bone metabolic diseases. Patients using medications that alter calcium metabolism (steroids, calcium carbonate, vitamin D, drugs for the treatment of osteoporosis, serotonin reuptake inhibitors, anticonvulsants, thiazide diuretics, and hormone replacement therapy) were also excluded.
Clinical characterization of patients with IBD
Patients with CD were classified according to the Montreal classification [20] regarding age at disease onset, location, and behavior. Disease clinical activity was assessed using the criteria of the Crohn’s Disease Activity Index (CDAI) [21]. Patients with CDAI <150 points were considered to be in clinical remission. Patients with UC were also classified according to the Montreal classification [20] regarding disease extent and clinical activity. Patients completely free of symptoms since the last visit were considered to be in clinical remission. Data for these classifications were obtained personally by one of the investigators (CMB) during an actual visit and complemented with a further review of each patient’s medical records prior to inclusion in the study. More specifically, patient notes were reviewed to obtain data on current remission time, time since diagnosis, previous operations, current and recent use of drugs, and previous use of steroids.
In the CD group, age at the disease diagnosis was between 17 and 40 years in 18 (85.7%) patients, disease was located in terminal ileum exclusively or ileocolon in 17 (81.0%) patients, and 14 (66.7%) patients had either structuring or penetrating disease behavior. In the UC group, 13 (86.7%) patients had either a left-sided or extensive large bowel involvement.
All patients with CD and UC were in clinical remission (CD 42.8 ± 52.1 months; UC 14.9 ± 16.7 months; p = 0.15). Although 19/21 CD patients (90.5%) and UC 12/15 patients (80.0%) had used steroids, they had all been free from them for 72.9 ± 112.9 months (CD) and 29.3 ± 25.6 months (UC) (p = 0.42). Eight CD and three UC patients had not used steroids for more than 36 months, one CD and three UC patients were free from steroids for at least 24 months, and five CD and four UC patients had not used any glucocorticoid for at least 12 months. Only five CD and one UC patients had a course of steroids during the 12 months preceding their inclusion in the study.
The mean time from the initial diagnosis was 118.6 ± 59.4 months for patients with CD and 88.0 ± 55.3 months for patients with UC. Among patients with CD, 6/21 (28.6%) had a previous partial bowel resection. Eleven CD (52.4%) and three UC (20%) patients were on anti-TNF agents (p = 0.08), while the remaining cases were on azathioprine or mesalazine.
Dual X-ray energy absorptiometry
Participants of the three groups underwent BMD determination by dual X-ray energy absorptiometry (DEXA) (Discovery Wi, QDR series, Hologic, Waltham, MA, USA) in the lumbar spine (L1–L4), femoral neck, total hip, one-third radius, and total body. The precision error was 1.2, 2.3, 2.7, and 1.7% for L1–L4, femoral neck, total hip, and one-third radius, respectively. BMD values are reported as gram per square centimeter. The equipment was calibrated daily with a phantom provided by the manufacturer. BMD was expressed as absolute values of both Z-score and T-score. Bone mass was considered to be adequate in relation to age for values of Z-score >−2.0 [22]. We also utilized T-scores to classify results as normal (T-score ≥−1.0) or indicating the presence of osteopenia (−1.0 > T-score > −2.5) or osteoporosis (T-score ≤−2.5) [7]. Body composition regarding total fat (percentage) and lean mass (kg/m2) was also estimated.
Magnetic resonance imaging acquisition and analysis
Patients with CD and UC and controls underwent spine magnetic resonance imaging (MRI) on a 1.5-T system (Philips ACHIEVA, Philips Medical Systems; Best, Netherlands). For lumbar spine spectroscopy acquisition, we used a sagittal T2-weighted fast spin echo (FSE) sequence as a reference for the placement of a 1.5 × 1.5 × 1.5 cm3 voxel in the center of the third lumbar (L3) vertebral body. Proton magnetic resonance spectroscopy (1H-MRS) was performed by the Point-Resolved Spectroscopy (PRESS) technique, and the spectroscopy parameters were echo time (TE) = 40/60/80 ms, repetition time (TR) = 2000 ms, 8 average, without fat suppression, and 1-min duration. The coefficient of variation of the BMAT measurements was 4.5%. For the calculation of the coefficient of variation, BMAT measurements were performed in 12 individuals, with three replicates each. Data were processed with the LC Model software, and the values obtained were used to calculate the water and fat fractions [23]. We also calculated the fat fraction/BMD ratio at L3.
Biochemistry
Blood samples were obtained from all subjects for the determination of circulating levels of osteocalcin (MicroVue ™ Osteocalcin, Quidel, San Diego, USA), CTX (Serum CrossLaps®, IDS, Tyne and Wear, UK), osteoprotegerin (OPG MicroVue ™, Quidel, San Diego, USA), and RANKL (Ampli-sRANKL human, Biomedica, Wien, Austria). The intra- and inter-assay errors were less than 10 and 20%, respectively, for all measurements. Circulating levels of 25-hidroxi vitamin D [25(OH)D] (Liaison® 25 OH Vitamin D Total, Diasorin, Vercelli, Italy), parathyroid hormone (PTH), total calcium, and C-reactive protein (CRP) were also measured. Laboratory reference values were 11–48 ng/ml for osteocalcin, <0.85 ng/ml (men) and <0.65 ng/ml (women) for CTX, 10–69 pg/ml for PTH, 8.4–10.5 mg/dl for total calcium, ≤0.5 mg/dl for CRP, and ≥20 ng/ml (normal) and <20 ng/ml (deficient) for 25(OH)D.
Statistical analysis
Continuous quantitative variables are reported as mean ± standard deviation (SD). Normality of data distribution was preliminarily tested using the Kolmogorov-Smirnov test. For normally distributed data, analysis of variance (ANOVA) was used, followed by Bonferroni’s post hoc test for comparison between groups when p ≤ 0.05. When normality was rejected, data were analyzed using the Kruskal-Wallis test with Dunn’s post hoc test for comparison between groups when p ≤ 0.05. Correlations between two normally distributed continuous variables were analyzed calculating the Pearson linear correlation coefficient (R). The nominal quantitative and ordinal qualitative variables are reported as absolute values and percentages. Comparisons between groups regarding proportions were performed using the chi-squared test or Fisher’s exact probability test. All data were analyzed using the GraphPad Prism version 5.0 software for Windows (GraphPad Software, San Diego, CA). Differences were considered to be statistically significant when p ≤ 0.05.
Results
Bone mineral density
The values of BMD at L1–L4, femoral neck, total hip, one-third radius, and total body are shown in Table 2. There were no statistically significant differences between groups for any bone site. There was no correlation between time since diagnosis or current remission time and BMD.
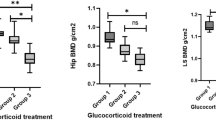
There were no statistically significant differences between groups regarding Z-score at L1–L4 (C −0.2 ± 1.3; CD −0.8 ± 1.3; UC −0.6 ± 1.2; p = 0.13), femoral neck (C 0.3 ± 1.0; CD −0.3 ± 1.4; UC −0.0 ± 1.1; p = 0.13), total hip (C 0.1 ± 0.8; CD −0.4 ± 1.4; UC −0.1 ± 0.7; p = 0.08), or one-third radius (C −0.4 ± 1.0; CD −0.6 ± 1.4; UC −0.5 ± 1.1; p = 0.75) (Table 2 and Fig. 1). According to the classification based on Z-score, the proportions of CD (19.05%) and UC (20.0%) patients with inadequate bone mass at L1–L4 were significantly higher (p = 0.02) than among controls (1.54%). There were no statistically significant differences in the proportions of inadequate bone mass between groups at other sites.
There was a statistically significant difference between CD and controls regarding T-score in the femoral neck (C −0.1 ± 1.0; CD −0.7 ± 1.4; UC −0.4 ± 1.1; p = 0.05) (Table 2 and Fig. 2). There were no statistically significant differences between groups regarding T-score for L1–L4 (C −0.4 ± 1.2; CD −1.0 ± 1.2; UC −0.8 ± 1.3; p = 0.22), total hip (C −0.1 ± 0.8; CD −0.6 ± 1.3; UC −0.3 ± 0.8; p = 0.06), and one-third radius (C −0.7 ± 0.9; CD −0.9 ± 1.4; UC −0.8 ± 1.1; p = 0.67) (Table 2 and Fig. 2). According to the classification based on T-score, there were statistically significant differences in the proportions of individuals with osteopenia and osteoporosis between CD and control groups at femoral neck (CD 47.62%; C 18.46%; p = 0.02) and at total hip (CD 42.86%; C 16.92%; p = 0.03). There were ten patients with osteoporosis (seven CD, three UC), but this subgroup was similar to the remaining patients regarding demographic and clinical characteristics, except for the duration of remission time, which tended to be shorter (median 9 months; range 2–108 months). In particular, 4 of the 10 patients had never used steroids, while in the 6 remaining patients, the duration of the steroid-free period ranged from 9 to 492 months (median 25 months). There were no statistically significant differences between groups at L1–L4 (C 40.0%; CD 52.38%; UC 46.67%; p = 0.59) or one-third radius (C 30.77%; CD 38.09%; UC 40.0%; p = 0.70).
There were no statistically significant differences between groups regarding total fat (C 31.9 ± 8.0%; CD 29.4 ± 9.1%; UC 30.8 ± 10.8%; p = 0.51) or lean mass (C 17.3 ± 2.9 kg/m2; CD 16.4 ± 3.1 kg/m2; UC 16.5 ± 2.5 kg/m2; p = 0.37). There were positive, statistically significant correlations between total body BMD and lean mass in the three groups (C: R = 0.28; CI 95% 0.04–0.49; p = 0.02; CD: R = 0.74; CI 95% 0.45–0.89; p = 0.0001; UC: R = 0.62; CI 95% 0.16–0.86; p = 0.01). There was a negative, statistically significant correlation between total body BMD and total fat in UC patients only (R = −0.61; CI 95% − 0.86 to −0.15; p = 0.01).
Bone marrow adipose tissue
There were no statistically significant differences between groups regarding BMAT variables at the L3 vertebral body. Values for fat fraction (Fig. 3) (C 28.6 ± 8.1%; CD 29.8 ± 6.9%; UC 27.3 ± 9.8%; p = 0.67) and for the ratio between fat fraction and BMD (C 27.4 ± 9.2; CD 30.5 ± 8.2; UC 27.5 ± 11.8; p = 0.43) were similar in the three groups. There was no correlation between fat fraction and BMD at L3 in any group. There was no correlation between BMAT and total fat in any group. There was a positive, statistically significant correlation between BMAT and lean mass in UC patients only (r = 0.53; CI 95%; 0.03–0.82; p = 0.04).
Biochemistry
Table 3 shows the distribution of CTX, osteocalcin, osteoprotegerin, RANKL, 25(OH)D, and CRP in the three groups. CTX levels were significantly higher (p = 0.01) in CD patients (0.85 ± 0.35 ng/ml) than in the UC (0.66 ± 0.21 ng/ml) and control (0.62 ± 0.27 ng/ml) groups. PTH levels were significantly higher (p = 0.001) in CD patients (58.19 ± 18.47 pg/ml) than in controls (40.49 ± 18.94 pg/ml). There were no statistically significant differences between groups regarding osteocalcin, osteoprotegerin, RANKL, 25(OH)D, total calcium, or CRP.
There was a statistically significant correlation between fat fraction at L3 and osteoprotegerin in CD patients (r = −0.44; CI 95% 0.0–0.74; p = 0.0476), but not in UC patients or in controls. There were no correlations between fat fraction at L3 and osteocalcin, or CTX or RANKL in any group. There were no correlations between total body BMD and 25(OH)D or CRP in any group. There were no correlations between BMAT and 25(OH)D in any group. There was a negative, statistically significant correlation between BMAT and CRP in CD patients only (r = −0.65; CI 95% −0.85 to −0.27; p = 0.003).
Discussion
Bone, the hard mesenchymal tissue in charge of supporting the body and protecting more delicate organs and tissues, has also a remarkable role in mineral metabolism. In the last decade, evidence has emerged indicating that bone cells are also part of the intricate physiological network for the modulation of energy metabolism [24]. In addition, great effort has been devoted to the determination of the mutual influence between osteoblasts and adipocytes, which might have a key role in bone development and maintenance [14]. In several disorders and conditions associated with osteoporosis, namely anorexia nervosa and glucocorticoid therapy, bone loss and increased marrow adipose tissue are coexisting processes [17, 25]. The present study was designed to investigate for the first time BMD and BMAT in a special group of IBD patients, i.e., those in long-term, glucocorticoid-free remission. Although these patients had similar mean values of BMD and BMAT compared to control, they had a higher prevalence of low lumbar spine BMD for their age (IBD = 19% vs C = 1.5%) and there was no correlation between BMD and BMAT. Our results confirm the heterogeneity of bone disorders in IBD and show that these patients have preserved marrow adiposity even with low bone mass.
Determination of BMD with DEXA allowed the identification of a subgroup of IBD patients with inadequate bone mass. Osteoporosis, in particular, was detected in 7/21 CD and in 3/15 UC patients who, however, were similar to the remaining patients regarding demographic and clinical characteristics. Moreover, four of these 10 patients had never utilized steroids to control disease activity. Considering that all the patients included in this study were in remission and not using glucocorticoids, other factors may have contributed for bone mass loss. Since this is a cross-sectional study, it is not possible to rule out previous flares of inflammatory disease activity and the use of glucocorticoids as causes of bone loss in these patients.
Several studies have detected an increased prevalence of osteoporosis and osteopenia in IBD patients [1], which warrant their inclusion within the list of causes of secondary osteoporosis [4, 26, 27]. Malabsorption of nutrients (including protein, calcium, and vitamin D), weight loss, inflammatory status, secondary hyperparathyroidism, and decreased physical activity are common detrimental factors directly involved in bone loss in IBD [1]. Moreover, glucocorticoid therapy, still used to control active IBD, controls inflammation and alleviates intestinal manifestations but, on the other hand, may deeply impair bone mass and bone quality itself. Consequently, IBD patients may be exposed to long-lasting threatening conditions for the bone. This may be the case of the patients in the present study with low bone mass, which might be seen as a scar detected even after many years of remission and glucocorticoid withdrawal. Indeed, these data mirrored previous studies in patients harboring active disease, which showed a modest effect of IBD on BMD with a pooled Z-score of −0.5, but a higher prevalence of low bone mass [28].
In the present study, results are reported as BMD, Z-score, and T-score, since both patients and controls were predominantly younger than 50 years and, due to the inclusion criteria, all women were in the premenopause period, but few IBD and control males were older than 50 years. IBD patients showed higher rate of osteoporosis and osteopenia. Two aspects need to be highlighted concerning these results: firstly, although the risk of fractures drops after glucocorticoid interruption [29], it may persist at higher levels than basal in prior glucocorticoid therapy independently of bone mass [30]; second, low bone mass in addition to the previous use of glucocorticoid amplifies the fracture risk. Another point that cannot be neglected is the higher circulatory levels of CTX observed in IBD patients, which may indicate a persistent tendency to increased bone resorption resulting in bone loss. However, the cross-sectional design of the present study represents a limitation and does not permit a definitive conclusion about the rate of bone loss in these patients.
Since the comprehension that BMAT expansion can be intimately linked to the occurrence of osteoporosis, great interest emerged in full characterization of this tissue in different physiological conditions, as well as in disease-associated osteoporosis. The magnetic resonance spectroscopy (MRS) is the main tool currently used for BMAT quantification due to its important advantages of noninvasiveness and no exposition to radiation. Moreover, several studies have documented that MRS is a highly precise and accurate method for measurement of BMAT, allowing the collection of reproducible data and yielding results closely related to those obtained by bone biopsy [31], which is regarded as the gold standard method, and comparable to those obtained with dual-energy computed tomography [32]. In a recent study performed in our laboratory in healthy individuals, we were able to detect not only the age-related effect on BMAT (age <50 years 32.4 ± 7.9% vs age >50 years 49.5 ± 8.9%; p < 0.05) but also sex-related difference in lipid quality, as women were shown to have higher amount of unsaturated lipids than men (women = 3.18 ± 1.05% vs men = 1.67 ± 0.65%; p < 0.05) [33].
The expansion of BMAT seems to be an inherited, age-related process, starting early after birth and progressing continuously in heterogeneous but predictable steps. It is well documented that the distal portions of the small peripheral bones are occupied by BMAT earlier than axial bones [34]. Additionally, previous studies showed that there is a progressive increase in BMAT in the inferior vertebral bodies in comparison to their superior counterparts [35]. Thus, in the present study, the localization of vertebral body L3 was strictly standardized based on the crest iliac as the indication for L5.
Our results showed smaller values of BMAT in controls, in comparison to those from a previous study [17] (28.6 ± 8.1 vs 52 ± 18%). This may have been due to site-related differences for measuring BMAT (L3 vs L4), as well as to the utilization of different formulas to calculate BMAT. It is also important to point out that there is no consensus in literature concerning the estimation of BMAT. While in the present study, the formula lipid-to-water plus lipid ratio was used, Bredella and colleagues utilized the equation lipid-to-water ratio [17]. Nevertheless, most of the published studies used the same formula that was utilized in the present study [16, 36].
The subgroup of patients with decreased bone mass was further compared with the subgroup showing normal BMD. There was no significant difference concerning relevant clinical characteristic (e.g., age of disease onset, duration of the disease, time and dose of glucocorticoid use, and disease severity).
Experimental [37] (caloric restriction of mice) and clinical [17] (women with anorexia nervosa) studies have shown that, in conditions of active feeding restriction, bone loss and enhancement of marrow adipose tissue are concurrent processes. Moreover, the comparison of anorexic women with active and inactive disease showed that the group who gained weight had BMD and BMAT similar to the control group [38]. Our data for patients with IBD in remission are in line with the results of this study [38]. Unfortunately, neither the Z-score values nor correlations between the BMD and BMAT in their patients were presented. The relationships between peripheral white adipose tissue and BMAT are not yet completely understood. Recent studies in patients with obesity and diabetes mellitus, in whom peripheral white adipose tissue was markedly expanded, failed to show any change in BMAT [23, 39]. Since our IBD patients did not have increased energy expenditure and had values similar to controls for body total fat and lean mass, BMAT was not expected to be increased due to this particular mechanism.
The present study did not find statistically significant differences between IBD and control individuals regarding circulatory levels of RANKL or OPG. Although these results are apparently in conflict with the increased serum levels of CTX, indeed, they most likely reflect the incapacity of the peripheral measurements of RANKL and OPG to reproduce the status of cell activity within the bone microenvironment [40, 41].
Currently, there is a great interest in the investigation of the endocrine role of BMAT. Recent evidence [42] has been collected showing that BMAT putatively contributes to the circulatory levels of adiponectin, an insulin sensitizer molecule, in condition of caloric restriction. However, as long as we know, the present study is the first to report an inverse relationship between BMAT and CRP.
It is reported that patients with CD may have lactose malabsorption [43], with consequent decreased intake of milk products. This might explain the significantly higher PTH values in the group of patients with CD in the present study. Nevertheless, we have not collected data on milk consumption and tolerance, since this was not the aim of the present study.
This study has a main limitation, which is the relatively small number of patients included, who came from a single institution. This small number has precluded the separate analysis of data from male and female patients, which could provide additional valuable information. Nevertheless, the characteristics of our patients are comparable to those of patients included in similar studies and, due to the strict inclusion and exclusion criteria, the sample was rather homogeneous, including only patients in remission and without glucocorticoid use. Also, other factors associated with risk of bone loss, such as malnutrition, smoking, medications that alter calcium metabolism, and menopause in women, were ruled out. Additionally, a relatively large control group was included and a comprehensive assessment of bone status was performed, including the measurement of biochemical factors involved in bone remodeling and BMAT, which have never been performed in patients with IBD. Both the CD and UC patients included in this study had demographic and clinical characteristics concerning age at IBD diagnosis, disease location and extension, and clinical behavior similar to those described by others [5, 44], indicating that the main findings could be generalized. Another possible limitation is that 14 out of the 65 participants in the control group (patients with minor digestive disease) were in use of a proton pump inhibitor (PPI). Since there are data in the literature pointing to an association between chronic use of PPI and loss of bone mass [45], the differences between controls and the IBD groups may have been attenuated.
Additionally, we were not able to determine the influence of medications to treat IBD in the results concerning bone mass loss. Although all participants of the CD and UC groups were free from steroids, the time since they last used them varied hugely among individual patients. However, 4 out of the 7 IBD patients with definite bone mass impairment had never taken steroids, which do not support a prominent previous effect of glucocorticoids. Also, 14 IBD patients were in use of anti-TNF agents, which are thought to have a protective effect on bone mass, considering the block of cytokine of osteoclastic action [1]. Nevertheless, five out of these 14 patients had inadequate bone mass, which may point to a greater contribution of previous inflammation, since TNF agents are used preferentially in more severe cases.
Regarding possible practical implications of our findings, it is uncertain whether bone protection measures should be used for IBD patients in long-term, corticosteroid-free remission. The present study shows that the mean values of BMD in individuals with IBD are similar to the control group; however, it should be highlighted that a considerable number of patients (approximately 20%) exhibit inadequate bone mass. These preliminary results encourage further investigation that may allow uncovering risk factors that hamper bone mass development and maintenance in IBD.
In summary, the present study confirms that IBD patients pertain to a very heterogeneous group regarding BMD and shows that some patients present with inadequate bone mass even during long-term, steroid-free remission. Low bone mass remains as a single impairment of bone composition for this group of patient, since BMAT seems to be preserved and has no correlation with BMD. Previous flares of inflammatory disease activity and treatment with glucocorticoids, which are largely accepted as independent risk factors for low bone mass [1], may have contributed to our findings. Nevertheless, only further studies with a greater number of patients, stratified by different forms of clinical presentation and treatment, will be able to determine the contributions of different factors to impaired bone mass in IBD in remission, as well as the relationships between bone mass and bone marrow adipose tissue in this condition.
References
Ali T, Lam D, Bronze MS et al (2009) Osteoporosis in inflammatory bowel disease. Am J Med 122:599–604
Ghosh S, Cowen S, Hannan WJ et al (1994) Low bone mineral density in Crohn’s disease, but not in ulcerative colitis, at diagnosis. Gastroenterology 107:1031–1039
Tilg H, Moschen AR, Kaser A et al (2008) Gut, inflammation and osteoporosis: basic and clinical concepts. Gut 57:684–694
Jahnsen J, Falch JA, Aadland E et al (1997) Bone mineral density is reduced in patients with Crohn’s disease but not in patients with ulcerative colitis: a population based study. Gut 40:313–319
Ardizzone S, Bollani S, Bettica P et al (2000) Altered bone metabolism in inflammatory bowel disease: there is a difference between Crohn’s disease and ulcerative colitis. J Intern Med 247:63–70
Silvennoinen A, Karttunen TJ, Niemelã SE et al (1995) A controlled study of bone mineral density in patients with inflammatory bowel disease. Gut 37:71–76
Kanis JA (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 4:368–381
Pigot F, Roux C, Chaussade S et al (1992) Low bone mineral density in patients with inflammatory bowel disease. Dig Dis Sci 37:1396–1403
Roux C, Abitbol V, Chaussade S et al (1995) Bone loss in patients with inflammatory bowel disease: a prospective study. Osteoporos Int 5:156–160
Targownik LE, Bernstein CN, Leslie WD (2014) Risk factors and management of osteoporosis in inflammatory bowel disease. Curr Opin Gastroenterol 30:168–174
Pietschmann P, Resch H, Woloszczuk W et al (1990) A circadian rhythm of serum osteocalcin levels in postmenopausal osteoporosis. Eur J Clin Investig 20:310–312
Yavropoulou MP, Tomos K, Tsekmekidou X et al (2011) Response of biochemical markers of bone turnover to oral glucose load in diseases that affect bone metabolism. Eur J Endocrinol 164:1035–1041
Sanchez Cano D, Ruiz-Villaverde R, Olvera Porcel MC et al (2011) Evaluation of bone mineral density, bone turnover markers, the OPG/RANKL system and sTNF-RI in Crohn’s disease. Gastroenterol Hepatol 34:3–9
de Paula FJ, Rosen CJ (2013) Bone remodeling and energy metabolism: new perspectives. Bone Res 1:72–84
Bianco P, Riminucci M, Gronthos S et al (2001) Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 19:180–192
Li X, Kuo D, Schafer AL et al (2011) Quantification of vertebral bone marrow fat content using 3 tesla MR spectroscopy: reproducibility, vertebral variation, and applications in osteoporosis. J Magn Reson Imaging 33:974–979
Bredella MA, Fazeli PK, Miller KK et al (2009) Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab 94:2129–2136
Liu Y, Tang GY, Tang RB et al (2010) Assessment of bone marrow changes in postmenopausal women with varying bone densities: magnetic resonance spectroscopy and diffusion magnetic resonance imaging. Chin Med J 123:1524–1527
Tang GY, Lv ZW, Tang RB et al (2010) Evaluation of MR spectroscopy and diffusion-weighted MRI in detecting bone marrow changes in postmenopausal women with osteoporosis. Clin Radiol 65:377–381
Silverberg MS, Satsangi J, Ahmad T et al (2005) Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 19(Suppl A):5a–36a
Best WR, Becktel JM, Singleton JW et al (1976) Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 70:439–444
Schousboe JT, Shepherd JA, Bilezikian JP et al (2013) Executive summary of the 2013 ISCD position development conference on bone densitometry. J Clin Densitom 16:455–467
de Paula FJA, de Araújo IM, Carvalho AL et al (2015) The relationship of fat distribution and insulin resistance with lumbar spine bone mass in women. PLoS One 10:e0129764
Zhang Q, Riddle RC, Clemens TL (2015) Bone and the regulation of global energy balance. J Intern Med 277:681–689
Rosen CJ, Bouxsein ML (2006) Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2:35–43
Cosman F, de Beur SJ, LeBoff MS et al (2014) Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 25:2359–2381
van Hogezand RA, Hamdy NA (2006) Skeletal morbidity in inflammatory bowel disease. Scand J Gastroenterol Suppl 243:59–64
Bernstein CN, Leslie WD, Leboff MS (2003) AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology 124:795–841
Compston J, Reid DM, Boisdron J et al (2008) Recommendations for the registration of agents for prevention and treatment of glucocorticoid-induced osteoporosis: an update from the Group for the Respect of Ethics and Excellence in Science. Osteoporos Int 19:1247–1250
Seguro LP, Rosario C, Shoenfeld Y (2013) Long-term complications of past glucocorticoid use. Autoimmun Rev 12:629–632
Cohen A, Shen W, Dempster DW et al (2015) Marrow adiposity assessed on transiliac crest biopsy samples correlates with noninvasive measurement of marrow adiposity by proton magnetic resonance spectroscopy (1H-MRS) at the spine but not the femur. Osteoporos Int 26:2471–2478
Bredella MA, Daley SM, Kalra MK et al (2015) Marrow adipose tissue quantification of the lumbar spine by using dual-energy CT and single-voxel 1H MR spectroscopy: a feasibility study. Radiology 277:230–235
Maciel JG, de Araújo IM, Carvalho AL et al (2016) Marrow fat quality differences by sex in healthy adults. J Clin Densitom. doi:10.1016/j.jocd.2016.08.002
Blebea JS, Houseni M, Torigian DA et al (2007) Structural and functional imaging of normal bone marrow and evaluation of its age-related changes. Semin Nucl Med 37:185–194
Liney GP, Bernard CP, Manton DJ et al (2007) Age, gender, and skeletal variation in bone marrow composition: a preliminary study at 3.0 tesla. J Magn Reson Imaging 26:787–793
Schwartz AV (2015) Marrow fat and bone: review of clinical findings. Front Endocrinol 6:1–6
Devlin MJ, Cloutier AM, Thomas NA et al (2010) Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res 25:2078–2088
Fazeli PK, Bredella MA, Freedman L et al (2012) Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa. J Bone Miner Res 27:1864–1871
de Araújo IM, Salmon CE, Nahas AK et al (2017) Marrow adipose tissue spectrum in obesity and type 2 diabetes mellitus. Eur J Endocrinol 176:21–30
Schett G, Kiechl S, Redlich K et al (2004) Soluble RANKL and risk of nontraumatic fracture. JAMA 291:1108–1113
Vega D, Maalouf NM, Sakhaee K (2007) CLINICAL review #: the role of receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/osteoprotegerin: clinical implications. J Clin Endocrinol Metab 92:4514–4521
Cawthorn WP, Scheller EL, Leaman BS et al (2014) Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab 20:368–375
Pironi L, Callegari C, Cornia GL et al (1988) Lactose malabsorption in adult patients with Crohn’s disease. Am J Gastroenterol 83:1267–1271
Dinca M, Fries W, Luisetto G et al (1999) Evolution of osteopenia in inflammatory bowel disease. Am J Gastroenterol 94:1292–1297
Arj A, Razavi Zade M, Yavari M et al (2016) Proton pump inhibitors use and change in bone mineral density. Int J Rheum Dis 19:864–868
Acknowledgements
The authors are indebted to Marta T. Nakao and Rodrigo A. Pessini for their competent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional Ethics Committee (HCRP Case No. 3836/2011) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Funding
This study was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Bastos, C.M., Araújo, I.M., Nogueira-Barbosa, M.H. et al. Reduced bone mass and preserved marrow adipose tissue in patients with inflammatory bowel diseases in long-term remission. Osteoporos Int 28, 2167–2176 (2017). https://doi.org/10.1007/s00198-017-4014-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4014-3