Abstract
Summary
We conducted an observational cross-section study to investigate the status of bone mineral mass of Tibetan postmenopausal women with type 2 diabetes and the possible predictors for osteoporosis. We found that prevalence of osteoporosis was 27.0% and blood pressure was an independent risk factor for bone mass loss.
Introduction
The aims of this study is to investigate the prevalence of osteoporosis in postmenopausal women with type 2 diabetes dwelling in Tibet and the possible risk factors for bone mass loss.
Methods
We recruited 99 Chinese Tibetan postmenopausal women with type 2 diabetes from the department of endocrinology of People’s Hospital Tibet Autonomous Region. Multiple sites of bone mineral density (BMD) were measured by dual-energy X-ray absorptiometry (DXA). The subjects were divided into three groups based on BMD T-score: osteoporosis, osteopenia, and normal. The clinical characteristics were compared between groups. The risk factors for bone mass loss were assessed by multiple linear regression analysis.
Results
Among diabetic postmenopausal women dwelling in high altitude, mean age was 62 ± 8 years, the median postmenopausal period was 12 years (5, 20), the median duration of diabetes mellitus was 3 years (1, 8), and mean BMI was 27.6 ± 4.2 kg/m2. Patients (52.5%) had hypertension. The percentages of patients with osteoporosis, osteopenia and normal BMD were 27.3, 42.4, and 30.3%, respectively. HbA1c and systolic blood pressure (SBP) were independently associated with T-scores of spine; ages and SBP were independently associated with T-scores of femoral neck or hip.
Conclusions
Among diabetic postmenopausal women dwelling in high altitude, 27.3% patients have osteoporosis, 42.4% patients have osteopenia, and 30.3% are normal. The BMD T-score of spine was inversely associated with SBP and positively associated with HbA1c, while the BMD T-score of femoral neck or hip was inversely associated with ages and SBP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the decreased production of estrogen after menopause and with aging, the prevalence rate of osteoporosis in postmenopausal women was higher than in men the same age and in women with menstruation. It has been estimated that osteoporosis contributes to ≥90% of hip and spine fractures in women beyond 65 years old, thus linking with a significant burden on the healthcare systems of countries worldwide [1].
High altitude is defined as 2440 m above sea level. An elevation of over than 5490 m belonged to extreme high altitude. People living at high altitude bear hard environment including hypobaric hypoxia, low temperature, less humidity, and limited nutritional resources [2]. The high-altitude stress increased with altitude [3]. Hypoxia may stimulate secretion of many hormones that have affected bone mineral metabolisms. It is well documented that thyroid hormones, cortisol, catecholamines, and growth factors increased with altitude [4]. Increased glucocorticoids are known to inhibit bone growth and cause osteoporosis by inhibiting osteoblast proliferation [5]. While increased thyroid hormones is known to stimulate bone resorption more than formation and cause bone mass reduction [6].
Diabetes and osteoporosis are both frequent conditions and they may occur simultaneously. Although patients with type 2diabetes are known to have various changes in the BMD, a growing body of evidence suggested that patients with type 2 diabetes had increased risk for fracture of the femur [7,8,9,10]. The prevalence of osteoporosis in different regions for patients with type 2 diabetes has been largely reported previously [10,11,12,13]. However, no report is available on the prevalence of osteoporosis for those dwelling in high altitude. The Tibet plateau has an average elevation of more than 4000 m above sea level and counts 2.62 million inhabitants [14]. It is valuable to explore the prevalence of osteoporosis in Tibetan diabetic patients since both diabetes and high altitude may affect bone metabolisms.
Based on the foregoing information, we carried out an observational cross-sectional study to investigate the status of bone mineral mass of postmenopausal women with type 2 diabetes dwelling in Tibet and the possible predictors for osteoporosis. To our best knowledge, this is the first study to report the prevalence of osteoporosis and explore the risk factors of osteoporosis in diabetic postmenopausal women in high altitude.
Methods
Study population
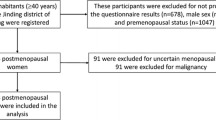
The participants were Tibet-dwelling, postmenopausal women with type 2 diabetes who were admitted to department of endocrinology of People’s Hospital of Tibet Autonomous Region for occurrence of typical symptoms including polyuria, polydipsia, polyphagia, and weight loss or bad glucose control (fasting serum glucose ≥10 mmol/l or HbA1c ≥ 9.0%) from January 2013 to August 2016. The inclusion criteria were as follows: (1) subjects were diagnosed as type 2 DM according to WHO 1999 criteria; (2) subjects were native dwellers in high altitude (2500∼4500 m); (3) women who were at least 12 months past their last menstrual period; (4) not receiving treatment for osteoporosis or drugs known to affect bone and mineral metabolism. A total of 99 postmenopausal women with type 2 diabetes were enrolled in our study. The study was approved by the Institutional ethnic committee of People’s Hospital of Tibet Autonomous Region.
Physical examinations and laboratory measurements
Histories of duration of diabetes and hypertension were obtained from inpatient medical records. BMI was calculated as weight (kg) divided by the square of height (m). All blood samples were obtained at fasting state. Fasting blood glucose (FBG), serum total cholesterol (TC), triglyceride (TG), low- and high-density lipoprotein cholesterol (LDL-C and HDL-C), uric acid (UA), glutamic-oxaloacetic transaminase (ALT), glutamic-pyruvic transaminase (AST), and serum creatinine (CRE) were determined with enzymatic methods on automatic biochemical analyzer (Architech-C1600, USA). HbA1c was measured by high-performance liquid chromatography method (SYSMEX G8, Japan) with reference range from 4.0 to 6.0%. The coefficients of variation (CVs) of intra-assay and inter-assay are 0.004 and 1.73%, respectively, which suggests excellent reproducibility. Blood routine examinations were performed by automated hematology analyzer (Sysmex XE-2100, Japan).
Bone mineral density measurements
Multiple sites of BMD examination was routinely performed by a DXA (Hologic Discovery W S/N 86724, Bedford, USA) for each postmenopausal woman in our institution. Daily calibration was carried out with a manufacture-provided phantom, and the maximum coefficient of variation (CV%) of BMD was 0.1%. Bone density was measured at the lumbar spine (L1-L4) and proximal femur on a postero-anterior scan. BMD was expressed in g/cm2 and as peak bone mass percentage in normal subjects (T-score), depending on the software used in the device. The reference range of normal subjects in our study was adopted from that in mainland, China.
Definitions
According to WHO criteria, osteoporosis was diagnosed on the basis of measurements at the spine and the right and left femoral neck. Osteoporosis was diagnosed if T-score ≤−2.5, osteopenia was diagnosed if −2.5 < T-score < −1.0, and normal BMD was diagnosed if T-score ≥−1.0. Hypertension was defined if systolic pressure exceeded 140 mmHg and/or diastolic pressure exceeded 90 mmHg, or if the patients are on antihypertensive drugs. Frequent pregnancies were defined as numbers of pregnancy ≥4.
Statistical analyses
All statistical analysis was performed using SPSS 16.0 (IBM, Inc., New York, USA). Continuous variables were expressed as mean ± SD. Since duration of DM did not follow a normal distribution, the values were Log transferred (e.g., Ln duration of DM) and displayed as median and interquartile range. The differences of continuous parameters that had normal distribution among groups were analyzed by one-way ANOVA analysis. Multiple stepwise linear regression analysis was performed to determine the independent risk factors associated with bone mass loss.
Results
Prevalence and risk factors of osteoporosis of diabetic postmenopausal women in high altitude
Baseline characteristics of 100 subjects were summarized in Table 1. The mean age was 61 ± 8 years and the median menopause period was 13 years (5, 18) with a median duration of diabetes of 3 years. The mean BMI was 27.6 ± 4.2 kg/m2 and the median duration of menopause was 12 years. Patients (29.3%) were normal BMD, 44.4% of patients were osteopenia, and 27.3% of patients were osteoporosis.
Patients were divided according to BMD measurements in three groups: 30 with normal BMD (T-score ≥ −1.0), 43 with osteopenia (−2.5 < T-score < −1.0), and 27 osteoporosis (T-score ≤ −2.5). From normal BMD, osteopenia to osteoporosis, patients had significantly older age (p = 0.000), longer menopause period (p = 0.001), higher percentage of frequent pregnancies (p = 0.024), longer duration of diabetes (p = 0.036), and higher SBP level (p = 0.011). Other variables including smoking, BMI, Hgb, HbA1c, UA, and CRE did not show significant differences among three groups.
Associations between bone mineral loss and baseline characteristics
Multiple linear regression analyses were performed to determine the strongest predictors for BMD T-score at different sites. When BMD T-score of spine was entered as dependent variable and age, BMI, menopause period, HbA1c, SBP, frequent pregnancies, and smoking as independent variables, the strongest predictors of BMD T-score of spine were HbA1c and SBP (Table 2). The ages and SBP were significantly independently associated with T-score of femoral neck or hip (Tables 3 and 4).
Discussion
In the present retrospective cross-sectional study, we showed that among diabetic postmenopausal women who dwelled in high altitude, 27.3% patients have osteoporosis, 42.4% patients have osteopenia, and 30.3% are normal. HbA1c was positively associated with BMD T-score at spine; age was inversely associated with BMD T-score at femoral neck and hip; and SBP was inversely associated with BMD T-score at multiple sites.
The incidence of osteoporosis varies widely between populations. It has been reported that approximately 30% of postmenopausal American women and 23% of postmenopausal Australian women have osteoporosis. In Germany, 23.3% of postmenopausal German women had osteoporosis [13]. In Asia, the prevalence of osteoporosis and osteopenia in postmenopausal women in Oman were 40 and 47%, respectively. The prevalence of osteoporosis among women 50 years and older ranged from 25.7 to 37.0% in Hong Kong and 11.4% in Taiwan [11, 12]. A survey in central south of China showed 37.5% Chinese postmenopausal women suffered from osteoporosis. Few reports are available for prevalence of osteoporosis in postmenopausal women in high altitude. Our study firstly showed for diabetic postmenopausal women in Tibet plateau, the prevalence of osteoporosis and osteopenia was 27.3 and 42.4%, respectively, which is comparative to that at sea level.
High altitude-induced hypoxia may impair bone health. Basu M et al. investigated bone mass change of healthy males who migrated from an altitude of 3542 m to an extreme altitude (5400–6700 m) in India where they stayed for 4 months. The bone impairment was detected at the proximal phalanx. Bone-specific alkaline phosphatase activities and 25(OH) vitamin D declined significantly [4]. Another study compared the alkaline phosphatase activity of dwellers in between moderately high altitude and low altitude in Meghalaya, India. The raised alkaline phosphatase activity was found among highlanders, which may elevate the risk of osteoporosis [15]. Insufficient supplementation of nutritional elements such as calcium and vitamin D may cause dysfunction of musculoskeletal system [2, 16]. Very low vitamin D supplement in dietary, repeated pregnancies and lacking of calcium supplement during pregnancy may be partially responsible for the low BMD measurement obtained in these diabetic postmenopausal women at high altitude. The National Osteoporosis Foundation (NOF) recommends 1200 mg calcium and 800 to 1000 IU vitamin D daily for women aged 50 and beyond [17]. Tibetans fall far below this recommendation. Previous studies showed that vitamin D was alarmingly low, and bone-related diseases were significantly higher in Tibet [2, 18]. Serum 25(OH)D is the best indicator of vitamin D status. The mean 25(OH)D concentration in our study was 10.8 ± 7.2 ng/ml. The prevalence was 95.2% for 25(OH)D < 30 ng/ml and 90.5% 25(OH)D < 20 ng/ml, which was much higher than postmenopausal women in mainland. The food habit that is lacking of vitamin D and rarely exposure more of their body than hands and face in whole life contribute to the vitamin D insufficiency in Tibet. However, the potential reasons for the apparently comparable osteoporosis prevalence at high altitude and sea level under the circumstances of lower vitamin D in high altitude is still unknown. A larger-scale epidemiological study covering mainland and high altitude will be needed in the future.
Identification of risk factors will help healthcare providers to diagnose and treat patients with osteoporosis at the earliest. The risk factors for osteoporosis have been documented for population at sea level. Few studies are available of the risk factors of osteoporosis in highlanders. In our study, the risk factors of bone mass loss were different at different sites. HbA1c was positively associated with bone mass loss in spine. The positive association between HbA1c and BMD was also proved by other studies [8]. Mechanisms accounted for type 2 diabetic patients with increasing BMD are complex and largely unclear. Adipocytokines dysfunction and hyperinsulinemia may play a role [19]. Large prospective studies are needed to explore the mechanisms underlying the association. Advancing age, an absolute risk factor for osteoporosis and evidenced by a majority of studies, was also a risk factor for bone mass loss in femoral neck and hip in our study. In addition, blood pressure was significantly associated with bone mass loss of multiple sites. Our result was in accordant with previous finding of strong association between osteoporosis and hypertension [20,21,22,23,24]. Cappuccion et al. studied 3676 white postmenopausal women and found that higher blood pressure was associated with increased bone loss at the femoral neck [21]. Similarly, for Korean and Chinese postmenopausal women [22, 23], the strong association between osteoporosis and hypertension were also evidenced by researchers in large cohorts, respectively.
The main limitations of this study are: first, we have no data for the prevalence of osteoporosis in the general population at Tibet and are not able to compare the differences between people with and without diabetes. Second, due to the nature of cross-section study, we cannot conclude the causal relationship between the risk factors and osteoporosis. Third, results from a single-center may not be generalized and small sample size may diminish its statistical power. Therefore, prospective studies with large samples and intervention strategies are further needed to verify our results.
In conclusion, our study reported that prevalence of bone mass loss in postmenopausal women with type 2 diabetes dwelling in high altitude was 27.0%. Blood pressure was an independent risk factor for bone mass loss at multiple sites. HbA1c was independently positively associated with BMD T-score in spine, while advancing age was independently inversely associated with BMD T-score in femoral neck and hip.
References
NIH Consensus Development Panel on Osteoporosis Prevention D, Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–795
Norsang G, Ma L, Dahlback A, Zhuoma C, Tsoja W, Porojnicu A, Lagunova Z, Moan J (2009) The vitamin D status among Tibetans. Photochem Photobiol 85:1028–1031
Lankford HV (2014) Extreme altitude: words from on high. Wilderness Environ Med 25:346–351
Basu M, Malhotra AS, Pal K, Chatterjee T, Ghosh D, Haldar K, Verma SK, Kumar S, Sharma YK, Sawhney RC (2013) Determination of bone mass using multisite quantitative ultrasound and biochemical markers of bone turnover during residency at extreme altitude: a longitudinal study. High Alt Med Biol 14:150–154
Seibel MJ, Cooper MS, Zhou H (2013) Glucocorticoid-induced osteoporosis: mechanisms, management, and future perspectives. Lancet Diabetes Endocrinol 1:59–70
Tuchendler D, Bolanowski M (2014) The influence of thyroid dysfunction on bone metabolism. Thyroid Res 7:12
Leidig-Bruckner G, Ziegler R (2001) Diabetes mellitus a risk for osteoporosis? Exp Clin Endocrinol Diabetes 109(Suppl 2):S493–S514
Ma L, Oei L, Jiang L, Estrada K, Chen H, Wang Z, Yu Q, Zillikens MC, Gao X, Rivadeneira F (2012) Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol 27:319–332
Qorbani M, Bazrafshan HR, Aghaei M, Dashti HS, Rezapour A, Asayesh H, Mohammadi R, Mohammadi Y, Ansari H, Mansourian M (2013) Diabetes mellitus, thyroid dysfunctions and osteoporosis: is there an association? J Diabetes Metab Dis 12:38
Rakel A, Sheehy O, Rahme E, LeLorier J (2008) Osteoporosis among patients with type 1 and type 2 diabetes. Diabetes Metab 34:193–205
Lo SS (2015) Bone health status of postmenopausal Chinese women. Hong Kong Med J 21:536–541
Wang Y, Tao Y, Hyman ME, Li J, Chen Y (2009) Osteoporosis in China. Osteoporos Int 20:1651–1662
Haussler B, Gothe H, Gol D, Glaeske G, Pientka L, Felsenberg D (2007) Epidemiology, treatment and costs of osteoporosis in Germany—the BoneEVA study. Osteoporos Int 18:77–84
Petousi N, Robbins PA (2014) Human adaptation to the hypoxia of high altitude: the Tibetan paradigm from the pregenomic to the postgenomic era. J Appl Physiol (1985) 116:875–884
Ranhotra HS, Sharma R (2010) Moderately high altitude habitation modulates lipid profile and alkaline phosphatase activity in aged Khasis of Meghalaya. Indian J Clin Biochem 25:51–56
Polly P, Tan TC (2014) The role of vitamin D in skeletal and cardiac muscle function. Front Physiol 5:145
Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R, National Osteoporosis F (2014) Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 25:2359–2381
Rooze S, Dramaix-Wilmet M, Mathieu F, Bally P, Yangzom D, Li JZ, Goyens P (2012) Growth, nutritional status, and signs of rickets in 0-5-year-old children in a Kashin-Beck disease endemic area of Central Tibet. Eur J Pediatr 171:1185–1191
Starup-Linde J, Vestergaard P (2015) Management of endocrine disease: diabetes and osteoporosis: cause for concern? Eur J Endocrinol 173:R93–R99
Afghani A, Johnson CA (2006) Resting blood pressure and bone mineral content are inversely related in overweight and obese Hispanic women. Am J Hypertens 19:286–292
Cappuccio FP, Meilahn E, Zmuda JM, Cauley JA (1999) High blood pressure and bone-mineral loss in elderly white women: a prospective study. Study of Osteoporotic Fractures Research Group. Lancet 354:971–975
Park JS, Choi SB, Rhee Y, Chung JW, Choi EY, Kim DW (2015) Parathyroid hormone, calcium, and sodium bridging between osteoporosis and hypertension in postmenopausal Korean women. Calcif Tissue Int 96:417–429
Zhang J, Zhang K, Shi H, Tang Z (2015) A cross-sectional study to evaluate the associations between hypertension and osteoporosis in Chinese postmenopausal women. Int J Clin Exp Med 8:21194–21200
Yazici S, Yazici M, Korkmaz U, Engin Erkan M, Erdem Baki A, Erden I, Ozhan H, Ataoglu S (2011) Relationship between blood pressure levels and bone mineral density in postmenopausal Turkish women. Arch Med Sci 7:264–270
Acknowledgements
This work was supported by the Special Scientific Research Fund of Public Welfare Profession of China (201402005) and the Special Scientific Research Fund of Clinical Medicine of Chinese Medical Association (15010010589).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics statement
The study was approved by the Institutional ethnic committee of People’s Hospital of Tibet Autonomous Region.
Conflicts of interest
None.
Additional information
L. Zhou and J. Song contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhou, L., Song, J., Yang, S. et al. Bone mass loss is associated with systolic blood pressure in postmenopausal women with type 2 diabetes in Tibet: a retrospective cross-sectional study. Osteoporos Int 28, 1693–1698 (2017). https://doi.org/10.1007/s00198-017-3930-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-3930-6