Abstract
Summary
We compared bone mineral density (BMD) and trabecular bone score (TBS) in postmenopausal women with a distal radius fracture older than 50 years with controls. Total hip BMD was significantly different, but TBS was not different between two groups, suggesting TBS does not reflect microarchitectural changes of the distal radius.
Introduction
The purpose of this study was to determine whether trabecular bone score (TBS) has additive value for discriminating distal radius fracture (DRF) independent of BMD.
Methods
We compared BMD and TBS in 258 postmenopausal women with a DRF older than 50 years of age with age- and body mass index (BMI)-matched controls who had no history of osteoporotic fracture. BMD was measured at the lumbar spine and hip using dual energy X-ray absorptiometry scans (GE Lunar Prodigy). TBS was calculated on the same spine image. A multivariate logistic regression analysis was used to analyze the odds ratio (OR) for the occurrence of DRF using age, BMI, lumbar spine BMD, total hip BMD, and TBS.
Results
Patients with a DRF had significantly lower BMDs at hip (neck, trochanter and total) than those of controls: 0.752 ± 0.097, 0.622 ± 0.089, and 0.801 ± 0.099 in patients and 0.779 ± 0.092, 0.648 ± 0.089, 0.826 ± 0.101 in controls. However, lumbar spine BMD and TBS were not significantly different between the groups (p = 0.400 and 0.864, respectively). The multivariate analysis indicated that only total hip BMD was significantly associated with the occurrence of DRF (OR, 10.231; 95 % confidence interval, 1.724–60.702; p = 0.010).
Conclusions
TBS was not different between women with a DRF and those without a history of osteoporotic fracture, suggesting that TBS measured at the lumbar spine does not reflect early microarchitectural changes of the distal radius. Only total hip BMD is associated with the risk of DRF in Korean women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis and fragility fractures are a major public health issues with considerable social and economic costs [1]. The diagnosis of osteoporosis is based on dual energy X-ray absorptiometry (DXA), currently considered the reference standard for determining bone mineral density (BMD) [2]. However, BMD does not always accurately reflect fracture risk and up to 50 % of those who sustain fragility fractures do not have osteoporosis based on BMD testing [3]. In particular, BMD is not consistently lower than normal controls in patients with a distal radius fracture (DRF) [4–6], suggesting that factors other than BMD, such as bone microarchitecture, bone geometry, micro-damage, mineralization, bone turnover, and propensity to fall, influence bone strength and fracture risk [7–9]. However, these structural properties of bone cannot be evaluated by using DXA [10].
The trabecular bone score (TBS) is a textural index that evaluates pixel gray-level variations in a lumbar spine DXA image, providing an indirect index of trabecular microarchitecture and bone quality [11]. TBS is significantly associated with vertebral and non-vertebral fractures in several cross-sectional and prospective studies involving a large number of postmenopausal women [12–15]. However, no studies have evaluated the result of TBS for patients with only a DRF.
An osteoporosis evaluation of patients with a DRF is important. DRF is the most common upper extremity fracture in women aged equal to or more than 50 years [16] as it is a predictor of osteoporosis and subsequent fractures [17] and typically occurs earlier than a hip fracture by an average of 15 years [18]. Therefore, patients with a DRF offer physicians an opportunity to diagnose osteoporosis and start secondary prevention [19]. However, because BMD determined by DXA does not reflect microstructural changes in trabecular bone, some osteoporotic fragile patients can be misdiagnosed as normal or osteopenia according to DXA results [14]. TBS, which evaluates the trabecular bone quality, would have a complementary role for the evaluation of osteoporosis in the patients with a DRF.
In this study, we examined the additive value of TBS for discriminating a DRF independent of BMD. We assessed TBS and BMD in patients with a DRF and compared the values with controls which were selected from a large cohort of women with use of propensity score-matched analysis.
Materials and methods
Study population
We obtained approval for this study from the Institutional Review Board of our institute. We enrolled women (age, 50–79 years) who suffered a DRF between May 2010 and December 2014 and who met the following inclusion criteria: (1) acute DRF caused by minor trauma, such as a fall from standing height, (2) underwent a DXA scan (Lunar Prodigy; GE Lunar, WI, USA) within 2 weeks after the fracture. We excluded patients with other conditions, such as renal insufficiency, adrenal insufficiency, rheumatoid arthritis, thyroid disease, parathyroid disease, Parkinson’s disease, chronic obstructive pulmonary disease, malignancy, history of osteoporotic fracture, or those who received medication known to affect bone quality, such as steroids, menopausal hormone therapy, or medications for osteoporosis. Patients who had undergone spine surgery, or had severe scoliosis, spondylitis, or vertebral infection were also excluded.
Figure 1 illustrates the patient and control group enrollment process. Most of the patients who had a previous osteoporotic fracture, previous medication affecting bone surgery, previous lumbar spine surgery, and other spine problems affecting bone quality, had BMD examinations before sustaining DRF. These patients were excluded by the exclusion criteria. Among the 804 consecutive women who visited our hospital with a DRF, 269 who met the inclusion criteria were enrolled as the patient group. During the same period, 16,889 women (age, 50–79 years) had DXA scans in our hospital; 7993 subjects were excluded from the cohort using the patient exclusion criteria and 8896 remained as controls. To reduce bias between patients and controls, we used a propensity score analysis to select the 269 case-matched controls. The propensity score is the conditional probability of being selected given the covariates; thus, it can be used to balance confounding factors between two groups [20]. The propensity score was calculated for each group based on a logistic regression analysis, using subject age and body mass index (BMI) for matching. From each groups, subjects who were in premenopausal status were excluded from cohort to avoid the bias. Eleven patients (4.1 % of 269 patients) were excluded from patient group and 14 subjects (5.2 % of 269 controls) were excluded from control group. The proportion of premenopausal women was not significantly different between two groups (p = 0.533). Finally, 258 DRF patients and 255 controls were enrolled in the cohorts.
Bone mineral density and trabecular bone score
In our institute, BMD (g/cm2) was measured at the lumbar spine, femoral neck, trochanter, Ward’s triangle, and the total hip with DXA scans and analyzed with Encore Software ver. 11.0. The BMD precision error (percentage of the coefficient of variation) was 1.7 % for lumbar spine, 1.8 % for femoral neck, and 1.7 % for total hip, which were measured by assessing 30 individuals with two scans at our institution. Calculated from the formula 2.77 × precision error, the least significant change of BMD was 0.044 g/cm2 for lumbar spine, 0.038 g/cm2 for femoral neck, and 0.038 g/cm2 for total hip in a 95 % confidence level. For lumbar spine BMD, the L1–4 value was used for analysis. All TBS measurements were performed retrospectively using TBS iNsight Software, ver. 2.0.0.1 (Med-Imaps, Needham, MA, USA) using spine DXA files from the database to ensure blinding of the investigators to all clinical parameters. The software uses the raw DXA images of the anteroposterior spine for the same region of interest as the lumbar spine BMD measurement.
Statistical analysis
All statistical analyses, except the propensity score analysis, were performed using IBM-SPSS ver. 22.0 software (IBM Corp., Armonk, NY, USA) and a p value <0.05 was considered significant. The propensity score analysis was performed with R statistical software (Foundation for Statistical Computing, Vienna, Austria, ver. 3.1.0; http://cran.r-project.org/). Descriptive statistics, including means and 95 % confidence intervals, were estimated for both groups. The chi-square test was used to compare the proportion of premenopausal women and incidence of osteoporosis between the groups. Differences in subject characteristics, BMDs, and TBS between the groups were assessed with the Student’s t test or the Mann–Whitney U test after assess normality of the distribution for the tested parameter. Correlations between all parameters were evaluated with Pearson correlation test. Linear regression was used to study the relationships between TBS and age, BMI, and BMDs in each group. A multivariate logistic regression analysis was used to analyze the odds ratio (OR) for the occurrence of DRF using age, BMI, lumbar spine BMD, total hip BMD, and TBS (binary logistic regression, forward conditional method). Femoral neck BMD and trochanter BMD were excluded from the multivariate analysis because these variables are correlated with total hip BMD.
Result
Bone mineral density
A total of 128 of the 258 DRF patients (49.6 %) had BMD in the osteoporotic range (lowest T-score among measured sites was ≤−2.5), whereas 39.2 % of controls (100 of 255 subjects) were in the osteoporotic range (p = 0.018). The DRF patient group had significantly lower BMD values at femoral neck, femoral trochanter, and total hip than those of the control group: 0.752 ± 0.097, 0.622 ± 0.089, and 0.801 ± 0.099 in patient group and 0.779 ± 0.092, 0.648 ± 0.089, 0.826 ± 0.101 in control group. However, lumbar spine BMD was not significantly different between the groups (p = 0.400) (Table 1).
Trabecular bone score
TBS was not significantly different between the patient and control groups (p = 0.864). When the subjects were divided into two age categories: 50 to 64 and 65 to 79, TBS was not different between the groups in either category (p = 0.867 and 0.988, respectively). The patient group had 128 subjects (46.1 %) in the osteopenic range and the control group had 145 subjects (54.9 %) in the osteopenic range. No difference in TBS was detected between the osteoporosis and osteopenia groups (p = 0.449 and 0.307, respectively) (Table 2).
Correlation between TBS and BMDs
TBS was positively correlated with all BMD values, but the correlation coefficients were lower than the r-value between femur BMDs and lumbar spine BMD. A moderate correlation was observed between TBS and lumbar spine BMD (r = 0.445, p < 0.001). A negative correlation was observed between age and the BMD values or TBS (Table 3). TBS decreased linearly as age increased and increased linearly as total hip and lumbar BMD increased in each group (all p < 0.001) (Fig. 2).
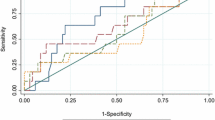
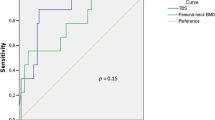
Relationship with the occurrence of DRF
The multivariate analysis indicated that only total hip BMD was significantly associated with the occurrence of DRF (OR, 10.231; 95 % confidence interval, 1.724–60.702; p = 0.010), meaning that a decrease of 1.000 g/cm2 in total hip BMD increases the risk of DRF by 10.231 times.
Discussion
Bone microarchitectural deterioration is a major contributing factor for fracture risk, and TBS has been studied as a convenient tool for evaluating the bone microarchitectural structure [21]. Although previous studies revealed that TBS has additive value for predicting vertebral and non-vertebral fractures in postmenopausal women [12–15, 22], our results demonstrate that TBS is not reliable to evaluate bone frailty in patients with a DRF.
This result contrasts with previous studies that reported microarchitectural deterioration of trabecular bone structure in the distal radius in pre- and postmenopausal women with a DRF, as assessed by high resolution peripheral quantitative computed tomography (HR-pQCT) [23, 24]. In addition, a few studies have shown a significant correlation between TBS and skeletal microarchitecture of the distal radius in human cadavers and postmenopausal women with primary hyperparathyroidism [21, 25]. However, these two studies were not comparative studies between patient and control groups.
The lack of relationship between TBS and the occurrence of a DRF could be explained in a few ways. First, frailty of the distal radius may be mainly associated with the cortical compartment, which cannot be assessed by the lumbar spine TBS. It has been reported that patients with a displaced DRF have lower cortical bone density than that of patients with a non-displaced DRF [26]. In addition, bicortical thickness of the distal radius on a radiograph and cortical thickness of the distal radius on HR-pQCT are studied as indicators of bone quality [24, 27]. Furthermore, metacarpal cortical bone density, which is assessed by digital X-ray radiogrammetry, is significantly associated with a DRF in elderly women [28, 29]. The occurrence of DRF is significantly related to hip BMD but not lumbar spine BMD [5, 27], and severity of a DRF and cortical thickness of the distal radius are not related to BMD of the lumbar spine, which is mainly trabecular [27, 30]. Second, even if trabecular microstructure affects frailty of the distal radius, it may not be accurately evaluated by the lumbar spine TBS due to anatomic site differences. Several studies have pointed out that the trabecular microstructure of the distal radius is weakly or not correlated with that of vertebra and iliac bone [21, 31]. Different loading properties, distribution of weight bearing forces, and aging patterns between the axial and appendicular skeleton could contribute to this discrepancy [27, 32].
Del Rio et al. reported a significant association between TBS and femoral fracture, although they are cortical [22]. However, mean patient age in their study was much higher than that of the current study. The relatively similar pattern of bone loss between the femur and lumbar spine during aging, which may be accelerated after peri-menopause, could contribute to the association between femoral fracture and lumbar TBS [33–35].
In this study, TBS was positively correlated with BMD at all sites and inversely correlated with age. The correlation (r = 0.45) between TBS derived from the GE Lunar device and lumbar BMD was similar with that of a Spanish case-control study (r = 0.52) [22]. Boutroy et al. also found a significant correlation (r = 0.58) between TBS derived from a Hologic scanner and lumbar BMD in the OFELY cohort [12]. However, a weak correlation (r = 0.33) was observed between TBS derived from a GE Lunar device and lumbar BMD in the Manitoba Study [36]. Further studies are needed to determine whether TBS differs according to scanner type.
Several limitations in this study should be mentioned. First, we compared our patients with our own hospital-based controls, who were not part of the community-based general population. Second, our institution is a tertiary referral hospital; thus, the included subjects may have more diseases and injuries, which may have affected both the patient and control groups. Third, the study subjects were ethnic Koreans; therefore, our results may not be representative of all women with a DRF. Fourth, although the patients were all postmenopausal and age-matched, we did not evaluate the years since menopause, which could have an impact on the study results. Finally, although we excluded subjects with diseases or who were taking medications affecting bone quality, we could not evaluate other parameters, such as bone turnover markers, due to incomplete control data.
In conclusion, TBS was not different between women with a DRF and those without a history of osteoporotic fracture, suggesting that TBS measured at the lumbar spine, which is mainly trabecular, may not reflect early microarchitectural changes of the distal radius. We found that only total hip BMD is associated with the risk of DRF in Korean women.
References
Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res 22(3):465–475
Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ 3rd, Khaltaev N (2008) A reference standard for the description of osteoporosis. Bone 42(3):467–475
Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E, Hofman A, Uitterlinden AG, van Leeuwen JP, Pols HA (2004) Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone 34(1):195–202
Itoh S, Ohta T, Samejima H, Shinomiya K (1999) Bone mineral density in the distal radius in a healthy Japanese population and in relation to fractures of the distal radius. J Hand Surg (Br) 24(3):334–337
Lee JO, Chung MS, Baek GH, Oh JH, Lee YH, Gong HS (2010) Age- and site-related bone mineral densities in Korean women with a distal radius fracture compared with the reference Korean female population. J Hand Surg [Am] 35(9):1435–1441
Nordvall H, Glanberg-Persson G, Lysholm J (2007) Are distal radius fractures due to fragility or to falls? A consecutive case-control study of bone mineral density, tendency to fall, risk factors for osteoporosis, and health-related quality of life. Acta Orthop 78(2):271–277
Dalle Carbonare L, Giannini S (2004) Bone microarchitecture as an important determinant of bone strength. J Endocrinol Investig 27(1):99–105
Rubin CD (2005) Emerging concepts in osteoporosis and bone strength. Curr Med Res Opin 21(7):1049–1056
Cho YJ, Gong HS, Song CH, Lee YH, Baek GH (2014) Evaluation of physical performance level as a fall risk factor in women with a distal radial fracture. J Bone Joint Surg Am 96(5):361–365
Seeman E (2007) Is a change in bone mineral density a sensitive and specific surrogate of anti-fracture efficacy? Bone 41(3):308–317
Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N, McCloskey EV, Kanis JA, Bilezikian JP (2014) Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res 29(3):518–530
Boutroy S, Hans D, Sornay-Rendu E, Vilayphiou N, Winzenrieth R, Chapurlat R (2013) Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporos Int 24(1):77–85
Briot K, Paternotte S, Kolta S, Eastell R, Reid DM, Felsenberg D, Gluer CC, Roux C (2013) Added value of trabecular bone score to bone mineral density for prediction of osteoporotic fractures in postmenopausal women: the OPUS study. Bone 57(1):232–236
Krueger D, Fidler E, Libber J, Aubry-Rozier B, Hans D, Binkley N (2014) Spine trabecular bone score subsequent to bone mineral density improves fracture discrimination in women. J Clin Densitom 17(1):60–65
Pothuaud L, Barthe N, Krieg MA, Mehsen N, Carceller P, Hans D (2009) Evaluation of the potential use of trabecular bone score to complement bone mineral density in the diagnosis of osteoporosis: a preliminary spine BMD-matched, case-control study. J Clin Densitom 12(2):170–176
Chung KC, Shauver MJ, Birkmeyer JD (2009) Trends in the United States in the treatment of distal radial fractures in the elderly. J Bone Joint Surg Am 91(8):1868–1873
Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, Eisman J, Fujiwara S, Garnero P, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A (2004) A meta-analysis of previous fracture and subsequent fracture risk. Bone 35(2):375–382
Owen RA, Melton LJ 3rd, Ilstrup DM, Johnson KA, Riggs BL (1982) Colles’ fracture and subsequent hip fracture risk. Clin Orthop Relat Res 171:37–43
Rozental TD, Makhni EC, Day CS, Bouxsein ML (2008) Improving evaluation and treatment for osteoporosis following distal radial fractures. A prospective randomized intervention. J Bone Joint Surg Am 90(5):953–961
D’Agostino RB Jr (1998) Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 17(19):2265–2281
Silva BC, Boutroy S, Zhang C, McMahon DJ, Zhou B, Wang J, Udesky J, Cremers S, Sarquis M, Guo XD, Hans D, Bilezikian JP (2013) Trabecular bone score (TBS)—a novel method to evaluate bone microarchitectural texture in patients with primary hyperparathyroidism. J Clin Endocrinol Metab 98(5):1963–1970
Del Rio LM, Winzenrieth R, Cormier C, Di Gregorio S (2013) Is bone microarchitecture status of the lumbar spine assessed by TBS related to femoral neck fracture? A Spanish case-control study. Osteoporos Int 24(3):991–998
Stein EM, Liu XS, Nickolas TL, Cohen A, Thomas V, McMahon DJ, Zhang C, Yin PT, Cosman F, Nieves J, Guo XE, Shane E (2010) Abnormal microarchitecture and reduced stiffness at the radius and tibia in postmenopausal women with fractures. J Bone Miner Res 25(12):2572–2581
Rozental TD, Deschamps LN, Taylor A, Earp B, Zurakowski D, Day CS, Bouxsein ML (2013) Premenopausal women with a distal radial fracture have deteriorated trabecular bone density and morphology compared with controls without a fracture. J Bone Joint Surg Am 95(7):633–642
Pothuaud L, Carceller P, Hans D (2008) Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone 42(4):775–787
Xie X, Barenholdt O (2001) Bone density and geometric properties of the distal radius in displaced and undisplaced Colles’ fractures: quantitative CT in 70 women. Acta Orthop Scand 72(1):62–66
Webber T, Patel SP, Pensak M, Fajolu O, Rozental TD, Wolf JM (2015) Correlation between distal radial cortical thickness and bone mineral density. J Hand Surg 40(3):493–499
Dhainaut A, Hoff M, Syversen U, Haugeberg G (2013) Cortical hand bone porosity and its association with distal radius fracture in middle aged and elderly women. PLoS One 8(7):e68405
Bouxsein ML, Palermo L, Yeung C, Black DM (2002) Digital X-ray radiogrammetry predicts hip, wrist and vertebral fracture risk in elderly women: a prospective analysis from the study of osteoporotic fractures. Osteoporos Int 13(5):358–365
Dhainaut A, Daibes K, Odinsson A, Hoff M, Syversen U, Haugeberg G (2014) Exploring the relationship between bone density and severity of distal radius fragility fracture in women. J Orthop Surg Res 9:57
Cohen A, Dempster DW, Muller R, Guo XE, Nickolas TL, Liu XS, Zhang XH, Wirth AJ, van Lenthe GH, Kohler T, McMahon DJ, Zhou H, Rubin MR, Bilezikian JP, Lappe JM, Recker RR, Shane E (2010) Assessment of trabecular and cortical architecture and mechanical competence of bone by high-resolution peripheral computed tomography: comparison with transiliac bone biopsy. Osteoporos Int 21(2):263–273
Riggs BL, Wahner HW, Dunn WL, Mazess RB, Offord KP, Melton LJ 3rd (1981) Differential changes in bone mineral density of the appendicular and axial skeleton with aging: relationship to spinal osteoporosis. J Clin Invest 67(2):328–335
Arlot ME, Sornay-Rendu E, Garnero P, Vey-Marty B, Delmas PD (1997) Apparent pre- and postmenopausal bone loss evaluated by DXA at different skeletal sites in women: the OFELY cohort. J Bone Miner Res 12(4):683–690
Ensrud KE, Palermo L, Black DM, Cauley J, Jergas M, Orwoll ES, Nevitt MC, Fox KM, Cummings SR (1995) Hip and calcaneal bone loss increase with advancing age: longitudinal results from the study of osteoporotic fractures. J Bone Miner Res 10(11):1778–1787
Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, Amin S, Rouleau PA, Khosla S (2008) A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res 23(2):205–214
Hans D, Goertzen AL, Krieg MA, Leslie WD (2011) Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res 26(11):2762–2769
Acknowledgments
This study was supported by a research fund (2015R1D1A1A01058562) from National Research Foundation of Korea. We also thank the Korean Society for Bone and Mineral Research for providing the software program of TBS for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Shin, Y.H., Gong, H.S., Gang, D.H. et al. Evaluation of trabecular bone score in patients with a distal radius fracture. Osteoporos Int 27, 3559–3565 (2016). https://doi.org/10.1007/s00198-016-3686-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3686-4