Abstract
Summary
We used the RAND UCLA appropriateness method to decide appropriateness of use of osteoporosis medication after incident fracture and potential for fracture healing and make suggestions for trial design for clinical and preclinical research.
Purpose
To develop appropriateness criteria to assist in the use and study of osteoporosis medications in patients with recent fracture and in the potential use of osteoporosis medications to enhance delayed fracture healing. To promote further research by suggesting preclinical and clinical trial design for studies where fracture healing is the endpoint.
Methods
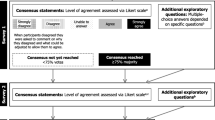
Design: RAND/UCLA appropriateness method (RUAM). Participants: A panel of experts, both members and non-members of the International Osteoporosis Foundation Fracture Working Group, were identified consisting of geriatricians, rheumatologists, orthopedists, endocrinologists, and internists. This resulted in a round 1 panel of 15 panelists, round 2 panel of 15 members, and a round 3 panel of 14 members. Main outcome measure: Agreement on statements and scenarios using RUAM. Three rounds of voting by panelists took place. Agreement in a third round was reached for 111 statements and scenarios, measured by median panel ratings and the amount of dispersion of panel ratings, based on the interpercentile range.
Results
An expert panel validated a set of statements and scenarios about the use of osteoporosis medications after incident fracture and use of these medications to enhance delayed fracture healing and made recommendations for study designs to investigate the effect of osteoporosis medications on fracture healing.
Conclusions
The result of this exercise is intended to assist in improving patient care by identifying the appropriateness of use of osteoporosis medications after fracture and in fracture healing and to make suggestions for further preclinical and clinical research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the number and variety of osteoporosis medications has grown rapidly in the recent past, we now have the option of individualizing our treatment choices to specific patient needs and preferences. Patients with osteoporosis with a recent fracture who are at risk of further fracture should have the opportunity to be on these medications. However, we do not yet know if it is safe to use these medications during fracture healing or if there is a positive effect on fracture healing.
In 2013, the International Osteoporosis Foundation Fracture Working Group (IOF FWG) convened a panel to review existing literature and vote on appropriateness of care for fracture healing using the RAND UCLA appropriateness methodology (RUAM). The working group chose the RUAM since it is one of the most methodologically studied panel processes in health care [1] and as it is almost as reliable and valid as common diagnostic tests [2]. The RUAM allowed the synthesis of the best available evidence with practice-based insights from experts. This unique combination ensured both clinical relevance and evidentiary support when available for the recommendations. Unlike other group-rating methods, the focus of the RUAM approach is not to ensure consensus but minimize artifactual disagreement that may arise from misunderstanding of the statements and scenarios that were being rated. Furthermore, because the RAND/UCLA method pairs clear instructions with a systematic, reliable, and reproducible rating system, the recommendations generated have high internal validity. When the panelists were voting on scenarios and statements, if there was insufficient detail to make an informed judgment regarding appropriateness, then the RUAM encouraged clarification by panelists so as to make them more relevant and precise.
The IOF FWG using the RUAM subsequently voted on statements and scenarios using three rounds of voting ending in 2014. This article is a summary of the panels’ findings.
The objectives of the panel included voting on the effect of osteoporosis medications on fracture healing, risk factors for delayed fracture healing, clinical and research goals, and guidelines for future trial design. The panel also voted on clinical scenarios of fracture healing to represent a spectrum of hypothetical patients requiring a treatment decision.
Methods
Study design
We used the RUAM, a formal group judgment method developed in the 1980s that contains elements of the Delphi technique and nominal group process [1].
Panel composition
Participants in the panels were international clinical members of the Fracture Working Group of the Council of Scientific Advisors (CSA) of the International Osteoporosis Foundation (IOF). Panelists included rheumatologists, endocrinologists, orthopedists, geriatricians, and general internal medicine physicians. There were 15 panelists in the first voting, 15 panelists in the second, and 14 panelists in the third voting. The panelists were supported by a member experienced in the RUAM.
Panel process
The process included three rounds of panel inquiry. The first round took place in 2013 at the IOF meeting in Rome. The panelists further added some statements and scenarios based on their experience and published papers. The second round took place by email prior to the 2013 American Society of Bone and Mineral Research (ASBMR) meeting in Baltimore with scientific discussion of the appropriateness of the statements and scenarios at that meeting. Fifteen panelists participated in this round of 43 statements and four different scenarios. The third round took place by email prior to the 2014 IOF meeting in Seville, Spain with results discussed at that meeting and reaching of a consensus. Fourteen panelists participated in this round of 19 new statements, seven modifications of old statements, and two completely new scenarios. The original statements and scenarios were written by Dr. Stuart Silverman who was helped in the writing of the second and third voting sheets by Drs. Susan Bukata and Eli Kupperman. Results of the second and third voting were analyzed by Dr. Kupperman.
Participants in the group process were asked to vote appropriateness on a 1 to 9 scale where 1 meant that the expected harms greatly outweighed the expected benefits and 9 meant that the expected benefits greatly outweighed the expected harms.
Analysis
Each indication was then considered as follows to determine if the statement could be deemed appropriate or inappropriate: A median score of 7 or greater with the panel members determined to be in agreement was termed “appropriate”; a median score of 3 or less with the panel members determined to be in agreement was termed “inappropriate”, and a median score of 3.5 to 6.5, or if the panel members were determined to be in disagreement, was termed “uncertain” [1]. To determine whether a panel was in disagreement, we used the interpercentile range adjusted for symmetry (IPRAS) as proposed by the RUAM. This method has been tested on more than 16,000 theoretical indications and more than 6000 real ones and has had very few discrepancies with the classic definition of agreement. In these cases, it has been widely determined that the IPRAS actually leads to more logical conclusions than the classic definition. We used this method not only because of its previous success but because our panel size was not constant throughout our three panels and we could not be guaranteed that the voting of the panelists would be symmetrical [1].
Results
Statements
Goals of fracture healing
Our experts agreed that the prevention of delayed fracture healing should be a goal of providers treating patients with fractures. Delayed fracture healing is common. Up to a third of femoral shaft or tibial fractures may develop delayed union or non-union [3]. Delayed fracture healing leads to considerable morbidity in patients, in particular increased pain and loss of function [3]. This, in turn, can lead to a lower quality of life as well as an increased time prior to return to work, resulting in a negative cost to society.
It is valuable to identify those patients at increased risk of delayed fracture healing as early as possible in order to consider intervention. Some treatments, such as BMP, are used at the time of surgery [4], and some medications may be more effective if administered earlier in the healing process. Earlier identification of those at risk may lead to closer follow-up, faster identification of slow healing, quicker intervention, and therefore decreased social health costs [3]. Our experts agreed with the literature that supports smoking [5, 6] and diabetes [7] as risk factors for delayed fracture healing. In addition, degree of soft tissue injury and vascular disease may increase risk of healing complications [8]. NSAID use has also been associated with delayed fracture healing [9].
Use of osteoporosis medications during bone healing
Our panelists reviewed treatment with bisphosphonates both injectable and oral, denosumab, teriparatide, and strontium. Treating patients with bisphosphonates during bone healing has been controversial because osteoclasts are important for remodeling callus into cortical bone [10]. Our panelists were in agreement that antiresorptives such as bisphosphonates may delay fracture healing; however, they found this risk to be low. Multiple preclinical studies demonstrate a modification of callus structure with bisphosphonates during fracture healing but no difference in biomechanical strength of the overall fracture callus due to the increased size of the callus [11]. There are reports of delayed healing of upper limb fractures [12, 13] not confirmed by others [14] and reports of delayed healing of lower limb fractures [15]. A recent meta-analysis of randomized controlled trials performed after our panel voting, however, did not show delay in radiologic or clinical fracture healing on bisphosphonates [16]. There is data from the FREEDOM trial to suggest that denosumab, an antiresorptive which is a RANK ligand inhibitor, does not delay fracture healing [17].
Our panel agreed that there was no evidence for delay in fracture healing when injectable bisphosphonates were given in the first 2 weeks after fracture. One randomized clinical trial of hip fracture showed no clinically evident effect on fracture healing, even when given in the first 2 weeks postoperatively [9]; however, in a closed rat fracture model, delaying the single dose of zoledronic acid 1 or 2 weeks postfracture improved mechanical strength and produced a larger and stronger callus [18]. Our panelists did voice theoretical concerns about whether a single injectable bisphosphonate would be bound avidly to the fracture site and not be taken up at other skeletal sites which would have benefited from the therapy.
Our panel agreed that bisphosphonates and denosumab were safe to use after a vertebral or non-vertebral fracture, but there were some concerns about using a bisphosphonate in a non-healing non-vertebral fracture after 3 months.
Our panel agreed that anabolic agents such as teriparatide which enhance osteoblastic bone formation may have a beneficial effect of fracture healing. In preclinical models. intermittent parathyroid hormone (1–34) treatment increased callus formation, bone mineral content and density, and mechanical strength of healing rat fractures [19–21]. Human parathyroid hormone (1–34) also accelerated natural fracture healing in the femoral osteotomy model of cynomologus monkeys [22]. In patients who are at risk of delayed fracture healing, such as the elderly, osteoporotic, postmenopausal women, and those with malnutrition, the use of parathyroid hormone (PTH) may improve fracture healing [23]. There have been case reports of accelerated healing in delayed unions of type III odontoid fractures [24]. In those with normal fracture healing, anabolic agents have not been shown to accelerate radial fracture healing [25] although in a post hoc analysis of another trial, teriparatide improved early callus formation in distal radial fractures [26]. Parathyroid hormone 1–84 did accelerate fracture healing in elderly osteoporotic women with pelvic fractures [27, 28]. Case reports have demonstrated enhanced healing with teriparatide treatment of lower extremity non-union fracture [29] and unstable peritrochanteric fractures [30].
Teriparatide (recombinant PTH) may enhance healing of vertebral fractures as it has been shown to prevent progression of collapse [31]. Several case reports have demonstrated enhanced healing with teriparatide treatment after the patient experienced delayed fracture healing [30, 31]. Teriparatide may also enhance healing of lumbar fusion [32].
The scenarios included use of strontium ranelate whose use was restricted by the European Medicine Agency in 2013 after the second round of our voting [33]. Strontium is incorporated into the callus and has a positive effect on bone healing in osteoporotic rat models [34–36]. In humans, strontium had a positive effect on 3D microarchitecture in bone biopsies [37]) and had positive effects on fracture healing in one model of surgically fixed tibial fractures [38] but had no effect on healing of wrist fractures [39]. In summary, although our panelists noted no solid scientific evidence to support acceleration of normal fracture healing with anabolic therapy, there were case reports to suggest improvement in fracture healing with anabolic therapy in individuals at high risk of delayed fracture healing which needs to be confirmed in randomized clinical trials.
In the scenario of a non-healing non-displaced diaphyseal non-vertebral fracture after 3 months, the panel agreed that treatment with anabolic therapy or strontium was safe to use, but not bisphosphonates or RANKL inhibitors. In the scenario of a non-healing instrumented spinal fusion after 3 months, our panel agreed that treatment with anabolic therapy was safe to use but was unable to come to a consensus on the safety of treatment with other osteoporosis medication. Our panel was in agreement that treatment with an anabolic therapy such as teriparatide could return a patient with delayed healing of a non-displaced non-vertebral fracture or of an instrumented spinal fusion to a more normal rate of fracture healing.
Although there is no evidence available, our panel agreed that treatment with an anabolic therapy such as teriparatide may enhance fracture healing in a patient with normal rate of fracture healing who is seen in the first 2–4 weeks after a new non-vertebral fracture or in the first 2 weeks after a compression fracture. The panel agreed that teriparatide and strontium will not delay healing of an uninstrumented spinal fusion after 3 months but was unable to agree that the other medications would not cause a healing delay.
In conclusion, our experts agreed that there was no negative effect of osteoporosis medications on fracture healing and that it is safe to start osteoporosis medications as soon as possible after both vertebral and non-vertebral fracture [11, 16]. The panel agreed in scenarios that treatment with any of the listed osteoporosis medications would not delay healing further in patients with non-healing non-vertebral fractures. However, the panelists felt that only anabolic therapy could return the patient to a more normal rate of healing.
We look forward to some of the newer medications that may safely improve the rate of fracture healing. Recombinant PTH or teriparatide has shown great success in building bone mineral and preventing osteoporotic fractures, and in both animal models and human clinical studies, it has had success in accelerating fracture healing. Newer medications such as anti-sclerostin and anti-DKK antibody are still in early stages of research. Although preclinical studies have shown positive effects of sclerostin on fracture healing [40, 41], the potential effects of sclerostin on fracture healing in humans are unclear and further preclinical and clinical studies of their role in fracture healing are needed. There is no data on the effects of abaloparatide on fracture healing. There is little data on newer antiresorptive agents such as cathepsin K which in one mouse model delayed callus remodeling [42].
Trial design
To facilitate further research in fracture healing, our experts agreed on some aspects of trial design. Identification of clinical markers of delayed fracture healing ought to be sought from history and clinical exam, imaging studies, as well as blood tests [43]. Serum TGFbeta1 levels appear to be an indicator of fracture healing [43]. New functional imaging techniques may also be helpful. While our panel agreed on the statement that the duration of a fracture healing study should be 12 months, there was disagreement on the statement that the duration of a fracture healing study should be 24 months.
Definition of delayed healing depends on the location of the fracture and varies among surgeons. Surveys of surgeons have shown considerable disagreement on criteria of delayed union (1–8 months) and non-union (2–12 months) [44]. Surveys of orthopedic surgeons have determined that a common definition of delayed healing would be beneficial to patient care [45]. Fragility fractures heal despite the remodeling anomalies seen with osteoporosis. Multiple clinical trials have not demonstrated a problem with the healing of fragility fractures in either the treatment or placebo groups [11].
Our panel members were in agreement that fracture healing trials should have co-primary endpoints of imaging, pain, and function, as well as clinical endpoints such as need for revision [46–49].
Sequential X-rays are currently used in clinical practice to follow fracture healing, and the consensus of our panel was that healing of three of four cortices was considered demonstrative of fracture healing. The most common clinical criteria use this radiographic measurement in the context of other clinical features including tenderness at the fracture site and pain with weight bearing to see if the fracture is healed [48]. In the evaluation of the X-rays, qualifications such as whether callus formation is visible and whether the fracture line is visible help to quantify the degree of fracture healing that has occurred at the fracture site. Scoring systems such as the radiographic union score for hip (RUSH) [50, 51] and radiographic union score for tibia (RUST) [52] are based on cortical scores. It is from these scoring systems that the concept that only two cortices could be completely radiographically healed (and get a healed level score) and yet the other two cortices could still show fracture lines and some or no callus. It is in this context that our panel also recognized that bridging and consolidation of two of four oppositional cortices may represent healing of a fracture site, and our panel felt that this pattern should be considered as a secondary endpoint in fracture healing studies.
Our panel felt that the primary endpoint of a fracture healing study should be reached within the first year. Delays in fracture healing, regardless of anatomic site, beyond 1 year are all considered to be delayed unions or non-unions and warrant secondary surgical intervention in many instances. Our panel felt that a single X-ray at the end of the second year is warranted to provide additional safety information and confirm persistence of the healed fracture site. In standard clinical practice, additional X-rays are rarely obtained after a fracture is considered healed and patients are generally discharged from care.
Panelists agreed that the optimal imaging modality for evaluation of fracture healing varied by fracture site. While CT scanning can provide detailed information regarding the progression of healing at a fracture site [53], the significant increase in radiation exposure to the patient, the increased cost of the modality, and the lack of access to the modality in some areas of the world [54] make CT scan less desirable as the primary imaging modality for a fracture healing study. The panel agreed that for fractures with a known site of infection associated with the fracture, intermediate endpoints including exam and radiographic studies are warranted to confirm no recurrence of the infection and no compromise of the healed fracture site.
Preclinical models
When considering preclinical models, our experts agreed that animal models may be appropriate for exploring mechanisms of fracture healing, most commonly in rodent models [55]. This is often easier and safer to do than in human models.
While the panel agreed that animal models may be appropriate, they also agreed that they may not predict efficacy of fracture healing in humans [55, 56]. Animal models are good surrogates but are not perfect and therefore may not prove highly predictive. Most animal studies use the ovariectomized rat model and a surgically induced fracture. However, postmenopausal OP is a complex, multifactorial disorder, and a surgically induced fracture is not the same as a fracture which results from bone fragility [57]. Some clinical findings are in contrast with preclinical studies, highlighting the need to develop better animal models, e.g., lack of efficacy for strontium [39] and teriparatide [25] for radial fractures despite efficacy in preclinical models [19–21, 34–36].
Results with different animal models of fracture healing may be conflicting as just as they are different than human models, different animal models can also differ in their results [55, 56].
Animal models have demonstrated a delay in fracture healing in older animals [55, 56]. A similar delay in fracture healing is suspected in older patients and is often discussed, but no evidence has clearly demonstrated this phenomenon in patients [57]. With clear definition of a healed fracture, further analysis of patient cohorts may be able to demonstrate a difference when stratified for age.
Atypical femoral fracture
Atypical femoral fractures have been associated with greater duration of bisphosphonate therapy [58] and denosumab therapy [59]. Causality is undetermined, and up to 15 % of fractures occur without exposure to osteoporosis medications [58, 60]. The panel agreed that after the occurrence of an atypical femur fracture, bisphosphonate therapy should be stopped. Atypical femoral fractures may be slow to heal. The panel agreed that treatment with an anabolic agent such as teriparatide should be considered to improve healing after an atypical femur fracture [60, 61]. Strontium has also been reported to be effective in delayed healing of atypical femur fracture [62].
Osteogenesis imperfecta
The panel agreed that bisphosphonates may delay healing of an osteotomy but do not delay healing of fractures in patients with osteogenesis imperfecta [63–65].
Strengths and limitations of this study
Strengths
We used a validated consensus method involving an expert panel of varied specialties interested in bone disease. We used published evidence where available but also relied on expert opinion where no evidence was available.
Limitations
Our conclusions may not be generalizable. Many of the conclusions are based upon expert opinion rather than based on clinical evidence because few randomized clinical trials for fracture healing safety or enhancement on osteoporosis medications have been done. This analysis does not consider cost-effectiveness or safety concerns of each osteoporosis medication discussed.
Conclusions
This study supported the utility of a structured expert opinion process as an effective strategy to evaluate the appropriateness of using osteoporosis medications in patients after fracture and their potential use in fracture healing.
References
The Rand UCLA Appropriateness method user’s manual (2001) Rand Corporation, Los Angeles
Hemingway H, Crook AM, Banerjee S, Dawson JR, Feder G, Magee PG, Wood A, Philpott S, Timmis A (2001) Hypothetical ratings of coronary angiography appropriateness: are they associated with actual angiographic findings, mortality, and revascularisation rate? The ACRE study. Heart 85:672–679
Tay WH, de Steiger R, Richardson M, Gruen R, Balogh ZJ (2014) Health outcomes of delayed union and nonunion of femoral and tibial shaft fractures. Injury 45(10):1653–1658
Schmidmaier G, Schwabe P, Wildemann B, Haas NP (2007) Use of bone morphogenetic proteins for treatment of non-unions and future perspectives. Injury 38(Suppl 4):S35–S41
Argintar E, Triantafillou K, Delahay J, Wiesel B (2012) The musculoskeletal effects of perioperative smoking. J Am Acad Orthop Surg 20:359–363
Sloan A, Hussain I, Maqsood M, Eremin O, El-Sheemy M (2010) The effects of smoking on fracture healing. Surgeon 8:111–116
Hernandez RK, Do TP, Critchlow CW, Dent RE, Jick SS (2012) Patient-related risk factors for fracture-healing complications in the United Kingdom General Practice Research Database. Acta Orthop 83:653–660
Bhandari M, Fong K, Sprague S, Williams D, Petrisor B (2012) Variability in the definition and perceived causes of delayed unions and nonunions: a cross-sectional, multinational survey of orthopedic surgeons. J Bone Joint Surg Am 94:e1091–e1096
Colon-Emeric CX, Nordslettern L, Olson S, Major N, Boonen S, Haentjens P, Mesenbrink P, Magaziner J, Adachi J, Lyles KW, et al. (2011) Association between timing of zoledronic acid infusion and hip fracture healing. Osteoporos Int 22(8):2329–36
Schindeler A, McDonald MM, Bokko P, Little DG (2008) Bone remodeling during fracture repair: the cellular picture. Semin Cell Dev Biol 19:459–466
Larsson S, Fazzalari NL (2014) Anti-osteoporosis therapy and fracture healing. Arch Orthop Trauma Surg 134(2):291–297
Molvik H, Khan W (2015) Bisphosphonates and their influence on fracture healing: a systematic review. Osteoporos Int 26:1251–1260
Ng AJ, Yue B, Joseph S, Richardson M (2014) Delayed/non-union of upper limb fractures with bisphosphonates: systematic review and recommendations. ANZ J Surg 84:218–224
Uchiyama S, Itsubo T, Nakamura K, Fujinaga Y, Sato N, Imaeda T, Kadoya M, Kato H (2013) Effect of early administration of alendronate after surgery for distal radial fragility fracture on radiological fracture healing time. Bone Joint J 95:1544–1550
Yue B, Ng A, Tang H, Joseph S, Richardson M (2015) Delayed healing of lower limb fractures with bisphosphonate therapy. Ann R Coll Surg Engl 97:333–338
Li YT, Cai HF, Zhang ZL (2015) Timing of the initiation of bisphosphonates after surgery for fracture healing: a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int 26:431–434
Adami S, Libanati C, Boonen S, Cummings SR, Ho PR, Wang A, Siris E, Lane J, FREEDOM Fracture-Healing Writing Group (2012) Denosumab treatment in postmenopausal women with osteoporosis does not interfere with fracture-healing: results from the FREEDOM trial. J Bone Joint Surg 94:2113–2119
Amanat N, McDonald M, Godfrey C, Bilston L, Little D (2007) Optimal timing of a single dose of zoledronic acid to increase strength in rat fracture repair. J Bone Miner Res 22:867–876
Andreassen TT,Ejersted C, Oxlund H (1999) Intermittent parathyroid (1–34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res 14:960–8
Alkhiary YM, Gerstenfeld LC, Krall E, Westmore M, Sato M, Mitlak B, Einhorn TA (2005) Enhancement of experimental fracture healing by systemic administration of human parathyroid hormnone (PTH 1–34). 87: 731–41
Nakajima A, SHimoji N, Shiomi K, Shimizu S, Moriya H, Einhorn TA, Yamazaki M (2002) Mechanisms for the enhancement of fracture healing in rats treated with intermittent low dose human parathyroid hormone (1–34). J Bone Miner Res 17:2038–2047
Manabe T, Mori S, Mashiba T, Kaji Y, Iwata K, Komatsubara S, Seki A, Sun YX, Yamamoto T (2007) Human parathyroid hormone (1–34) accelerates natural fracture healing process in the femoral osteotomy model of cynomolgus monkeys. Bone 40:1475–1482
Zhang D, Potty A, Vyas P, Lane J (2014) The role of recombinant PTH in human fracture healing: a systematic review. J Orthop Trauma 28:57–62
Rubery PT, Bukata SV (2010) Teriparatide may accelerate healing in delayed unions of type III odontoid fractures: a report of 3 cases. J Spinal Disord Tech 23:151–155
Aspenberg P, Genant HK, Johansson T, Nino AJ, See K, Krohn K, García-Hernández PA, Recknor CP, Einhorn TA, Dalsky GP, Mitlak BH, Fierlinger A, Lakshmanan MC (2010) Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res 25:404–414
Aspenberg P, Johansson T (2010) Teriparatide improves early callus formation in distal radial fractures. Acta Orthop 81:234–236
Peichl P, Holzer LA, Maier R, Holzer G (2011) Parathyroid hormone 1–84 accelerates fracture healing in pubic bones of elderly osteoporotic women. J Bone Joint Surg Am 93:1583–1587
Moon SW, Lee DH, Kim YC, Kim YB, Lee SJ, Kim JW (2012) Parathyroid hormone 1–34 (teriparatide) treatment in pelvic insufficiency fractures - a report of two cases. J Bone Metab 19:147–151
Mancilla EE, Brodsky JL, Mehta S, Pignolo RJ, Levine MA (2015) Teriparatide as a systemic treatment for lower extremity nonunion fractures: a case series. Endocr Pract 21:136–142
Huang TW, Yang TY, Huang KC, Peng KT, Lee MS, Hsu RW (2015) Effect of teriparatide on unstable pertrochanteric fractures. Biomed Res Int 2015:568390. doi:10.1155/2015/568390, Epub 2015 Feb 10
Park JH, Kang KC, Shin DE, Koh YG, Son JS, Kim BH (2014) Preventative effects of conservative treatment with short-term teriparatide on the progression of vertebral body collapse after osteoporotic vertebral compression fracture. Osteoporos Int 25:613–618
Ohtori S, Inoue G, Orita S, Yamauchi K, Eguchi Y, Ochiai N, Kishida S, Kuniyoshi K, Aoki Y, Nakamura J, Ishikawa T, Miyagi M, Kamoda H, Suzuki M, Kubota G, Sakuma Y, Oikawa Y, Inage K, Sainoh T, Takaso M, Ozawa T, Takahashi K, Toyone T (2012) Teriparatide accelerates lumbar posterolateral fusion in women with postmenopausal osteoporosis: prospective study. Spine 37:E1464–E1468
European Medicine Agency (2013) Recommendations to restrict the use of Protelos/Osseor (strontium ranelate). www.ema.europa.edu/docs/en_GB/document_library/Press_release/2013/04/wc500142507.pdfAccessed May 25,2015
Habermann B, Kafchitsas K, Olender G, Augat P, Kurth A (2010) Strontium ranelate enhances callus strength more than PTH 1–34 in an osteoporotic rat model of fracture healing. Calcif Tissue Int 86:82–89
Li YF, Luo G, Zhu S, Li JH, Hu J (2010) Systemic treatment with strontium ranelate promotes tibial fracture healing in ovariectomized rats. Osteoporos Int 21:1889–1897
Oztkuran KE, Demir B, Yucel I, Cakici H, Yilmaz F, Haberal A (2011) Effect of strontium ranelate on fracture healing in the osteoporotic rat. J Orthop Res 29:138–142
Arlot ME, Jiang Y, Genant HK, Zhao J, Burt-Pichat B, Roux JP et al (2008) Histomorphometric and microCT analysis of bone biopsies from postmenopausal woment treated with strontium ranelate. J Bone Miner Res 23:215–222
Aslam MZ, Khan MA, Chinoy MA, Jillani SA, Sultan SA, Ahmed SK (2014)Significance of strontium ranelate in healing of surgically fixed tibial diaphyseal fractures treated with strontium ranelate vs placebo; a randomised double blind controlled trial. 64(12 Suppl 2):S123-6
Scaglione M, Fabbri L, Casella F, Guido G (2015) Strontium ranelate as an adjuvant for fracture healing: clinical, radiological, and ultrasound findings in a randomized controlled study on wrist fractures. Osteoporos Int
Feng G, Chang-Qing Z, Yi-Min C, Xiao-Lin L (2015) Systemic administration of sclerostin monoclonal antibody accelerates fracture healing in the femoral osteotomy model of young rats. Int Immunopharmacol 24:7–13
Virk MS, Alaee F, Tang H et al (2013) Systemica dministration of sclerostin antibody enhances bone repair in a critical-sized femoral defect in a rat model. J Bone Joint Surg Am 95:694–701
Soung DY, Gentile MA, Duong LT, Drissi H (2013) Effects of pharmacologic inhibition of cathepsin K on fracture repair in mice. Bone 5:248–255
Hankenson KD, Zimmerman G, Marcucio R (2014) Biological perspectives of delayed fracture healing. Injury 45(Suppl 2):S8–S15
Bhandari M, Guyatt GH, Swiontkowski MF, Tornetta P, Sprague S, Schemitsch EH (2002) A lack of consensus in the assessment of fracture healing among orthopedic surgeons. J Orthop Trauma 16:562–566
Bhandari M, Fong K, Sprague S, Williams D, Petrisor B (2012) Variability in the definition and perceived causes of delayed unions and nonunions: a cross-sectional, multinational survey of orthopaedic surgeons. J Bone Joint Surg Am 94(15):e 1091–e 1096
Morshed S, Corrales L, Genant H, MIclau T (2008) Outcomes assessment in clinical trials of fracture-healing. J Bone Joint Surg 90(Suppl 1):62–67
Axelrad TW, Einhorn TA (2011) Use of clinical assessment tools in the evaluation of fracture healing. Injury 42(3):301–305
Corrales L, Morshed S, Bhandari M, Miclau T (2008) Variability in the assessment of fracture healing in orthopaedic trauma studies. J Bone Joint Surg Am 90:1862–1868
Kooistra BW, Sprague S, Bhandarim, Schemitsch EH (2010) Outcomes in fracture healing trials: a primer. J Orthop Trauma 24:S71–S75
Bhandari M, Chiavaras MM, Parasu N, et al. (2013) Radiographic union score for hip substantially improves agreements between surgeons and radiologists. BMC Musculoskelet Disord 14: article 70
Chiavaras MM, Bain S, Choudur H et al (2013) The radiographic union score for hip (RUSH); the use of a checklist to evaluate hip fracture healing improves agreement between radiologists and orthopedic surgeons. Skelet Radiol 42:1079–1088
Whelan DB, Bhandari M, Stephen D et al (2010) Development of the radiographic union score for tibial fractures for the assessment of tibial fracture healing after intramedullary fixation. J Trauma 68:629–632
Nakashima H, Yukawa Y, Ito K, Horie Y, Machino M, Kanbara S, Morita D, Imagama S, Ishiguro N, Kato F (2011) Extension CT scan: its suitability for assessing fusion after posterior lumbar interbody fusion. Eur Spine J 20(9):1496–1502
McLane HC, Berkowitz AL, Patenaude BN, McKenzie ED, Wolper E, Washlster S, Fink G, Mateen FJ (2015) Availability, accessibility and affordability of neurodiagnostic tests in 37 countries. Neurology 85:1614–1622
Mills LA, Simpson AH (2012) In vivo models of bone repair. J Bone Joint Surg 94:865–874
Peric M, Dumic-Cule I, Grcevic D, Matijasic M, Verbanac D, Paul R, Grgurevic L, Trkulia V, Bagi CM, Vukicevic S (2015) The rational use of animal models in the evaluation of novel bone regenerative therapies. Bone 70:73–86
Cortet B (2011) Bone repair in osteoporotic bone: postmenopausal and cortisone-induced osteoporosis. Osteoporos Int 22:2007–2010
Schilcher J, Koeppen V, Aspenberg P (2014) Risk of atypical femoral fracture during and after bisphosphonate use. N Engl J Med 371:974–976
Khow KS, Yong TY (2015) Atypical femoral fracture in a patient treated with denosumab. J Bone Miner Metab 33(3):355–358
Adler RA, Fuleihan GE, Bauer DC, Camacho PM, Clarke BL, Clines GA, Compston JE, Drake MT, Edwards BJ, Favus MJ, Greenspan SL, McKinney R, Pignolo RJ, Sellmeyer DE (2016) Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society of Bone and Mineral Research. J Bone Miner Res 31(1):16–35
Chiang CY, Zebaze RM, Ghasem-Zadeh A, Iuliano-Burns S, Hardidge A, Seeman E (2013) Teriparatide improves bone quality and healing of atypical femoral fracture associated with bisphosphonate therapy. Bone 52(1):360–365
Miyakoshi N, Aizawa T, Sasaki S, Ando S, Maekawa S, Aonuma H, Tsuchie H, Sasaki H, Kasukawa Y, Shimada Y (2015) Healing of bisphosphonate-associated atypical femoral fractures in patients with osteoporosis: a comparison between treatment with and without teriparatide. J Bone Miner Metab 33(5):553–559
Anam EA, Rach F, Gloriex FH, Fassier F, Hamdy R (2015) Osteotomy healing in children with osteogenesis imperfecta receiving bisphosphonate treatment. J Bone Miner Res 30(8):1362–1368
Munns CF, Rauch F, Zeitlin L, Fassier F, Glorieux FH (2004) Delayed osteotomy but not fracture healing in pediatric osteogenesis imperfecta patients receiving pamidronate. J Bone Miner Res 19(11):1779–1786
Pizones J, Plotkin H, Parra-Garcia JI, Alvarez P, Gutierrez P, Bueno A, Fernandez-Arroyo A (2005) Bone healing in children with osteogenesis imperfecta treated with bisphosphonates. J Pediatr Orthop 25(3):332–335
Conflicts of interest
Stuart Silverman is a consultant for Amgen, Alexion, Eli Lilly, and Pfizer. He is a member of the Speakers Bureau for Lilly and Pfizer. He has received research grants from Pfizer, Lilly, and Amgen. Susan V. Bukata is a consultant for Amgen and Eli Lilly and a member of the Speakers Bureau for Eli Lilly, Amgen, and Novartis. Eli Kupperman has no disclosures.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
IOF Fracture Working Group Members include K Akesson, S Boonen, ML Brandi, M Chandran, T Chevalley, C Cooper, S Goemaere, J Goldhahn, N Harvey, S Hough, MK Javaid, EM Lewiecki, GP Lyritis, N Napoli, DD Pierroz, S Silverman, and M Sosa.
Appendices
Appendix 1
Statements voted as appropriate
In the Results section, we only show statements and scenarios that were voted by our panels as “appropriate” (median categories 7–9).
Introduction
-
1.
An important goal in fracture healing is to reduce the number of individuals with delayed fracture healing (median rating of 9).
-
2.
It is valuable to identify patients at high risk of delayed fracture healing after a fracture so that intervention can be considered (median rating of 7).
Risk factors
-
1.
Smoking is a marker for increased risk of delayed fracture healing (median rating of 7).
-
2.
Diabetes is a marker for increased risk of delayed fracture healing (median rating of 8).
Clinical judgment
-
1.
The occurrence of fragility fracture while on osteoporosis treatment does not mean that the treatment was ineffective. Our current medications for osteoporosis reduce but do not eliminate fracture (median rating of 9).
-
2.
In a patient on an osteoporosis medication who fractures, one should not stop therapy but rather reassess and look for causes of secondary osteoporosis (median rating of 9).
-
3.
Patients who fracture on therapy may have had more fractures if they had not taken the medication (median rating of 9).
-
4.
Patients who fracture on therapy may be poorly adherent, and poor adherence should be considered (median rating of 9).
Impact
-
1.
In the absence of a systematic approach to delivery of secondary fracture prevention, the majority of patients will fail to receive treatment designed to reduce future fracture risk (median rating of 9).
-
2.
Pharmacologic intervention following fragility fracture has the potential to halve fracture incidence within 3 years, assuming good persistence and compliance (median rating of 9).
Medications
Bisphosphonates
-
1.
There is no evidence in preclinical studies that antiresorptive drugs impair the restoration of mechanical integrity following fracture, despite the fact that they may delay remodeling of the callus (median rating of 8).
-
2.
Antiresorptive therapy may delay fracture healing in claims data and marketing surveillance data (although the risk is low) (median rating of 8).
-
3.
There is no evidence for harm (median rating of 7) or delay in fracture healing (median rating of 8) when injectable bisphosphonates are given in the first 2 weeks after fracture4. Randomized clinical trials have shown no effect of antiresorptive therapy (both bisphosphonates and denosumab) on fracture healing (median rating of 8).
-
5.
During the time that a fracture callus is forming, there is increased sequestration of bisphosphonates at the fracture site (median rating of 7).
-
6.
When using an IV bisphosphonate after an acute fracture, one should wait 2 weeks before starting medication (median rating of 7).
-
7.
Bisphosphonate therapy is sequestered to callus when bone is forming (median rating of 7).
Denosumab
-
1.
There is some data to suggest that denosumab does not delay (median rating of 7) or interfere with fracture healing (median rating of 8).
Teriparatide
-
1.
Teriparatide may enhance healing of vertebral fractures (median rating of 8).
-
2.
Teriparatide may help the fracture healing in a non-vertebral fracture (median rating of 7).
-
3.
It is expected that anabolic agents used to treat osteoporosis would have a beneficial effect on fracture healing (median rating of 8).
-
4.
Currently known anabolic agents have not been shown to accelerate fracture healing in patients with normal fracture healing but may improve fracture healing in individuals at high risk of delayed fracture healing (median rating of 7).
Bone physiology
-
1.
The timing to assess delayed healing after fracture varies across skeletal sites (median rating of 8).
-
2.
Fracture repair involves different stages of tissue differentiation that resemble embryological skeletal development (median rating of 9).
-
3.
Clinical observations suggest that fragility fractures heal despite the abnormality of bone remodeling in osteoporosis (median rating of 8).
-
4.
Implant anchorage is impaired in bone based on biomechanical testing and clinical experience (median rating of 7).
Trial design
Research goals
-
1.
An important research goal is the identification of clinical markers of delayed fracture healing. These markers should be simple and reliable (median rating of 9).
-
2.
An important research goal is to develop simple, reliable clinical measures related to fracture healing (median rating 9).
Study design recommendations
-
1.
The duration of a fracture healing study should be 12 months (median rating of 7).
-
2.
Fracture healing trials should have co-primary endpoints of imaging, pain, function, and clinical endpoints such as need for revision (median rating of 8).
-
3.
It is recommended that adjudicated fracture healing should be considered as a secondary endpoint in all future clinical trials (median rating of 8).
Imaging endpoints
-
1.
The primary imaging endpoint for fracture healing should be bridging and consolidation of three out of four cortices, and the preferred imaging to technique is sequential X-rays (median ratings of 8 and 7).
-
2.
The primary endpoint for fracture healing should be reached within the first year after fracture. No additional endpoints are needed except a single follow-up X-ray at the end of the second year (median rating of 7.5).
-
3.
The preferred imaging endpoint for fracture healing depends on the fracture site (e.g., CT for sacrum and pelvis and plain X-rays for tibia) (median rating of 8).
-
4.
The preferred imaging endpoint for spinal fusions is CT scanning (median rating of 8).
-
5.
CT scanning may be used as an endpoint for studies of fracture healing but is expensive, has high radiation burden, and is not accessible everywhere in the world (median rating of 8).
-
6.
Intermediate endpoints (e.g., month 18) should be obtained in the second year after a fracture is considered healing in the setting of a known fracture site infection (median rating of 7).
-
7.
Secondary imaging endpoints should be bridging and consolidation of two out of four oppositional cortices (median rating of 7).
Preclinical models
-
1.
Animal models may be appropriate for exploring mechanisms of fracture healing pathophysiology (median rating of 7).
-
2.
Whole animal models may not predict efficacy of fracture healing in humans (median rating of 8).
-
3.
Results with different animal models of fracture healing may be conflicting (median rating of 8).
-
4.
It is recommended for a new agent which may influence fracture healing that both a rodent and a non-rodent large animal model be done (median rating of 8).
-
5.
In animal models, there is an effect of age on fracture healing. Fracture healing takes longer in older animals (median rating of 8).
Specialty statements
Atypical femur fractures
-
1.
When a patient has an atypical femoral fracture, one should stop the bisphosphonate (median rating of 8).
-
2.
When a patient has an atypical femoral fracture, after stopping the bisphosphonate, one should consider teriparatide (median rating of 8).
Osteogenesis imperfecta
-
1.
In osteogenesis imperfecta, bisphosphonates may delay healing of osteotomies (median rating of 8).
-
2.
In osteogenesis imperfecta, bisphosphonates do not delay healing of fractures (median rating of 8).
Scenarios
-
1.
In scenarios of
-
(a)
Non-healing non-displaced metaphyseal non-vertebral fractures after 3 months.
-
(b)
First 2 weeks after a new non-vertebral fracture without significant displacement.
-
(c)
First 2 weeks after a new vertebral compression fracture.
Our panel agreed that treatment with any of the antiosteoporosis medications (bisphosphonates, RANKL inhibitors, anabolic therapy or strontium) was safe to use (median ratings 7 to 8).
-
(a)
-
2.
In the scenario of a non-healing non-displaced diaphyseal non-vertebral fracture after 3 months, the panel agreed that treatment with anabolic therapy or strontium were safe to use, but not bisphosphonates or RANKL inhibitors (median rating of 7).
-
3.
In the scenario of a non-healing instrumented spinal fusion after 3 months, our panel agreed that treatment with anabolic therapy was safe to use but was unable to come to a consensus on the safety of treatment with other osteoporosis medications (median rating of 7).
-
4.
Our panel was in agreement that treatment with an anabolic therapy such as teriparatide could return a patient with delayed healing of a non-displaced non-vertebral fracture or of an instrumented spinal fusion to a more normal rate of fracture healing (median rating of 7).
-
5.
Our panel agreed that only treatment with an anabolic therapy such as teriparatide may enhance fracture healing in a patient with normal rate of fracture healing who is seen in the first 2–4 weeks after a new non-vertebral fracture or in the first 2 weeks after a compression fracture (median rating of 7).
-
6.
Treatment with any of the listed osteoporosis medications (bisphosphonates, RANKL inhibitors, anabolics, or strontium) would not delay fracture healing in patients with non-healing non-vertebral fractures without significant displacement after 3 months (median ratings 7 to 8). An example would be a femur fracture that is healing but is not completely healed but is showing progression to healing radiographically at 3 months.
-
7.
The panel agreed that teriparatide and strontium will not delay healing of an uninstrumented spinal fusion after 3 months (median rating of 8) but was unable to agree that the other medications would not cause a healing delay.
Comment: These statements were approved by our panel prior to the recent actions of the European Medical Evaluation Agency (EMEA) with regards to strontium (3).
-
8.
For patients with delayed healing of a non-displaced non-vertebral fracture after 3 months, only anabolic therapy will return them to a normal rate of healing (median rating of 8), a claim deemed untrue for bisphosphonate therapy and RANKL inhibitors (median rating of 3).
Rights and permissions
About this article
Cite this article
Silverman, S.L., Kupperman, E.S., Bukata, S.V. et al. Fracture healing: a consensus report from the International Osteoporosis Foundation Fracture Working Group. Osteoporos Int 27, 2197–2206 (2016). https://doi.org/10.1007/s00198-016-3513-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3513-y