Abstract
Summary
This study examines the impact of smoking and smoking cessation on fracture risk in 75-year-old women followed for 10 years. Smoking increased fracture risk, especially for vertebral fractures. Smoking cessation decreased the risk for vertebral fractures but not for other fracture types.
Introduction
The purpose of this study was to examine effects of smoking and smoking cessation on fracture risk.
Methods
This prospective observational population-based study followed 1033 women during 10 years from age 75. Data regarding smoking were collected at age 75. Hazard ratios (HRs) and 95 % confidence intervals for fracture were calculated using competing risks proportional hazards regression.
Results
Both former smokers and current smokers had an increased risk for any fracture (HR 1.30; 1.03–1.66, and HR 1.32; 1.01–1.73, respectively) and any osteoporotic fracture (hip, proximal humerus, distal radius, vertebra) (HR 1.31; 1.01–1.70 and HR 1.49; 1.11–1.98, respectively) compared to non-smokers. Former smokers had an increased risk for proximal humerus fractures (HR 2.23; 1.35–3.70), and current smokers had an increased risk for vertebral fractures (HR 2.30; 1.57–3.38) compared to non-smokers. After adjustment for weight, previous fractures, alcohol habits, bone mineral density (BMD), use of corticoids, vitamin D, bisphosphonates, and previous falls, former smokers had an increased risk for proximal humerus fracture (HR 2.07; 1.19–3.57) and current smokers had an increased risk for osteoporotic (HR 1.47; 1.05–2.05) and vertebral fractures (HR 2.50; 1.58–3.95) compared to non-smokers. Former smokers had a decreased risk for vertebral fractures, but not for other types of fractures, compared to current smokers.
Conclusions
Smoking increased the risk for fracture among elderly women, especially vertebral fractures. Smoking cessation decreased the risk for vertebral fractures but not for other types of fractures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporotic fractures compose a major public health issue, associated with great morbidity and increased mortality [1] as well as high costs for society [2]. The incidence of osteoporosis, and consequently the fracture risk, varies greatly in different parts of the world [3]. Sweden holds one of the highest incidences of osteoporotic fractures, with an overall lifetime risk in a 50-year-old woman at about 50 % [4]. There are several identified risk factors associated with osteoporotic fractures including natural ageing, diseases, and lifestyle factors. Postmenopausal women are at increased risk because of the accelerated bone loss due to the decrease of estrogen production at menopause [5]. Smoking is one of several lifestyle factors associated with increased fracture risk. An association with lower bone mineral density (BMD) and increased risk of osteoporotic fractures in a dose- and duration-related manner has been implicated [6–8]. In fact, recent studies have suggested that smoking is an independent risk factor for osteoporotic fracture [7].
The global prevalence of smoking has decreased since 1980. However, due to population growth, the number of smokers has increased. Sweden distinguishes itself by being the only country in the world where smoking is more common among women than men [9]. This, along with the extensive tobacco marketing for women [10], will bring increased tobacco-related health problems among women in the future.
Studies have observed the relationship between smoking cessation and bone health, with various results. Positive effects on BMD among elderly women have been seen as early as <10 years after smoking cessation [11], and in a study of postmenopausal women (mean age 58 years), smoking cessation was shown to improve markers of bone turnover [12]. In previously smoking men (mean age 50 years at baseline), fracture risk has been seen to slowly decrease after cessation, but fracture risk was still elevated after 30 years compared to non-smokers [13]. A meta-analysis from 2003 concluded that smoking cessation was associated with a decrease in fracture risk regarding all fractures and hip fractures [8]. However, the relationship between smoking cessation and fracture risk at different fracture sites in elderly women, the group that is the most affected by osteoporosis and osteoporotic fractures, has not been fully explored.
The purpose of this study is to examine effects of smoking and smoking cessation on fracture risk among elderly women in Sweden. Does smoking increase the fracture risk also among elderly women? And if so, does smoking cessation decrease the increased risk?
Material and methods
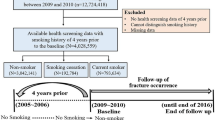
We followed elderly women in a prospective population-based observational study for 10 years. In the Osteoporosis Risk Assessment (OPRA) study, 1604 women in the city of Malmö, Sweden, were randomly selected using the population registry, during the years 1995–1999. The women were invited to participate in the study by mail 1 week after their respective 75th birthday. Of those invited, 1044 (65 %) participated. Main reasons for not participating was lack of interest (n = 376) and illness (n = 139). Additionally, a few women died shortly after invitation (n = 13) and some could not be reached despite several attempts (n = 32). No exclusion criteria were used. Mortality was continuously registered by using national population registry, which is considered to be complete in Sweden. Details of the OPRA study have been described previously [11, 14–18].
Questionnaire
At baseline, the participating women answered an extensive self-assessment questionnaire regarding previous and present lifestyle and health, including questions on smoking, alcohol habits, previous fractures, and history of falls the previous year [15]. Current or previous medications with glucocorticoids, vitamin D, and bisphosphonates were also registered.
Data concerning smoking habits include whether the subjects were smokers, former smokers, or non-smokers. Information on when subjects started smoking and stopped smoking was registered. Smokers and former smokers estimated their average cigarette consumption/day, making it possible to estimate their total cigarette consumption. A few women had not answered the question regarding smoking habits (n = 11). These were excluded from the analyses.
Alcohol habits were registered in four groups depending on the frequency of alcohol intake into total abstainer, drinks alcohol a few times a month, drinks alcohol every week, and drinks alcohol regularly almost every day.
The subjects also answered a shortened version of the original questionnaire at the 5-year follow-up which included a question on current smoking status. This question was answered by 819 women.
Fractures
Using the Swedish personal identification number, fractures were continuously registered by searching the radiological archives of the Malmö University Hospital. In these archives, the records of all persons undergoing conventional X-rays, computer tomography, and magnetic resonance imaging are available. In addition, questionnaire data for those women attending the follow-up 1, 3, 5, and 10 years after the baseline investigation were compared with the radiological data to capture fractures not found in the archives. Fracture registration was in this study 10 years from inclusion at the age of 75. The number of fracture cases missed by using solely radiology files in Malmö has previously been determined to be less than 3 % [19]. Information on previous fractures was collected from questionnaire and radiological archives [16].
Fracture data was analyzed as any fracture and as typical osteoporotic fracture (hip, symptomatic vertebral fracture, distal radius, and proximal humerus), which were also considered separately. Excluded were pathologic fractures and fractures due to high-energy trauma.
Bone mineral density
Bone mineral density (BMD) was measured with a Lunar DPX-L (Lunar Corporation, Madison, WI, USA). The coefficient of variation in elderly women was 1.4 % in the lumbar spine and 4.0 % in the femoral neck [17]. Lumbar spine BMD at L1–L2 was used since degenerative changes are more pronounced at the lower segments [18]. Femoral neck and vertebral BMD was measured in 937 (91 %) and 966 (94 %) women, respectively.
Statistics
Descriptive statistics (median, 25th and 75th percentile) were used to describe smoking habits; number of fractures; and weight, height, BMI, and BMD. Pack-years were used to describe the amount that an individual had smoked until the age of 75 years. One pack-year is the equivalent of smoking one package (20 cigarettes) daily for 1 year. Categorical data were compared using chi2 tests, and continuous non-parametric data was compared with the Kruskal-Wallis non-parametric ANOVA or the Mann-Whitney U test, in order to identify statistically significant differences between the groups. By applying the phreg procedure in SAS 9.4, competing risks proportional hazards regression [20] was used to calculate the hazard ratio (HR) for fracture with follow-up time and mortality taken into account. The variables weight, previous fracture, alcohol habits, BMD, ever use of glucocorticoids, use of vitamin D and bisphosphonates, and falls the year before baseline (yes/no) were included for the calculation of multivariate-adjusted HR. Femoral neck BMD was used in all calculations except those regarding vertebral fractures, in which lumbar spine BMD was used. Smoking parameters were analyzed by dichotomizing the groups of former smokers and current smokers by the median time as a smoker, as well as by amount of cigarettes smoked, and calculating the HR for the upper half compared to the lower half. Former smokers were analyzed through dichotomization by the median time from smoking cessation to see how fracture risk changed by time without tobacco. A change in smoking status after the baseline investigation could possibly have an effect on the results. Therefore, we compared the HR for fracture for women that were smokers both at baseline and at the 5-year follow-up with women that were in the non-smoking group both at baseline and at the 5-year follow-up. A p value less than 0.05 was considered significant.
Results
Of the 1033 participants with registered smoking habits, 679 (66 %) were non-smokers, 209 (20 %) were former smokers, and 145 (14 %) were current smokers at baseline. During the 10-year follow-up time, 420 (41 %) of the women sustained a total of 1142 fractures. Of these fractures, 720 were in non-smokers, 232 in former smokers, and 190 in current smokers. Detailed crude data regarding fractures and smoking habits for the three groups are shown in Table 1. Around half of the participants had previously sustained fractures, but there was no significant difference between the groups. Current smokers had smoked longer compared to the former smokers, resulting in more pack-years. BMI was lower and mortality higher among smokers. Alcohol consumption in all three groups was moderate. Drinking alcohol a few times a month was the most common answer alternative, ranging from 57 to 63 % in all three groups.
Fracture risk
Taking mortality into account, the mean follow-up time was 8.8 years for the whole cohort of 1033 women. The mean yearly fracture rate for the whole cohort was 4.6 % for any type of fracture, 3.9 % for osteoporotic fracture, and 1.4 % for hip fracture.
Competing risks proportional hazards regression for the risk of fracture among the participants in the three groups is shown in Table 2. Both former smokers and current smokers had a higher risk for any fracture and osteoporotic fractures compared to non-smokers. Former smokers had, in addition, a higher risk for fractures of the proximal humerus, and current smokers had a higher risk for vertebral fractures when compared to non-smokers.
After adjustment for possible confounding variables (weight, previous fracture, alcohol habits, BMD, glucocorticoids, vitamin D, bisphosphonates, and history of fall), there was an increased risk of osteoporotic and vertebral fracture among current smokers and proximal humerus fracture among former smokers (Table 2). Both unadjusted and adjusted vertebral fracture risk was increased in smokers when compared to former smokers (data not shown).
Analyzing time at risk, vertebral fracture risk increased with time as a smoker, and distal radius fracture risk decreased with time as a smoker (Table 3). Time as a smoker had no effect on fracture risk for any fracture, osteoporotic fractures, proximal humerus fractures, and hip fractures (Table 3). The total amount smoked dichotomized by the median, measured in pack-years, did not affect fracture risk for any fracture type (Table 3). In former smokers, there was no statistically significant correlation between time from smoking cessation and fracture risk (Table 3).
The group of women who smoked both at baseline and at the 5-year follow-up had an increased univariate and multivariate-adjusted risk for vertebral fractures compared to the group of women that were non-smokers at baseline and at the 5-year follow-up. The point estimates for other fractures were similar to the analysis with only baseline smoking data but did not reach significance (data not shown).
Discussion
In this study, we confirmed that smoking increases the risk for fractures in elderly women, particularly in terms of clinical vertebral fractures. Smoking cessation decreased the risk for vertebral fractures but not for other types of fractures.
Current smoking increased the general fracture risk as well as the risk for typical osteoporotic fractures as a group and for vertebral fractures alone. The risk for distal radius fractures, proximal humerus fractures, or hip fractures alone was not increased. After adjustments for other risk factors, the risk of osteoporotic and vertebral fractures persisted.
Our results are somewhat in agreement with other studies in the field. Vestergaard et al. concluded, in their meta-analysis based on results from 50 different cohort, case-control, and cross-sectional studies, that current smoking is a risk factor for all fractures pooled together and for hip and spine fractures, but not for wrist fractures [8]. Many reports found statistically significant associations between smoking and hip fractures [21–24], but several others failed to report such a relationship [25–27]. Of the studies that did not find an increased fracture risk, however, many had small sample sizes, relatively young participants (less than 50 years old), or short follow-up time (less than 5 years), resulting in few cases of hip fracture. Another meta-analysis with ten prospectively studied cohorts (mean age 63 years) found current smoking to be a risk factor for all fractures, osteoporotic fractures, and hip fractures in women [7]. However, the increased risk of any fracture as well as for osteoporotic fractures was no longer significant when adjusting for BMD [7].
The fact that we did not find an increased hip fracture risk among smokers was rather unexpected, since most studies suggest such a relationship and the women in the present study were at the age at which the peak number of hip fractures occurs. Also, measurements of BMD in the same cohort showed that current smokers had significantly lower BMD in the hip than non-smokers [11]. The lack of association between smoking and hip fracture risk in this study could be explained by the relatively few fractures, and the results may have been different if the study population was larger. Also, the women in this study had been postmenopausal for a long period of time and therefore had lost a large part of their bone reserve, resulting in less effect of environmental factors such as smoking. This theory is supported by the fact that the meta-analysis by Vestergaard et al. found that age was not associated with increased hip fracture risk in current smokers compared to non-smokers [8]. Most of the included studies with participants over 70 years of age did not find any increased fracture risk [8]. As discussed by Baron et al., adjustments for weight might also remove some of the biological effect of smoking and may thus underestimate the fracture risk in terms of hip fractures [28].
Another potential explanation could be that other risk factors, such as falling, seem to influence fracture rates more with age [29], thus decreasing the impact of smoking. This could explain the increased risk of vertebral fracture observed with smoking in this population, as vertebral fractures are not as strongly associated with falls as fractures of the extremities [30].
In accordance with Vestergaard et al. as well as several other studies, we did not find an increased risk for distal radius or proximal humerus fracture in current smokers [8, 31–34].
We found no evidence to support the hypothesis that former smokers have a lower general fracture risk when compared to current smokers. The risk of clinical vertebral fractures was however increased among current smokers when compared to former smokers.
One theory that may explain these findings is that the vertebra consists of more trabecular bone compared to other fracture sites. Trabecular bone has a higher rate of turnover and is therefore more susceptible to osteoporosis [35]. This could mean that smoking has a direct effect on bone remodeling and bone cells, as suggested by Lappin et al. [36], and therefore affect the highly transformative bone in the vertebra to a larger extent than other bones.
Smoking cessation increased the risk for proximal humerus fracture. However, this increase in risk lost significance in the multivariate analysis. The results regarding humerus fractures must be interpreted with caution due to the low number of fractures.
Other studies that examined the effect of smoking cessation on fracture risk show diverse results. Vestergaard et al. concluded in their meta-analysis that smoking cessation was associated with a decrease in fracture risk regarding all fractures and hip fractures, but not wrist fractures [8]. Too few studies investigated vertebral fractures in former smokers to be analyzed [8]. A large cohort study with over 100,000 female nurses also found a decrease in hip fracture risk after smoking cessation [24]. However, this effect was not seen until 10 years after smoking cessation. Additionally, the subjects were 34 to 59 years old at baseline, considerably lower than the age of the peak fracture rate for hip fractures. Smoking has also been shown to have long-lasting effects on fracture risk in men. Olofsson et al. reported that fracture risk in men slowly decreases after smoking cessation, but an increased risk still is present 30 years after smoking cessation [13]. Furthermore, a Danish study by Høidrup et al. was not able to distinguish any benefit, in terms of hip fracture rate, of smoking cessation in women (mean age 50 years at baseline) who had quit smoking more than 5 years ago [37].
The time from smoking cessation did not seem to affect fracture risk in the present study. While vertebral fractures had an increased fracture risk with time as a smoker, the opposite effect could be seen regarding fractures of the distal radius.
We did not find any dose-response effect between amount smoked and fractures. Previous studies have seen a dose-dependent relationship between smoking and decrease in BMD [38, 6], but few studies have investigated how the amount that a person has smoked affects the fracture risk. Our results suggest that smoking affects the bone and fracture risk independently of dose.
Our findings that neither smoking cessation nor amount of smoked cigarettes seemed to affect the general fracture risk suggest that smoking causes long-lasting detrimental effects on bone metabolism and, consequently, a persistent increased fracture risk after smoking cessation. The results also propose that smoking affects something else in the bone than the BMD, since BMD was higher in former smokers than in smokers in the same cohort [11].
The advantage with this study is that it is a prospective population-based observational study performed in randomly selected 75-year-old women followed for 10 years. The study was deliberately designed to include women of the same age, thus eliminating the need to adjust for age in the analyses. This age was chosen because osteoporosis and osteoporotic fractures increase steeply after the age of 75. Fracture registration can be regarded as complete, and multiple possible competing risk factors were available and accounted for in the statistical analyses.
A major limitation of the study is that there were relatively few current and former smokers, resulting in few fractures of some of the fracture types. For example, there were only eight fractures of the proximal humerus in the current smoking group during the 10-year period.
Smoking habit before baseline, at age 75 years, was based on recall, which might be a source of misclassification. However, others have studied reliability of recall in prospective studies and have found a good correlation between recall and previous self-assessed smoking status up to several decades [39, 40]. However, data on amount of cigarettes smoked may be less exact [39, 40], especially in heavy smokers [39]. The analyses of amount of cigarettes smoked should be less sensitive to misclassifications by dichotomization, as done in the present study.
In addition, we have only studied older women, and the results from the present study may not be applicable in younger cohorts or men.
Conclusions
In this study, smoking increased the risk for fracture among elderly women, especially vertebral fractures. Smoking cessation decreases the risk for vertebral fractures but not for other types of fractures. The multifactorial effect of smoking on bone metabolism is very complex, and more studies are needed to fully understand how smoking affects fracture risk.
References
Cummings SR, Melton LJ (2002) Epidemiology and outcomes of osteoporotic fractures. Lancet 359(9319):1761–1767. doi:10.1016/s0140-6736(02)08657-9
Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8(1–2):136. doi:10.1007/s11657-013-0136-1
Kanis JA, Oden A, McCloskey EV, Johansson H, Wahl DA, Cooper C (2012) A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int 23(9):2239–2256. doi:10.1007/s00198-012-1964-3
Kanis JA, Johnell O, Oden A, Sembo I, Redlund-Johnell I, Dawson A, De Laet C, Jonsson B (2000) Long-term risk of osteoporotic fracture in Malmo. Osteoporos Int 11(8):669–674
Kanis JA (2002) Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359(9321):1929–1936. doi:10.1016/s0140-6736(02)08761-5
Yoon V, Maalouf NM, Sakhaee K (2012) The effects of smoking on bone metabolism. Osteoporos Int 23(8):2081–2092. doi:10.1007/s00198-012-1940-y
Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA, Fujiwara S, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A (2005) Smoking and fracture risk: a meta-analysis. Osteoporos Int 16(2):155–162. doi:10.1007/s00198-004-1640-3
Vestergaard P, Mosekilde L (2003) Fracture risk associated with smoking: a meta-analysis. J Intern Med 254(6):572–583
Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, Wollum A, Sanman E, Wulf S, Lopez AD, Murray CJ, Gakidou E (2014) Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA 311(2):183–192. doi:10.1001/jama.2013.284692
Centers for Disease Control and Prevention (2001) Women and smoking: a report of the surgeon general. Available at www.cdc.gov/tobacco/data_statistics/sgr/2001/index.htm. Accessed April 2015
Gerdhem P, Obrant KJ (2002) Effects of cigarette-smoking on bone mass as assessed by dual-energy x-ray absorptiometry and ultrasound. Osteoporos Int 13(12):932–936. doi:10.1007/s001980200130
Oncken C, Prestwood K, Cooney JL, Unson C, Fall P, Kulldorff M, Raisz LG (2002) Effects of smoking cessation or reduction on hormone profiles and bone turnover in postmenopausal women. Nicotine Tob Res 4(4):451–458. doi:10.1080/1462220021000018399
Olofsson H, Byberg L, Mohsen R, Melhus H, Lithell H, Michaelsson K (2005) Smoking and the risk of fracture in older men. J Bone Miner Res 20(7):1208–1215. doi:10.1359/jbmr.050208
Wihlborg A, Akesson K, Gerdhem P (2014) External validity of a population-based study on osteoporosis and fracture. Acta Orthop 85(4):433–437. doi:10.3109/17453674.2014.920987
Wihlborg A, Englund M, Akesson K, Gerdhem P (2015) Fracture predictive ability of physical performance tests and history of falls in elderly women: a 10-year prospective study. Osteoporos Int 26(8):2101–2109. doi:10.1007/s00198-015-3106-1
Gerdhem P, Akesson K (2007) Rates of fracture in participants and non-participants in the Osteoporosis Prospective Risk Assessment study. J Bone Joint Surg (Br) 89(12):1627–1631. doi:10.1302/0301-620x.89b12.18946
Lenora J, Akesson K, Gerdhem P (2010) Effect of precision on longitudinal follow-up of bone mineral density measurements in elderly women and men. J Clin Densitom 13(4):407–412. doi:10.1016/j.jocd.2010.04.004
Tenne M, McGuigan F, Besjakov J, Gerdhem P, Akesson K (2013) Degenerative changes at the lumbar spine--implications for bone mineral density measurement in elderly women. Osteoporos Int 24(4):1419–1428. doi:10.1007/s00198-012-2048-0
Jónsson B (1993) Life style and fracture risk. University of Lund, Lund
Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94(446):496–509. doi:10.2307/2670170
Forsen L, Bjorndal A, Bjartveit K, Edna TH, Holmen J, Jessen V, Westberg G (1994) Interaction between current smoking, leanness, and physical inactivity in the prediction of hip fracture. J Bone Miner Res 9(11):1671–1678. doi:10.1002/jbmr.5650091102
la Vecchia C, Negri E, Levi F, Baron JA (1991) Cigarette smoking, body mass and other risk factors for fractures of the hip in women. Int J Epidemiol 20(3):671–677
Cumming RG, Klineberg RJ (1994) Case–control study of risk factors for hip fractures in the elderly. Am J Epidemiol 139(5):493–503
Cornuz J, Feskanich D, Willett WC, Colditz GA (1999) Smoking, smoking cessation, and risk of hip fracture in women. Am J Med 106(3):311–314
Hemenway D, Colditz GA, Willett WC, Stampfer MJ, Speizer FE (1988) Fractures and lifestyle: effect of cigarette smoking, alcohol intake, and relative weight on the risk of hip and forearm fractures in middle-aged women. Am J Public Health 78(12):1554–1558
Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, Cauley J, Black D, Vogt TM (1995) Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med 332(12):767–773. doi:10.1056/nejm199503233321202
Meyer HE, Tverdal A, Falch JA (1993) Risk factors for hip fracture in middle-aged Norwegian women and men. Am J Epidemiol 137(11):1203–1211
Baron JA, Farahmand BY, Weiderpass E, Michaelsson K, Alberts A, Persson I, Ljunghall S (2001) Cigarette smoking, alcohol consumption, and risk of hip fracture in women. Arch Intern Med 161(7):983–988
Jarvinen TL, Sievanen H, Khan KM, Heinonen A, Kannus P (2008) Shifting the focus in fracture prevention from osteoporosis to falls. BMJ 336(7636):124–126. doi:10.1136/bmj.39428.470752.AD
Kelsey JL, Samelson EJ (2009) Variation in risk factors for fractures at different sites. Curr Osteoporos Rep 7(4):127–133
O’Neill TW, Marsden D, Adams JE, Silman AJ (1996) Risk factors, falls, and fracture of the distal forearm in Manchester, UK. J Epidemiol Community Health 50(3):288–292
Hemenway D, Azrael DR, Rimm EB, Feskanich D, Willett WC (1994) Risk factors for wrist fracture: effect of age, cigarettes, alcohol, body height, relative weight, and handedness on the risk for distal forearm fractures in men. Am J Epidemiol 140(4):361–367
Kelsey JL, Browner WS, Seeley DG, Nevitt MC, Cummings SR (1992) Risk factors for fractures of the distal forearm and proximal humerus. The Study of Osteoporotic Fractures Research Group. Am J Epidemiol 135(5):477–489
Hagino H, Fujiwara S, Nakashima E, Nanjo Y, Teshima R (2004) Case–control study of risk factors for fractures of the distal radius and proximal humerus among the Japanese population. Osteoporos Int 15(3):226–230. doi:10.1007/s00198-003-1543-8
Clarke B (2008) Normal bone anatomy and physiology. Clin J Am Soc Nephrol 3(Suppl 3):S131–S139. doi:10.2215/cjn.04151206
Lappin DF, Sherrabeh S, Jenkins WM, Macpherson LM (2007) Effect of smoking on serum RANKL and OPG in sex, age and clinically matched supportive-therapy periodontitis patients. J Clin Periodontol 34(4):271–277. doi:10.1111/j.1600-051X.2007.01048.x
Hoidrup S, Prescott E, Sorensen TI, Gottschau A, Lauritzen JB, Schroll M, Gronbaek M (2000) Tobacco smoking and risk of hip fracture in men and women. Int J Epidemiol 29(2):253–259
Ward KD, Klesges RC (2001) A meta-analysis of the effects of cigarette smoking on bone mineral density. Calcif Tissue Int 68(5):259–270
Pickett KE, Kasza K, Biesecker G, Wright RJ, Wakschlag LS (2009) Women who remember, women who do not: a methodological study of maternal recall of smoking in pregnancy. Nicotine Tob Res 11(10):1166–1174. doi:10.1093/ntr/ntp117
Krall EA, Valadian I, Dwyer JT, Gardner J (1989) Accuracy of recalled smoking data. Am J Public Health 79(2):200–202
Acknowledgments
We thank Per Näsman for providing statistical advice. We also thank Margareta Hedström for helpful comments and thoughts.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thorin, M.H., Wihlborg, A., Åkesson, K. et al. Smoking, smoking cessation, and fracture risk in elderly women followed for 10 years. Osteoporos Int 27, 249–255 (2016). https://doi.org/10.1007/s00198-015-3290-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3290-z