Abstract
Summary
Trabecular bone score (TBS) seems to provide additive value on BMD to identify individuals with prevalent fractures in T1D. TBS did not significantly differ between T1D patients and healthy controls, but TBS and HbA1c were independently associated with prevalent fractures in T1D. A TBS cutoff <1.42 reflected prevalent fractures with 91.7 % sensitivity and 43.2 % specificity.
Introduction
Type 1 diabetes (T1D) increases the risk of osteoporotic fractures. TBS was recently proposed as an indirect measure of bone microarchitecture. This study aimed at investigating the TBS in T1D patients and healthy controls. Associations with prevalent fractures were tested.
Methods
One hundred nineteen T1D patients (59 males, 60 premenopausal females; mean age 43.4 ± 8.9 years) and 68 healthy controls matched for gender, age, and body mass index (BMI) were analyzed. The TBS was calculated in the lumbar region, based on two-dimensional (2D) projections of DXA assessments.
Results
TBS was 1.357 ± 0.129 in T1D patients and 1.389 ± 0.085 in controls (p = 0.075). T1D patients with prevalent fractures (n = 24) had a significantly lower TBS than T1D patients without fractures (1.309 ± 0.125 versus 1.370 ± 0.127, p = 0.04). The presence of fractures in T1D was associated with lower TBS (odds ratio = 0.024, 95 % confidence interval (CI) = 0.001–0.875; p = 0.042) but not with age or BMI. TBS and HbA1c were independently associated with fractures. The area-under-the curve (AUC) of TBS was similar to that of total hip BMD in discriminating T1D patients with or without prevalent fractures. In this set-up, a TBS cutoff <1.42 discriminated the presence of fractures with a sensitivity of 91.7 % and a specificity of 43.2 %.
Conclusions
TBS values are lower in T1D patients with prevalent fractures, suggesting an alteration of bone strength in this subgroup of patients. Reliable TBS cutoffs for the prediction of fracture risk in T1D need to be determined in larger prospective studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 1 diabetes (T1D) is characterized by autoimmune destruction of pancreatic beta cells and impaired insulin secretion. This results in hyperglycemia and long-term impairment of different organs, especially kidneys, peripheral nerves, and retina. It is also known that T1D leads to an increased risk of fractures [1] and that patients with T1D have a lower bone mineral density (BMD). However, it has also been observed that the actual fracture rates largely exceed the fracture risk calculated from the measurement of BMD [2]. Thus, BMD measurements do not entirely reflect the reduction of bone strength caused by diabetes. Major mechanisms potentially contributing to the increased fracture risk in T1D are the insulin deficiency and the increased accumulation of non-enzymatic cross-links (AGEs) in bone matrix, with consequent impairment of bone strength [3]. Indeed, serum levels of AGEs are associated with prevalent fractures in T1D, independently from BMD values [4].
The trabecular microarchitecture is an important component of bone quality; however, this is not measured by conventional dual-energy absorptiometry (DXA). The recently proposed trabecular bone score (TBS), in contrast, is able to evaluate tri-dimensional (3D) characteristics of bone micro architecture in the spine. Previous studies have reported that TBS is not significantly correlated with BMD, but significantly correlated with trabecular number, trabecular separation, and structure model index (SMI) [5–8]. TBS measurements are based on experimental variograms of two-dimensional (2D) projections of existing DXA assessments and—when used in addition to BMD measurements taken at the same anatomical site—help to predict bone quality and osteoporotic fractures independently of clinical risk factors [9]. It has recently been demonstrated that in premenopausal women, the microstructural parameters of bone, as evaluated by high-resolution peripheral quantitative computed tomography (HR-pQCT), correlate with TBS [10]. In general, higher TBS reflect a denser bone with a higher resistance to fracture. Unlike DXA, notably, TBS values are not significantly affected by osteoarthritic changes, making TBS potentially of even greater value for the evaluation of bone quality in the spine [8, 11].
It has already been demonstrated that TBS is reduced in type 2 diabetes (T2D) and inversely associated with poor glycemic control [12]. Furthermore, TBS predicts fracture risk in T2D patients [13].
The present cross-sectional study was initially conducted and powered to investigate differences in BMD between patients with T1D and controls [14]. We now re-investigated the initial cohort, and focused on the indirect evaluation of the lumbar spine trabecular microarchitecture by means of TBS measurements obtained from conventional DXA. We aimed to elucidate factors that influence TBS in T1D and hypothesized that TBS enabled the discrimination of T1D patients with prevalent fractures from those without fractures.
Methods and materials
The study was approved by the local ethics committee and was performed according to the principles of the Declaration of Helsinki. All subjects gave written informed consent before study participation.
The cross-sectional study was designed to calculate the TBS by means of 2D gray-scale DXA images of the lumbar spine obtained from a large cohort of male and female patients with long-standing T1D and controls [14]. Associations of various parameters, including individual patient characteristics, BMD and TBS, with prevalent fractures were also investigated.
Patient population
This cohort was initially recruited to investigate the association between glycemic control, BMD, and risk of fractures in T1D [14]. Exclusion criteria were a disease duration <3 years, co-medication with corticosteroids, menopause or no menstrual period within the last 12 months, pregnancy, chronic inflammatory disease, malnutrition, renal failure [glomerular filtration rate (modification of diet in renal disease formula) <30 ml/min], and severe mental or somatic disease. The initial cohort consisted of 128 patients with T1D (63 males, age 45.2 ± 9.7 years; 65 females, age 41.5 ± 7.5 years) and 77 controls (39 males, age 44.9 ± 11.2 years; 39 females, age 41.1 ± 8.1 years). For the present analysis of TBS, a body mass index (BMI) >35 kg/m2 was introduced as additional exclusion criterion because this factor has been shown to interfere with the accuracy of the measurements. This resulted in the exclusion of nine controls and nine patients with T1D, leading to the analysis of 119 T1D patients and 68 controls.
Clinical evaluation
A physical examination and a standardized interview on medical history, including fracture history, were performed. Previous fractures were retrieved from the patient’s history and confirmed by medical notes of primary care physicians. Only fractures occurring after the age of 20 years were included. Fractures of the ribs, fingers, and toes were excluded from analysis.
Laboratory analysis
HbA1c (normal 24.6–41.0 mmol/mol) and 25-hydroxyvitamin D were measured by high-performance liquid chromatography (HPLC; Tosoh glycohemoglobin-analyzer-HLC-723-GHbV; Tosoh Corporation, Tokyo, Japan and Chromosystems Instruments and Chemicals, Munich, Germany). The levels of HbA1c and 25-hydroxy vitamin D were available for all study participants.
Measurement of BMD
The DXA scans were performed using a Prodigy Advance densitometer (General Electric Medical Systems Lunar, Madison, WI, USA). BMD measurements were recorded in lumbar spine (L1 through L4), femoral neck, and total hip. Regular scans of a body-composition phantom were performed to control for inter-day variations (variation coefficient 0.46 %).
Measurement of TBS
The TBS calculations were performed at the Bone Disease Unit of the University of Lausanne, Lausanne, Switzerland, using the TBS iNsight Software, Version 1.8 (Med-Imaps, Pessac, France). This software version was optimized for women and not yet for men. Pre-existing spine DXA files were anonymized to ensure blinding of the TBS investigators to all clinical parameters and outcomes. The TBS was evaluated by determining the variograms of the trabecular bone projected image, calculated as the sum of the squared gray-level differences between pixels at a specific distance and angle. TBS were calculated as the slope of the log-log transformation of the variograms, as previously published [15]. The software used the anterior-posterior spine raw images from the densitometer, so that the TBS calculation was performed exactly over the same region of interest as the BMD measurement.
Statistical analysis
Descriptive statistics were expressed as mean ± SD or absolute and relative frequencies as appropriate. Group comparisons of continuous variables were performed using independent sample t tests, and categorical variables were compared by Fisher’s exact test.
Pearson’s correlation coefficients were calculated to measure the association between TBS, BMD, and anthropometric parameters. The influence of different covariates (gender, age, HbA1c, and BMI) on TBS was estimated with a multivariate linear regression model. Binary logistic regression was used to evaluate the influence of diabetes status on BMD and TBS measurements (lowest versus highest tertile). Logistic regression models were also fitted to estimate adjusted odds ratios (OR) of fracture prevalence as measure of associations between potentially influencing variables and fractures. Receiver operator characteristics (ROC) analyses were performed to discriminate between individuals with and without prevalent fractures by the different TBS and BMD measures. A p value of <0.05 was considered to be statistically significant. Statistical analyses were performed using IBM SPSS Statistics for Macintosh, Version 20.0 (IBM Corporation, Armonk, NY, USA).
Results
The baseline descriptive data, as well as data on BMD, bone turnover markers, and prevalent fractures of the initial cohort have been published elsewhere [14]. The baseline characteristics of 119 patients with T1D and of 68 controls matched for gender, age, and BMI are described in Table 1. Mean duration of diabetes was 21.5 years. Diabetes-related complications (nephropathy, neuropathy, or retinopathy) were present in 46.2 % of all T1D patients (Table 1). As expected, the levels of HbA1c were significantly higher in T1D patients than in controls, and those of 25-hydroxy vitamin D significantly lower in T1D patients than in controls (Table 1).
The total hip BMD values—but not those of lumbar spine or femoral neck—were significantly lower (p = 0.019) in T1D patients than in controls (Table 1). Females (whether T1D patients or controls) had significantly higher mean TBS than males (1.411 versus 1.367, p = 0.028; 1.406 versus 1.308, p < 0.001, respectively) (data not shown).
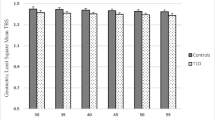
Lumbar spine TBS were inversely correlated with age, in T1D patients as well as in controls (Table 2, Fig. 1). In the control group, there were significant correlations between TBS and BMD of lumbar spine, total hip, and femoral neck, whereas in T1D patients the correlation was limited to the BMD of lumbar spine (Table 2).
We also investigated the influence of gender, age, HbA1c, and BMI on TBS by multivariate analysis. Mean TBS significantly depended on gender and age in controls (decrease of 0.004 per year, p < 0.001; difference females versus males = 0.039, p = 0.049) and on gender in T1D patients (difference females versus males = 0.089, p < 0.001). Adding diabetes duration to the analysis in the T1D group had no influence on the results (data not shown).
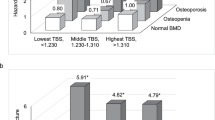
The adjusted odds ratio (aOR) for lumbar spine TBS in the lowest tertile (versus highest tertile reference) was significantly above 1 in the comparison between T1D patients and controls (aOR 2.59, 95 % confidence interval (CI) = 1.08–6.21; p = 0.033) (Fig. 2). In contrast, the aORs for BMD at lumbar spine, femoral neck or total hip did not differ between the two groups (Fig. 2).
Odds ratios (OR) and 95 % confidence intervals for bone mineral density (BMD) (LS lumbar spine, FN femoral neck, TH total hip) or trabecular bone score (TBS) of lumbar spine (L1–L4) in the lowest tertile (reference: highest tertile) associated with type 1 diabetes. Results are adjusted for age and body mass index
Twenty-four of the 119 T1D patients (20.2 %) had experienced previous fractures. One to four fractures were reported per patient, resulting in a fracture frequency of 0.018 per observational year. Most patients experienced fractures in distal extremities (15 in forearm/hand and 13 in lower leg/foot). Hip fractures occurred only in two cases, clinically apparent vertebral fractures in four cases. Patients with fractures were characterized by significantly higher levels of HbA1c and by significantly lower levels of total hip BMD and lumbar spine TBS (Table 3).
Eight of 68 controls (11.8 %) reported at least 1 prevalent fracture (maximum 3), resulting in a fracture frequency of 0.006 per observational year. In most individuals, fractures occurred in lower extremities (five in forearm/hand and two in lower leg/foot), whereas one individual had a vertebral fracture. No hip fractures were reported.
The logistic regression analysis showed that the presence of fractures in T1D patients was associated with a reduction of TBS (OR = 0.024, 95 % CI = 0.001–0.875, p = 0.042), but not with advancing age (OR = 1.046, 95 % CI = 0.991–1.103, p = 0.101) or increasing BMI (OR = 1.025, 95 % CI = 0.902–1.165, p = 0.704). After adjustment for lumbar spine BMD, the association with TBS remained significant (OR = 0.021, 95 % CI = 0–0.940, p = 0.046); however, it failed the level of significance when TBS was adjusted for total hip BMD (OR = 0.033, 95 % CI = 0.001–1.519, p = 0.081). For healthy controls, there was no association of prevalent fractures with TBS, BMI, or age (data not shown).
In a multivariate model, TBS (OR = 0.026, 95 % CI = 0.003–0.979, p = 0.049) and HbA1c (OR = 1.040, 95 % CI = 1.003–1.078, p = 0.036) were independently associated with prevalent fractures in T1D.
The discriminative value for prevalent non-vertebral fractures of TBS and BMD of various skeletal sites was also assessed by the area under the ROC curve. The ROC curve showed that only TBS and total hip BMD significantly differentiated between diabetic patients with and without fractures (AUC 0.63, 95 % CI = 0.51–0.74, p = 0.048 and AUC 0.64, 95 % CI = 0.51–0.78, p = 0.032, respectively) (Fig. 3). When mean TBS and total hip BMD values were combined, the AUC increased to 0.68 (95 % CI = 0.55–0.81; p = 0.007).
The cutoff of TBS with the best compromise between sensitivity and specificity by ROC curve analysis was set at 1.42. This cutoff had a sensitivity of 91.7 % (95 % CI = 73.0–99.0) and a specificity of 43.2 % (95 % CI = 33.0–53.8). The positive predictive value to identify individuals with prevalent fractures was 28.9 % (95 % CI = 19.1–40.5), the negative predictive value 95.3 % (95 % CI = 84.1–99.5). In a separate analysis for gender, the cutoff values were 1.38 (sensitivity 92.3 %) for males and 1.48 (sensitivity 90.9 %) for females.
The cutoff of 1.42 was used also to calculate the OR able to detect fractures in patients with T1D. The subgroup with a TBS <1.42 had an OR of 8.35 (95 % CI = 1.85–37.56) compared with the subgroup with TBS >1.42 (reference group).
Discussion
Microarchitectural abnormalities are probably the most important determinants of fractures [16]. Based on the current definition, both bone density and quality—which includes structural and material properties of the bone—are important factors of bone strength. However, bone density alone does not entirely reflect the bone microarchitecture, and BMD values considerably overlap between individuals with and without fractures [17]. Also, in T1D and T2D patients, there are discrepancies between the calculated fracture risk based on BMD measurements and the actually observed fracture rate [2]. Furthermore, the observed fracture rate in older T2D patients exceeds the risk predicted by the World Health Organization Fracture Risk Algorithm (FRAX) as composite score [18].
In contrast to postmenopausal women and elderly individuals, the calculation of fracture risks and the indication for osteoporosis treatment in young adults (i.e., between 20 and 50 years of age) remain poorly defined [19]. As T1D typically has an early onset in childhood or adolescence, a cumulative higher fracture risk can develop already at a young age. Owing to the lack of valid screening procedures in young adults, frequently, the risk of fractures becomes evident only after the first fracture has manifested. Since the BMD alone does not provide sufficient information to predict the fracture risk in T1D, additional or alternative methods need to be explored. The TBS, developed as a clinical tool for the indirect assessment of bone microarchitecture [6], may fulfill this expectation.
To the best of our knowledge, this is the first study investigating the clinical value of TBS in assessing skeletal fragility in T1D. We first compared TBS in T1D patients and controls, and found that T1D patients had a lower TBS than controls, if only borderline significant. The TBS were weakly correlated with the BMD of lumbar spine, femoral neck and total hip in the controls, and only with lumbar spine BMD in T1D patients. This is in accordance with previous findings from larger cohorts suggesting that TBS is measuring different bone properties [9].
We then compared BMD and TBS from the lower bound with the upper bound of the range, and found that only the highest tertile of TBS—but not BMD values—significantly discriminated T1D patients from controls. TBS depended on gender, with higher values in females, but an independent association with age was seen only in controls. Thus, if TBS reflects bone microarchitecture, there appear to be differences between T1D patients and healthy individuals that BMD measurements alone cannot depict. As a matter of fact, only one study has investigated the bone microstructure in T1D patients [20], specifically by histomorphometry of biopsies obtained from the iliac crest. Interestingly, this study failed to show differences in structural parameters between T1D patients and controls, although the younger age of the T1D patients and the shorter disease duration may limit the comparability with our cohort.
TBS has been shown to predict fractures in postmenopausal women [9]. In our study, we attempted to test the discriminative power of TBS in the T1D patients with prevalent fractures and found indeed that a low TBS was significantly associated with a fracture history in patients with T1D. Furthermore, TBS had a sufficient accuracy to identify individuals with prevalent fractures when tested by ROC curve analysis. When a TBS cutoff of >1.42 was used to exclude patients with fractures, a specificity of 43.2 % and a negative predictive value of 95.3 % could be achieved. The discriminative power of TBS to identify individuals with T1D that have experienced fractures was greater than that of BMD at lumbar spine or femoral neck, and nearly as good as those of BMD at total hip.
The present cutoff of 1.42, showing the best compromise between sensitivity and specificity, resulted higher than in studies with other conditions [21–23]. This is not surprising because our cohort was substantially younger than in other studies, and it is well known that TBS decreases with age [24]. A normative mean TBS of 1.328 was determined in a cohort of individuals aged between 45 and 55 years [24]. The mean age (43.4 years) of our cohort is at the lower end of this range. As we analyzed only a small number of fractures in a comparatively small cohort, the power of this threshold needs to be tested in a large prospective analysis. This study indicates that TBS is associated with prevalent fractures in T1D patients, as are increased Hb1Ac and AGEs.
Major limitations of this study are the cross-sectional design, the relatively small sample size, and the retrospective analysis of fractures. The initial power analysis was performed to detect differences in BMD [14]. There was no option to calculate the power for a study of TBS to discriminate individuals with fractures in T1D because data were lacking. We cannot draw any conclusions on causality between low TBS and occurrence of fractures, and the study was not powered to detect differences in mean TBS values.
In conclusion, TBS values are lower in female and male patients with T1D who experienced fractures, suggesting an alteration of bone strength in this subgroup of patients. TBS appears to be more accurate than BMD of the lumbar spine, and can be used in addition to total hip BMD in predicting the risk of peripheral fractures. HbA1c does not influence the association of TBS with prevalent fractures. Reliable TBS cutoffs for the prediction of fractures in T1D patients need to be tested in a large, prospective trial.
Abbreviations
- BMD:
-
Bone mineral density
- BMI:
-
Body mass index
- HbA1c :
-
Glycated hemoglobin A 1c
- SD:
-
Standard deviation
- TBS:
-
Trabecular bone score
References
Nicodemus KK, Folsom AR (2001) Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care 24:1192–1197
Vestergaard P (2007) Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int 18:427–444
Hofbauer LC, Brueck CC, Singh SK, Dobnig H (2007) Osteoporosis in patients with diabetes mellitus. J Bone Miner Res 22:1317–1328
Neumann T, Lodes S, Kastner B, Franke S, Kiehntopf M, Lehmann T, Muller UA, Wolf G, Samann A (2014) High serum pentosidine but not esRAGE is associated with prevalent fractures in type 1 diabetes independent of bone mineral density and glycaemic control. Osteoporos Int 25:1527–1533
Bousson V, Bergot C, Sutter B, Levitz P, Cortet B (2012) Trabecular bone score (TBS): available knowledge, clinical relevance, and future prospects. Osteoporos Int 23:1489–1501
Pothuaud L, Carceller P, Hans D (2008) Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone 42:775–787
Roux JP, Wegrzyn J, Boutroy S, Bouxsein ML, Hans D, Chapurlat R (2013) The predictive value of trabecular bone score (TBS) on whole lumbar vertebrae mechanics: an ex vivo study. Osteoporos Int 24:2455–2460
Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N, McCloskey EV, Kanis JA, Bilezikian JP (2014) Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res 29:518–530
Hans D, Goertzen AL, Krieg MA, Leslie WD (2011) Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res 26:2762–2769
Popp AW, Buffat H, Eberli U, Lippuner K, Ernst M, Richards RG, Stadelmann VA, Windolf M (2014) Microstructural parameters of bone evaluated using HR-pQCT correlate with the DXA-derived cortical index and the trabecular bone score in a cohort of randomly selected premenopausal women. PLoS ONE 9:e88946
Winzenrieth R, Dufour R, Pothuaud L, Hans D (2010) A retrospective case–control study assessing the role of trabecular bone score in postmenopausal Caucasian women with osteopenia: analyzing the odds of vertebral fracture. Calcif Tissue Int 86:104–109
Dhaliwal R, Cibula D, Ghosh C, Weinstock RS, Moses AM (2014) Bone quality assessment in type 2 diabetes mellitus. Osteoporos Int 25:1969–1973
Leslie WD, Aubry-Rozier B, Lamy O, Hans D (2013) TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab 98:602–609
Neumann T, Samann A, Lodes S et al (2011) Glycaemic control is positively associated with prevalent fractures but not with bone mineral density in patients with type 1 diabetes. Diabet Med 28:872–875
Hans D, Barthe N, Boutroy S, Pothuaud L, Winzenrieth R, Krieg MA (2011) Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom 14:302–312
(2001) NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, March 7–29, 2000: highlights of the conference. South Med J 94:569–573
Cummings SR (1985) Are patients with hip fractures more osteoporotic? Review of the evidence. Am J Med 78:487–494
Schwartz AV, Vittinghoff E, Bauer DC et al (2011) Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA 305:2184–2192
Ferrari S, Bianchi ML, Eisman JA, Foldes AJ, Adami S, Wahl DA, Stepan JJ, de Vernejoul MC, Kaufman JM (2012) Osteoporosis in young adults: pathophysiology, diagnosis, and management. Osteoporos Int 23:2735–2748
Armas LA, Akhter MP, Drincic A, Recker RR (2012) Trabecular bone histomorphometry in humans with type 1 diabetes mellitus. Bone 50:91–96
Breban S, Briot K, Kolta S, Paternotte S, Ghazi M, Fechtenbaum J, Roux C (2012) Identification of rheumatoid arthritis patients with vertebral fractures using bone mineral density and trabecular bone score. J Clin Densitom 15:260–266
Romagnoli E, Cipriani C, Nofroni I et al (2013) "Trabecular Bone Score" (TBS): an indirect measure of bone micro-architecture in postmenopausal patients with primary hyperparathyroidism. Bone 53:154–159
Boutroy S, Hans D, Sornay-Rendu E, Vilayphiou N, Winzenrieth R, Chapurlat R (2013) Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporos Int 24:77–85
Dufour R, Winzenrieth R, Heraud A, Hans D, Mehsen N (2013) Generation and validation of a normative, age-specific reference curve for lumbar spine trabecular bone score (TBS) in French women. Osteoporos Int 24:2837–2846
Acknowledgments
The authors thank Ernesta Palombo-Kinne (Jena) for critical revision of the manuscript.
Conflict of interest
TBS iNsight Software is a product of Med-Imaps. Didier Hans is co-owner of the TBS patent and has corresponding ownership shares. Thomas Neumann, Sabine Lodes, Bettina Kästner, Thomas Lehmann, Olivier Lamy, Ulrich Alfons Müller, Gunter Wolf, and Alexander Sämann declare that they have no conflict of interest.
Funding
The study was funded by a grant of Merck Pharma GmbH, Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
The study was conducted according to the principles of the Declaration of Helsinki.
Rights and permissions
About this article
Cite this article
Neumann, T., Lodes, S., Kästner, B. et al. Trabecular bone score in type 1 diabetes—a cross-sectional study. Osteoporos Int 27, 127–133 (2016). https://doi.org/10.1007/s00198-015-3222-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3222-y