Abstract
Summary
The percent and absolute lumbar spine and femoral neck bone mineral densities and absolute procollagen type I N-terminal propeptide (PINP) increases following a 20-μg/day teriparatide treatment for 12 months were similar in men and women regardless of sex differences.
Introduction
Several placebo-controlled studies have measured the effects of daily teriparatide in men and postmenopausal women with osteoporosis but none have directly compared the effects between these groups. We retrospectively compared the effects of daily teriparatide therapy in men and postmenopausal women with osteoporosis and investigated biochemical markers of bone turnover to detect possible sex differences.
Methods
Patients (563; 75 men and 488 women) with osteoporosis were retrospectively investigated. All patients were administered with teriparatide at 20 μg/day for 12 months. The primary efficacy measure was changed in lumbar spine (LS) and femoral neck (FN) bone mineral density (BMD) after 12 months of treatment. The change in serum levels of procollagen type I N-terminal propeptide (PINP) and urinary N-telopeptide (uNTX) excretion after 4, 8 and 12 months of treatment were also measured.
Results
In men, the percent LS BMD significantly increased by 11.3 ± 9.9 % (mean ± standard deviation (SD)) and the FN BMD increased by 0.4 ± 6.4 % without a significant difference at 12 months. In postmenopausal women, the percent LS BMD significantly increased by 9.6 ± 8.1 % and the FN BMD significantly increased by 2.4 ± 7.8 % at 12 months. The percent and absolute BMD increases in LS and FN between men and women were similar. The absolute increases in PINP were similar in both groups at 4, 8 and 12 months. However, the absolute increases in uNTX were significantly lower in men than in women at 8 and 12 months.
Conclusion
Daily teriparatide treatment was as effective in men as in postmenopausal women regardless of sex differences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is an increasing concern for older adults because fragility fractures can significantly affect overall health and quality of life representing a public health challenge. Although less common than in women, osteoporosis in men is still a substantial public problem. One third of all hip fractures worldwide occur in men [1]. Between 30 and 40 % of fractures due to osteoporosis occur in men; the lifetime risk of fracture for men aged ≥50 is between 13 and 30 % [2]. Men with hip fractures have a mortality rate two- to three-times higher than women [3].
The pathophysiology of osteoporosis in men, as in postmenopausal women, is multifactorial. Theoretically, lower rates of fracture in men than in women are a consequence of men’s larger bone structure, reduced architectural disruption, greater bone accrual during skeletal growth, earlier mortality, fewer falls and lower bone turnover rates [4]. In women with osteoporosis, trabecular number is reduced, whereas in men with osteoporosis, trabecular thickness is reduced [5]. Osteoporosis in men is currently being increasingly recognised as an important health issue, but few potential treatments for osteoporosis in men have been adequately studied.
Recombinant human parathyroid hormone (rhPTH) (1–34), teriparatide, is a bone anabolic agent that increases bone mineral density (BMD) and reduces vertebral and non-vertebral fracture incidences in postmenopausal women [6]. Very few placebo-controlled studies have been performed to assess the effects of daily teriparatide treatment in men with osteoporosis [7, 8]. But none have directly compared the effects between men and postmenopausal women. In this study, we retrospectively investigated the effects of daily teriparatide treatment for 12 months in men and postmenopausal women with osteoporosis on BMD and attempted to identify biochemical markers of bone turnover to detect possible sex differences.
Materials and methods
Study subjects
The inclusion criteria were men and postmenopausal women diagnosed with osteoporosis and at high risk of fracture. A high risk of fracture was defined as when patients met at least one of the following criteria by referring to the previous reports [9, 10]: (1) BMD at lumbar spine (LS) L1–L4 <80 % of the young adult mean (YAM; for all subjects reported in the Japanese Normative Female Database [11]), with a minimum of one prevalent fragility fracture; (2) BMD at L1–4 <70 % of YAM and age ≥65 years; (3) BMD at L1–4 <65 % of YAM and age ≥55 years or (4) more than three previous osteoporotic fractures. The exclusion criteria were patients with illnesses affecting bone and calcium metabolism or bone disorders other than osteoporosis as well as patients with serious cardiovascular, renal or hepatic dysfunction. Patients with a high concentration of serum calcium (>11 mg/dl) at baseline were also excluded. Eighty percent, 70 and 65 % of YAM are approximately equivalent to a T score of −1.9, −2.8 and −3.2, respectively in Japanese women.
We performed a retrospective analysis of 381 of 563 patients (68 %) diagnosed with osteoporosis and who completed the 12-month teriparatide treatment (47 men and 334 women) (Fig. 1). Following are the reasons for 28 men to discontinue teriparatide treatment: lost to follow-up, 13 patients; loss of motivation for teriparatide treatment, 6 patients; death from a cause unrelated to teriparatide treatment, 4 patients; discontinuation for illness unrelated to teriparatide treatment, 2 patients; relocation, 1 patient; expensive medical expenses, 1 patient and fatigue, 1 patient. The reasons for 154 women to discontinue teriparatide treatment were the following: lost to follow-up, 44 patients; loss of motivation for teriparatide treatment, 26 patients; discontinuation for illness unrelated to teriparatide treatment, 22 patients; relocation, 13 patients; death from a cause unrelated to teriparatide treatment, 10 patients; nausea, 8 patients; dizziness, 6 patients; palpitation, 3 patients; hypercalcaemia, 2 patients; expensive medical expenses, 2 patients; dry mouth, 2 patients and others, 16. There were no differences in the completion rate between men and women (p = 0.32; chi-square test).
Measurements
We measured the BMD of the LS (L1–L4) and femoral neck (FN) using dual-energy X-ray absorptiometry (DXA) on a DPX-BRAVO instrument (GE Healthcare, Madison, WI, USA) at baseline, 4, 8 and 12 months after starting treatment. The coefficients of variation (%CV) for DXA were 0.6 % for LS and 0.9 % for FN. The concentration of procollagen type I N-terminal propeptide (PINP) and urinary N-telopeptide (uNTX) at baseline, 4, 8 and 12 months after starting treatment, were measured. Serum PINP was measured by a radioimmunoassay (Orion Diagnostica, Espoo, Finland). uNTX was measured by an enzyme-linked immunosorbent assay (ELISA; Alere Medical Co., Ltd., Tokyo, Japan). Additional details regarding the methods have been published elsewhere [12, 13].
Statistical analysis
To determine the response variables associated with BMD changes, univariate analyses were performed by Spearman correlation coefficient analysis and the Mann–Whitney U test. The longitudinal changes in PINP and uNTX after starting treatment were assessed by the Wilcoxon signed-rank test. Differences in categorical variables were assessed by the chi-square test and Fisher’s exact test. Data are expressed as means ± standard deviations (SDs). P values of <0.05 were considered to indicate statistical significance. The StatView statistical software package (version 5.0; SAS Institute, Cary, NC, USA) was used to perform statistical analyses.
Compliance
The participants were queried regarding the number of missed doses of medication and were considered compliant if they consumed ≥85 % of the study drug. The protocol was in compliance with the ethical principles stated in the Declaration of Helsinki and was approved by the Ethics Committee of Tomidahama Hospital. Written informed consent was obtained from the patients. Treatment compliance was evaluated by measurement of the remaining volume of teriparatide at each visit.
Results
Baseline characteristics
Table 1 shows the demographics and baseline characteristics of each group. Age, body mass index and serum calcium concentration did not differ significantly between the two groups. Height, body weight and serum uric acid concentration were higher in men. Serum phosphorus concentration was lower in men. LS and FN BMD were higher in men. Serum PINP concentration did not differ significantly between the two groups, and uNTX was higher in men.
Changes in LS and FN BMD in response to teriparatide treatment
The mean percent changes in BMD from baseline for LS and FN are shown in Fig. 2. In men, the LS percent BMD significantly increased by 11.3 ± 9.9 % (p < 0.01 vs. baseline; paired t test) (Fig. 2a), and the FN BMD increased by 0.4 ± 6.4 % (p = 0.90 vs. baseline; paired t test) without a significant difference at 12 months (Fig. 2b). In women, the LS percent BMD significantly increased by 9.6 ± 8.1 % (p < 0.01 vs. baseline; paired t test) (Fig. 2a), and the FN percent BMD significantly increased by 2.4 ± 7.8 % (p < 0.01 vs. baseline; paired t test) at 12 months (Fig. 2b). The discrepancies in the LS and FN BMD percent increases between men and women were not significant at 12 months (p = 0.53 for LS and p = 0.24 for FN, respectively; Mann–Whitney U test). In men, the mean absolute LS BMD change was 0.106 ± 0.121 g/cm2, and the mean absolute FN BMD change was 0.002 ± 0.036 g/cm2. In women, the mean absolute LS BMD change was 0.075 ± 0.059 g/cm2, and the mean absolute FN BMD change was 0.009 ± 0.037 g/cm2. The discrepancies in the LS and FN BMD absolute increases between the two groups were not significant at 12 months (p = 0.18 at LS and p = 0.31 at FN, respectively; Mann–Whitney U test).
Changes in serum PINP and uNTX concentration in response to teriparatide treatment
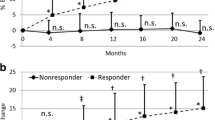
The changes in bone turnover markers are shown in Fig. 3. In men, the serum PINP level increased to 140.9 ± 98.5 μg/l at 4 months, 129.8 ± 97.4 μg/l at 8 months and 111.2 ± 85.5 μg/l at 12 months (p < 0.01 vs. baseline at 4, 8 and 12 months; Wilcoxon signed-rank test) (Fig. 3a). In women, the serum PINP level increased to 159.1 ± 118.3 μg/l at 4 months, 155.6 ± 116.8 μg/l at 8 months and 128.9 ± 88.7 μg/l at 12 months (p < 0.01 vs. baseline at 4, 8 and 12 months; Wilcoxon signed-rank test). There were no significant differences in the serum PINP levels between men and women (p = 0.29 at 4 months, p = 0.15 at 8 months, p = 0.14 at 12 months; Mann–Whitney U test). The difference in the serum PINP absolute changes between men and women were not significant (81.3 ± 87.6 μg/l in men versus 104.8 ± 110.3 μg/l in women at 4 months, p = 0.08; 70.0 ± 86.2 μg/l in men versus 101.3 ± 114.3 μg/l in women at 8 months, p = 0.12; 51.6 ± 79.9 μg/l in men versus 74.6 ± 92.8 μg/l in women at 12 months, p = 0.10; Mann–Whitney U test). Meanwhile, in men, the uNTX level increased to 87.6 ± 73.0 nmol BCE/mmol∙Cr at 4 months, 78.9 ± 64.3 nmol BCE/mmol∙Cr at 8 months and 74.3 ± 59.7 nmol BCE/mmol∙Cr at 12 months (p = 0.02 at 4 months, p = 0.15 at 8 months, p = 0.21 at 12 months; Wilcoxon signed-rank test) (Fig. 3b). In women, the uNTX level increased to 91.2 ± 70.5 nmol BCE/mmol∙Cr at 4 months, 93.4 ± 71.1 nmol BCE/mmol∙Cr at 8 months and 88.6 ± 70.1 nmol BCE/mmol∙Cr at 12 months (p < 0.01 at 4, 8 and 12 months; Wilcoxon signed-rank test). There were no significant differences in the uNTX levels between the men and women (p = 0.55 at 4 months, p = 0.09 at 8 months, p = 0.09 at 12 months; Mann–Whitney U test) (Fig. 2b). Interestingly, the absolute uNTX changes were lower in men than in women (27.9 ± 62.1 nmol BCE/mmol∙Cr in men versus 38.3 ± 63.7 nmol BCE/mmol∙Cr in women at 4 months, p = 0.06; 19.2 ± 62.5 nmol BCE/mmol∙Cr in men versus 40.5 ± 72.0 nmol BCE/mmol∙Cr in women at 8 months, p < 0.01 and 14.4 ± 53.9 nmol BCE/mmol∙Cr in men versus 35.7 ± 73.7 nmol BCE/mmol∙Cr in women at 12 months, p < 0.01; Mann–Whitney U test).
The mean changes in procollagen type I N-terminal propeptide (PINP) (a) and urinary N-telopeptide (uNTX) (b) during teriparatide treatment (*p < 0.01, **p < 0.05, n.s.; not significant versus baseline; Wilcoxon signed-rank test, n.s. (upper); not significant between men and women; Mann–Whitney U test). Data are means + SDs
Discussion
To the best of our knowledge, this is the first study to directly compare BMD and biochemical markers of bone turnover response in men and postmenopausal women. No significant differences were observed in the percent and absolute BMD increases in LS and FN between men and women by Mann–Whitney U test. But there were different responses in the longitudinal percent FN changes between men and women. The absolute increases in serum PINP were similar in both groups. However, the absolute increase in uNTX was significantly lower in men than in women. The treatment interruption rate and adverse events in men were not significantly different to those in women. Consequently, the clinical results of teriparatide therapy in men with osteoporosis were similar except for the longitudinal FN BMD increases and the absolute uNTX increase to those in women.
There have been a few reports on the efficacy of teriparatide in men. Kurland et al. reported that men treated for 12 months experienced a 9.6 % LS BMD increase but experienced no significant FN BMD increase [8]. Orwoll et al. reported that the LS BMD increased by 5.9 % and the FN BMD increased by1.5 % for a median duration of 11 months [7]. Langdahl et al. reported that men treated for 18 months experienced a 7.0 % LS BMD increase and postmenopausal women experienced a 7.8 % LS BMD increase [14]. However, none of these studies were specifically designed to directly compare the effects of teriparatide between men and women; therefore, it is unclear whether the effects of teriparatide were similar or different. In addition to these results, we believe that the magnitude of the LS BMD increase should be noted. Marcus et al. reported that similar absolute LS BMD increases were observed regardless of baseline BMD in postmenopausal women [15]. Although the relationship between baseline LS BMD and subsequent BMD increase in men is unclear, our results showed that the absolute LS BMD increase in men was similar to that in postmenopausal women regardless of baseline BMD. The noteworthy efficacy of teriparatide was demonstrated in this study because the percent LS BMD increase was dependent on the baseline LS BMD.
Regarding biochemical markers of bone turnover, Finkelstein et al. reported that serum PINP and serum NTX reached peak levels at 12 months after daily 37-μg teriparatide treatment in men [16]. Glüer et al. reported that serum PINP reached peak levels at 6 months in men [17]. This study showed that both the serum PINP level and uNTX level peaked at 4 months in men. The serum PINP level peaked at 4 months and the uNTX levels peaked at 8 months in postmenopausal women. The rapid increases in biochemical markers of bone turnover in men, which were similar to those in women, are consistent with an anabolic mode of action for teriparatide and indicate that teriparatide activated bone remodelling. The rapid and sustained gain in BMD during the 12-month teriparatide treatment implies a continuously positive coupling balance in favour of bone formation.
At the beginning of this study, we believed that the LS BMD increase was lower in men than in women because of differences in body weight. Neer et al. showed that teriparatide treatment resulted in significant dose-dependent increases in the BMD of the spine and hip [6]. However, our results showed that there was no significant difference in LS and FN BMD increases between in men and women by Mann–Whitney U test. Otherwise, there were different responses in the longitudinal percent FN changes between in men and women. The reason for different responses is unclear, but there might be a possibility of gender-specific difference or sample size effects (47 patients in men versus 334 patients in women). After referencing the differences in bone turnover markers, we speculate that there might be some differences between men and postmenopausal women in response to teriparatide. PINP response was similar in both groups, otherwise lower uNTX response was observed in men compared to women, which might produce an increased anabolic window and relatively higher BMD response in men.
This study has several limitations that should be considered when interpreting the results. First, because of the limited numbers of patients enrolled in this study, reduction in the fracture incidence could not be assessed. Second, we revealed the efficacy of teriparatide in BMD and identified biochemical markers of bone turnover, but we did not verify improvement in the microstructure of cortical and trabecular bone. Third, because of the observational and retrospective design of this study and the small number of subjects, the conclusions should be interpreted with caution. However, we believe that the results of this study are noteworthy because this is the first study to compare the efficacies of teriparatide treatment between men and women. Fourth, there were some differences in baseline characteristics between the men and women that, to some extent, depended on sex.
In conclusion, the study results in men showed that daily teriparatide treatment increased LS BMD and stimulated bone turnover. But there is a possibility of gender-specific difference in FN BMD response. Together with the previously reported results in women, the results provide evidence that teriparatide treatment is an effective option for osteoporosis in men.
References
Gullberg B, Johnell O, Kanis JA (1997) World-wide projections for hip fracture. Osteoporos Int 7:407–413
Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR (2009) Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 30:513–521
Watts NB, Adler RA, Bilezikian JP, Drake MT, Eastell R, Orwoll ES, Finkelstein JS, Endocrine Society (2012) Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 97:1802–1822
Olszynski WP, Davison KS (2008) Alendronate for the treatment of osteoporosis in men. Expert Opin Pharmacother 9:491–498
Mullender MG, Tan SD, Vico L, Alexandre C, Klein-Nulend J (2005) Differences in osteocyte density and bone histomorphometry between men and women and between healthy and osteoporotic subjects. Calcif Tissue Int 77:291–296
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez-Perez A, Kaufman JM, Clancy AD, Gaich GA (2003) The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 18:9–17
Kurland ES, Cosman F, McMahon DJ, Rosen CJ, Lindsay R, Bilezikian JP (2000) Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab 85:3069–3076
Miyauchi A, Matsumoto T, Sugimoto T, Tsujimoto M, Warner MR, Nakamura T (2010) Effects of teriparatide on bone mineral density and bone turnover markers in Japanese subjects with osteoporosis at high risk of fracture in a 24-month clinical study: 12-month, randomized, placebo-controlled, double-blind and 12-month open-label phases. Bone 47:493–502
Niimi R, Kono T, Nishihara A, Hasegawa M, Matsumine A, Nakamura T, Kono T, Sudo A (2014) An algorithm using the early changes in PINP to predict the future BMD response for patients treated with daily teriparatide. Osteoporos Int 25:337–384
Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S, Shiraki M, Kushida K, Miyamoto S, Soen S, Nishimura J, Oh-Hashi Y, Hosoi T, Gorai I, Tanaka H, Igai T, Kishimoto H, Osteoporosis Diagnostic Criteria Review Committee: Japanese Society for Bone and Mineral Research (2001) Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab 19:331–337
Niimi R, Kono T, Nishihara A, Hasegawa M, Matsumine A, Kono T, Sudo A (2014) Determinants associated with bone mineral density increase in response to daily teriparatide treatment in patients with osteoporosis. Bone 66C:26–30
Niimi R, Kono T, Nishihara A, Hasegawa M, Matsumine A, Kono T, Sudo A (2014) Cortical thickness of the femur and long-term bisphosphonate use. J Bone Miner Res. doi:10.1002/jbmr.2345
Langdahl BL, Marin F, Shane E, Dobnig H, Zanchetta JR, Maricic M, Krohn K, See K, Warner MR (2009) Teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: an analysis by gender and menopausal status. Osteoporos Int 20:2095–2104
Marcus R, Wang O, Satterwhite J, Mitlak B (2003) The skeletal response to teriparatide is largely independent of age, initial bone mineral density, and prevalent vertebral fractures in postmenopausal women with osteoporosis. J Bone Miner Res 18:18–23
Finkelstein JS, Leder BZ, Burnett SM, Wyland JJ, Lee H, de la Paz AV, Gibson K, Neer RM (2006) Effects of teriparatide, alendronate, or both on bone turnover in osteoporotic men. J Clin Endocrinol Metab 91:2882–2887
Glüer CC, Marin F, Ringe JD, Hawkins F, Möricke R, Papaioannu N, Farahmand P, Minisola S, Martínez G, Nolla JM, Niedhart C, Guañabens N, Nuti R, Martín-Mola E, Thomasius F, Kapetanos G, Peña J, Graeff C, Petto H, Sanz B, Reisinger A, Zysset PK (2013) Comparative effects of teriparatide and risedronate in glucocorticoid-induced osteoporosis in men: 18-month results of the EuroGIOPs trial. J Bone Miner Res 28:1355–1368
Acknowledgments
The authors thank Koji Fukuda, Hideki Ito, Takeshi Kato, Kana Nakanishi, Kenji Kuroda, Haruyoshi Mizuno, Yohei Takigawa, Yoshifumi Takahashi and Hiroaki Takeuchi for their diligence in preparing the clinical recordings. The authors thank Dr. Hideki Yamamoto and Dr. Takahito Saito for their cooperation to osteoporosis treatment. The authors acknowledge Akiko Mizutani and Nobuyuki Tanaka for their assistance.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niimi, R., Kono, T., Nishihara, A. et al. Analysis of daily teriparatide treatment for osteoporosis in men. Osteoporos Int 26, 1303–1309 (2015). https://doi.org/10.1007/s00198-014-3001-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-014-3001-1