Abstract
Introduction and hypothesis
Class action against Ethicon (J&J), manufacturer of transvaginal mesh devices, including mid-urethral slings (MUS), was brought to the Federal Court of Australia in 2016 by Shine Lawyers. As a result, subpoenas to all hospitals and networks were received, which overrode patient privacy concerns. This medical record search allowed a complete audit and communication with patients to offer clinical review. This enabled a review of complications, readmission and re-operation for women who underwent a MUS for stress urinary incontinence.
Methods
A cohort study of women who underwent MUS treatment for stress urinary incontinence (SUI) at a single tertiary teaching hospital between 1999 and 2017 was carried out. The main outcome measures were the rate of readmission and re-operation following MUS procedures. These include voiding dysfunction managed by sling loosening or sling division, mesh pain or exposure managed by mesh removal and reoperation for recurrent stress urinary incontinence.
Results
Between 1999 and 2017, a total of 1,462 women were identified as having a MUS; of these, 1,195 (81.7%) had full patient records available. Voiding dysfunction requiring surgical intervention with sling loosening or division was 3%, excision for mesh exposure was 2%, and partial or complete excision for pain was 1% at a median of 10 years from index surgery. The reoperation rate for recurrent stress urinary incontinence was 3%.
Conclusion(s)
This audit of all MUS procedures performed at a tertiary centre confirms an overall low rate of readmission for complications and recurrent SUI surgery; this justifies its continued availability with appropriate informed consent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stress urinary incontinence (SUI) has a reported prevalence up to 35% and can significantly affect a woman’s quality of life [1, 2]. Its management involves pelvic floor muscle training and surgery. The mid-urethral sling (MUS) was introduced to Australia in 1998; the first on the market was the TVT™ or tension-free tape by Johnson and Johnson (J & J), which replaced colposuspension as the most common surgical treatment option by 2004 [3]. A Cochrane review and a large meta-analysis showed superior or similar objective and subjective cure rates of MUS compared with colposuspension and pubovaginal sling [4, 5]. In the early 2000s the same device manufacturers also marketed transvaginal mesh devices for pelvic organ prolapse that utilised far larger amounts of mesh, often with anchors. The Food and Drug Administration (FDA) issued a warning regarding transvaginal mesh in 2008 and an update in 2011 with reports of increasing adverse events, especially associated with transvaginal prolapse mesh devices [6]. However, owing to the far greater number of mesh sling surgeries, there were greater numbers of cases brought against mesh sling versus mesh prolapse surgery [7]. Litigation in the USA followed and a class action against Ethicon (J&J), manufacturer of nine implantable transvaginal mesh devices, including MUS, was brought to the Federal Court of Australia in 2016 by Shine Lawyers [8].
A senate enquiry into transvaginal mesh was held in Australia in 2016 and a Parliamentary enquiry in the UK in 2018 [9]. In both, the main priority was to give a voice to mesh-affected women. Clinicians, however, had the sense that MUS for stress urinary incontinence were effective, with a relatively low rate of complications and adverse events [10].
To identify members of the class, health funds and health networks were subpoenaed to identify all women who had undergone any of the nine listed J&J devices from the time of their introduction in 1998 until July 2017.
Direct contact with patients or “group members”—those identified as having a claim in a class action—is often required, as class actions in Australia operate on an “opt-out” basis. The obligation imposed on health services by the subpoena(s) overrode the standard principles of confidentiality and privacy, which normally apply to a patient’s medical records. In this instance, subpoenaed documents—as provided to the Court by health services—were subsequently provided to Shine Lawyers, by the Federal Court. Although identifying and contacting group members is often a necessary step in class actions, patients were largely unaware that their health information could be used for such a purpose (personal comment: Peter Ryan and Emma Pelham).
This study is aimed at reviewing complications requiring readmission and re-operation of women who underwent a MUS procedure using J&J, Boston Scientific (BS) or American Medical Systems (AMS) devices at a single tertiary centre in Melbourne, Australia, from the time of their introduction until July 2017.
Materials and methods
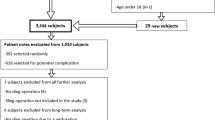
In 2017, Australian public hospitals and private health insurance providers received a subpoena to provide patient details to the Federal Court and subsequently Shine Lawyers of women who had received one of nine J&J transvaginal implants. Exhaustive searches of all relevant item number codes, electronic and paper records generated a database of women who had devices from J & J and other manufacturers. This study utilized the unique identifying code 35599 for MUS. All paper-based records prior to 2010 at this institution were archived in a storage facility. The class action pertaining to J&J required a manual review of all records, electronic and paper; the latter were made available from storage. AMS and BS were not subject to a class action and only electronic records (from 2010) were available for review. Women were informed by the health network about the expected letter from Shine Lawyers and offered the opportunity to attend for clinical review. Local ethics committee approval (QA/73022/MonH-2021-247433(v1)) was obtained and the database was created using Medicare Benefits Schedule coding number (35599). The type of sling, demographics, readmission rates and outpatient follow-up were included.
The slings were categorised as per the manufacturing company and the route of insertion: retropubic (RP), transobturator (TO) and single-incision slings (SIS). Categorical variables were expressed as number and percentage and compared between the groups using the Chi-squared test or Fisher’s exact test, as appropriate. Demographic continuous variables were expressed as median and interquartile range (IQR) and follow-up time was expressed as median and range.
Results
Between 1999 and 2017, a total of 1462 women were identified as having a MUS procedures; of these, 1,195 (81.7%) full patient records including all the J& J slings were available; the remainder had been archived (Table 1).
Johnson & Johnson
From 1999 onwards, 858 women had undergone 869 J&J MUS procedures; the majority (93%) were performed by Urogynaecology or Gynaecology consultants, fellows and/or trainees and 7% by the Urology unit. TVT and TVT Exact (both retropubic) made up 75%, TVT-O and TVT-Abbrevo (transobturator) the remainder. The median follow-up time from index surgery to chart review was 16 (IQR 4–22) years (Table 2)..
Boston Scientific and American Medical Systems
Between January 2003 and December 2017, a total of 130 Boston Scientific (BS) and 360 American Medical Systems (AMS) MUS procedures were performed; 120 BS and 206 AMS sling procedures were able to be audited.
The BS slings included Advantage and Advantage Fit, both RP devices (78%), SIS, Solyx (18%) and TO sling, Obtryx (4%). The AMS slings included the TO sling, Monarc (45%), the SIS, Miniarc (31%), and the retropubic slings (24%) Retroarc and rarely SPARC. The median time from index surgery to chart review for BS and AMS was 7 (IQR 4–15) and 9 (IQR 5–17) years respectively. The parent company of AMS made a commercial decision to withdraw from Women’s Health and all AMS products ceased to be available worldwide in 2016. BS withdrew all its women’s health mesh products only from the Australian and New Zealand markets in 2021.
Overall, a total of 790 RP (66%), 314 TO (26%) and 91 SIS (8%) procedures were performed. The median age at surgery was 56 (IQR 36–76) years. The women in the RP group were significantly older than in the TO or SIS groups (p≤0.001). Half of the incontinence procedures were performed together with prolapse surgery. The overall median length of stay for sling only was one night (afternoon operating list), and two nights when concomitant procedures were performed. The overall median length of clinic follow-up was 11 (IQR 27–49) months, and median length of chart review was 114 (IQR 35–193) months.
Readmission
Short-term voiding dysfunction
It was hospital policy to discharge routinely with an indwelling catheter if unable to void after 48 h and readmit 1 week later for a voiding trial. This occurred in 6.6% of women (79 out of 1,195) more often when concomitant prolapse surgery was performed versus sling alone (10.3% vs 2.9%, p<0.001). There was no significant difference in short-term voiding dysfunction between routes of sling (RP 7.6% vs TO 5.5% vs SIS 5.5%, p=0.157).
Sling loosening
Sling loosening within 30 days was performed in a total of 21 (1.8%) women. This was also more common in women who had a concomitant procedure (2.8% vs 0.7%, p=0.004). There was no significant difference between various routes of MUS (RP 2.3% vs TO 1.0% vs SIS 0%, p=0.195).
Sling division
Overall, the rate of sling division was low at 1.6% (19 out of 1,195) and this was also more common after a concomitant procedure than after a sling only procedure (2.7% vs 0.5%, p=0.003), There was no difference between the route of sling used (RP 1.8% vs TO 1.6%, SIS 0%, p=0.693), although it is noted that none of the women with an SIS required loosening or division.
Infection
Infection—either of the surgical site or of the urinary tract requiring admission—occurred in 13 patients (1.1%), and was higher in women who had undergone concomitant surgery (1.5% vs 0.7%, p=0.168).
Repeat cystoscopy or intervention
Cystourethroscopy was universally performed at the time of sling insertion. At any time, following MUS, cystoscopy occurred in 2% of women (n=24); of these, 12 were for urinary urgency (1%), 8 for voiding dysfunction (0.7%) and 4 for pelvic pain (0.3%). Interventions for pain were steroid injection along the sling path, bilateral pudendal nerve block and Botulinum toxin injection to the pelvic floor musculature in 1 (0.3%) patient each.
Other complications
A total of 9 (0.8%) patients required blood transfusion, which was more common in women with concomitant surgery, but not statistically significantly different (1.3% vs 0.2%, p=0.16). Two of these women required intensive care unit (ICU) admission; 1 had a sling alone. There was 1 patient death within 30 days unrelated to the sling procedure.
Mesh-specific complications
The overall repeat surgery rate for management of mesh complications, either pain and/or exposure, was 3.1% at a median of 9.5 years from index surgery. Surgical management for vaginal mesh exposure was 1.9%; of these, 3 patients had bladder/urethral erosion (0.3%). The rates were similar between different types of slings (TO 2.2% vs RP 1.8% vs SI 2.2%, p=0.733). The rate of partial or complete mesh excision for pain was 1.2% (14 out of 1,195) and was not significantly different between slings (SI 2.2% vs TO 1.6% vs RP 0.9%, p=0.244).
Recurrent stress urinary incontinence surgery
The rate of recurrent stress urinary incontinence surgery was 2.6% (31 out of 1195). There was a significant difference between types of slings (p<0.001) with the highest rate after SIS procedures (12%), followed by TO (3.2%) and RP (1.3%; (RP, TO p=0.043; RP, SIS p<0.01; TO, SIS p=0.002).
A repeat MUS surgery was performed in 77% (24 out of 31). Of these, 8 each underwent RP sling, TO sling and SIS. In a further 7 women, 4 (13%) underwent urethral bulking, and 3 (10%) pubovaginal sling.
Dedicated mesh support service
Women were offered the opportunity to attend a dedicated mesh support service for clinical review consisting of a validated questionnaire, clinical examination, uroflow measurement with post-void residual, and translabial ultrasound of the sling and urodynamic assessment if appropriate. Mesh complications were managed by utilising Australian and international guidelines [11, 12]. Eighty-one women who had received J&J MUS contacted the dedicated mesh support service after receiving a letter from the hospital and subsequently from Shine Lawyers, and 55 accepted the offer of a face-to-face review. Of these, 5 women were identified as having a mesh complication. Two (0.2%) have gone on to have total mesh removal for pain, 2 women with exposure declined surgical management, and 1 has chosen to postpone further review.
Three women reported recurrent SUI symptoms: 1 underwent urethral bulking, 1 had a pessary, and the other declined further management for symptoms that were not bothersome. Seventeen patients reported de novo or persistent overactive bladder symptoms and were medically managed.
Discussion
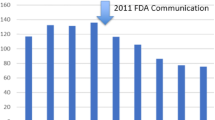
Medicare statistics between 1994 and 2009 showed that the MUS became the most common stress incontinence procedure by 2004 and the overall number of incontinence procedures increased over that time, especially in the older age group. The number of MUS and incontinence procedures overall has reduced since 2010, with a marked decrease after 2016 [3, 13]. The implication is that some women are not seeking treatment for their condition because of adverse mesh media reports (Fig. 1).
Stress urinary incontinence trends in Australia between 2008 and 2021. SUI stress urinary incontinence, MUS mid-urethral sling, BCS Burch colposuspension, UBA urethral bulking agent. Information and data obtained from Services Australia do not include services provided by hospital doctors to public patients
As a result of the Federal Court subpoena to the health network, an exhaustive paper and electronic search identified all women who had undergone a MUS. A comprehensive audit of all J&J, AMS and BS MUS procedures since their introduction was performed at a large tertiary health network. An overall low rate of short- and long-term complications across all MUS was found.
Short-term voiding dysfunction requiring catheterisation less than a week post-MUS surgery is common and generally spontaneously resolves. Our finding of a higher rate when concomitant surgery is performed is similar to others [14, 15]. Readmission for sling loosening for voiding dysfunction occurred in 1.8% and was more common in RP slings. The rate of sling division for voiding dysfunction was 1.6%; an earlier study reported 1.9% for TVT only up to 2 years [16]. This compares favourably with the 7% pubovaginal sling revision rate reported in the SISTER study [17].
Our findings of other complications such as urinary tract infection and blood transfusion are overall low and similar to the findings of a systematic review [5]. These complications are higher for the retropubic sling and may also be due to the concomitant prolapse surgery. The overall rate of reoperation for mesh exposure and/or pain was 3.1% at a median of 9.5 years’ follow-up, which is similar to a large cohort study (3.3% at 9 years) [18]. Indeed, the range of follow-up for all J&J slings was between 4 and 22 years, with a median of 15 years for the original TVT sling.
A limitation of this study is that follow-up was only captured through the health network, meaning that re-presentations or complications that were managed privately (this would be very unusual) or through another public health network (may have occurred if the woman had changed address) were not captured in this database. The Department of Health has funded the Australasian Pelvic Floor Procedure Registry, similar to the joint and breast implant registry, which currently captures mesh sling and bulking prostheses implanted by participating volunteer institutions.
This study was performed at a teaching tertiary health network; multiple consultants and very often fellows and trainees under supervision were the primary operators; there was no reliable information regarding the primary surgeon and their caseload. The learning curve of primary operators was therefore not captured; this is a known risk factor for operative and possibly post-operative complications [19].
In 2019, The Federal Court of Australia found that nine J&J devices including MUS were “defective” [20]. A revised Instruction for use and consumer information for MUS was issued by J&J in 2019 and included the wording of Judge Katzmann (Supplementary document). J&J MUS have been reclassified as class III and approved for use by the Therapeutic Goods Administration (TGA) [21]. Prior to obtaining consent for the J&J MUS, a patient is provided with a nine-page consumer information booklet, which includes the above wording. Many women will seek an alternative, either less effective (bulking) or somewhat more invasive (open or laparoscopic suture or fascial), surgical procedure. In performing this tertiary hospital audit, the aim was to provide a clinical perspective with useful estimates for patient counselling in terms of the risk of complications in contrast to the long list of potential adverse outcomes in the patient consumer information.
A second subpoena was issued in October 2021, which related to all AMS and BS transvaginal mesh devices, in addition to the nine J&J devices from July 2017 to 2021. The subpoenas again resulted in direct patient contact by Shine Lawyers via hospital records and medical insurance providers. The letter stated that the patient had been implanted with a defective product with an offer to register for the class actions and a 50-page questionnaire regarding possible symptoms. Both subpoenas and the Shine letter that followed have caused significant anxiety and concern among women.
All other manufacturers have withdrawn from Australia and New Zealand owing to legal costs and a relatively small commercial market. This also has had repercussions affecting the sacral colpopexy procedure for severe pelvic organ prolapse, which involves the abdominal placement of mesh and is the most effective procedure for this condition. There is no current TGA-approved prosthesis for this in Australia or New Zealand.
In conclusion, the overall readmission and complication rates following MUS surgery are low and numerically fewer than those attributable in the literature to the pubovaginal sling. The Senate enquiry and class action have led to a decline in women seeking and undergoing any surgical treatment for SUI and MUS. This study, which includes up to 22 years of clinical review, is as complete as possible with the available records and local data from a teaching tertiary hospital chart review. This is intended to provide “real-life” information to help counsel women regarding MUS as they consider their options for surgery for stress urinary incontinence.
References
Luber KM. The definition, prevalence, and risk factors for stress urinary incontinence. Rev Urol. 2004;6(Suppl 3):S3–9.
Margalith I, Gillon G, Gordon D. Urinary incontinence in women under 65: quality of life, stress related to incontinence and patterns of seeking health care. Qual Life Res. 2004;13(8):1381–90. https://doi.org/10.1023/B:QURE.0000040794.77438.cf.
Lee J, Dwyer PL. Age-related trends in female stress urinary incontinence surgery in Australia—Medicare data for 1994–2009. Aust N Z J Obstet Gynaecol. 2010;50(6):543–9. https://doi.org/10.1111/j.1479-828X.2010.01217.x.
Ford AA, Rogerson L, Cody JD, Aluko P, Ogah JA. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2017;7(7):CD006375. https://doi.org/10.1002/14651858.CD006375.pub4.
Fusco F, Abdel-Fattah M, Chapple CR, et al. Updated systematic review and meta-analysis of the comparative data on colposuspensions, pubovaginal slings, and midurethral tapes in the surgical treatment of female stress urinary incontinence. Eur Urol. 2017;72(4):567–91. https://doi.org/10.1016/j.eururo.2017.04.026.
FDA Public Health Notification: Serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. http://wayback.archiveit.org/7993/20170111190506/http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm.
Souders CP, Eilber KS, McClelland L, et al. The truth behind transvaginal mesh litigation: devices, timelines, and provider characteristics. Female Pelvic Med Reconstr Surg. 2018;24(1):21–5. https://doi.org/10.1097/SPV.0000000000000433.
Dyer O. Johnson and Johnson faces lawsuit over vaginal mesh devices. BMJ. 2016;353:i3045. https://doi.org/10.1136/bmj.i3045.
Dolan L. The controversy of polypropylene mesh. Obstet Gynaecol Reprod Med. 2018;28(10):329–31.
Thompson C, Faunce T. Australian Senate Committee report on transvaginal mesh devices. J Law Med. 2018;25(4):934–43.
Australian Commission on Safety and Quality in Health Care. Treatment options for complications of transvaginal mesh (including options for mesh removal). https://www.safetyandquality.gov.au/publications-and-resources/resource-library/treatment-options-complications-transvaginal-mesh-including-options-mesh-removal.
Cundiff GW, Quinlan DJ, van Rensburg JA, Slack M. Foundation for an evidence-informed algorithm for treating pelvic floor mesh complications: a review. BJOG. 2018;125(8):1026–37. https://doi.org/10.1111/1471-0528.15148.
Mathieson R, Kippen R, Manning T, Brennan J. Stress urinary incontinence in the mesh complication era: current Australian trends. BJU Int. 2021;128(1):95–102. https://doi.org/10.1111/bju.15302.
Norton PA, Nager CW, Chai TC, et al. Risk factors for incomplete bladder emptying after midurethral sling. Urology. 2013;82(5):1038–41. https://doi.org/10.1016/j.urology.2013.05.060.
Chung SM, Moon YJ, Jeon MJ, Kim SK, Bai SW. Risk factors associated with voiding dysfunction after anti-incontinence surgery. Int Urogynecol J. 2010;21(12):1505–9. https://doi.org/10.1007/s00192-010-1229-7.
Rardin CR, Rosenblatt PL, Kohli N, Miklos JR, Heit M, Lucente VR. Release of tension-free vaginal tape for the treatment of refractory postoperative voiding dysfunction. Obstet Gynecol. 2002;100(5 Pt 1):898–902. https://doi.org/10.1016/s0029-7844(02)02279-2.
Albo ME, Richter HE, Brubaker L, et al. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007;356(21):2143–55. https://doi.org/10.1056/NEJMoa070416.
Gurol-Urganci I, Geary RS, Mamza JB, et al. Long-term rate of mesh sling removal following midurethral mesh sling insertion among women with stress urinary incontinence. JAMA. 2018;320(16):1659–69. https://doi.org/10.1001/jama.2018.14997.
Hilton P, Rose K. The "learning curve" for retropubic mid-urethral sling procedures: a retrospective cohort study. Int Urogynecol J. 2016;27(4):565–70. https://doi.org/10.1007/s00192-015-2853-z.
Dyer C. Johnson & Johnson loses class action over vaginal mesh in Australia. BMJ. 2019;367:l6659. https://doi.org/10.1136/bmj.l6659.
Therapeutic Goods Administration (TGA). 2022. Information for medical practitioners on up-classification of surgical mesh devices. Available at: Accessed 23 Feb 2022.
Acknowledgements
The authors would like to thank Prof. Beverley Vollenhoven (Head of Gynaecology, Monash Health) Peter Ryan (Chief Legal Officer, Monash Health), Emma Pelham-Thorman (legal department, Monash Health) and Hayley Capiron (Medical Information Technology) for their involvement in the review.
Authors’ contributions
M. Kulkarni: study design, database, manuscript preparation; Y. Liu: database, manuscript preparation; M. Silagy: database, manuscript preparation; D. Rolnik: statistical analysis, manuscript preparation; A. Rosamilia: study design, database, manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 14 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kulkarni, M., Liu, Y., Silagy, M. et al. The transvaginal mesh class action: a tertiary teaching hospital experience of all mid-urethral sling procedures performed between 1999 and 2017. Int Urogynecol J 34, 2573–2580 (2023). https://doi.org/10.1007/s00192-023-05575-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-023-05575-5