Abstract
Introduction and hypothesis
Endovaginal ultrasound has long been hypothesized to have a significant effect on locations of what it visualizes. However, little work has directly quantified its effect. This study aimed to quantify it.
Methods
This cross-sectional study consisted of 20 healthy asymptomatic volunteers who underwent both endovaginal ultrasound and MRI. The urethra, vagina, rectum, pelvic floor, and pubic bone were segmented in both ultrasound and MRI using 3DSlicer. Then, using 3DSlicer’s transform tool the volumes were rigidly aligned based on the posterior curvature of the pubic bone. The organs were then split into thirds along their long axis to compare their distal, middle, and proximal sections. Using Houdini, we compared the location of the centroid of each of the urethra, vagina, and rectum and the surface-to-surface difference of the urethra and rectum. The anterior curvature of the pelvic floor was also compared. Normality of all variables was assessed by Shapiro–Wilk test.
Results
The largest amount of surface-to-surface distance was observed in the proximal region for the urethra and rectum. Across all three organs, the majority of the deviation was in the anterior direction for geometries obtained from ultrasound versus those from MRI. For each subject, the trace defining the midline of the levator plate was more anterior for ultrasound compared to MRI.
Conclusions
While it has often been assumed that placing a probe in the vagina probably distorts the anatomy, this study quantified the distortion and displacement of the pelvic viscera. This allows for better interpretation of clinical and research findings based on this modality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pelvic floor disorders such as stress urinary incontinence (SUI) and pelvic organ prolapse (POP) affect many women in the United States, with up to 24% of all women reporting at least one pelvic floor disorder [1]. These two disorders (SUI and POP) alone cost the healthcare system up to 12.4 billion and 412 million dollars annually respectively [2, 3]. The rise in prevalence of these disorders has made effective diagnosis and treatment imperative [4]. Diagnosis of pelvic floor disorders is often based on symptom evaluation and a pelvic exam. However, in recent years medical imaging procedures including MRI and ultrasound are increasing in popularity amongst clinicians as tools used to assess anatomy and function of the pelvic floor. Each modality comes with its own set of pros and cons, but the major differences between them are monetary cost, patient comfort, and accuracy [5].
Pelvic floor MRI provides a large field of view relative to ultrasound allowing for visualization of the entire pelvic floor, and it can be used to aid in the diagnosis of pelvic floor disorders [5]. In contrast, translabial ultrasound is cheaper, readily available and can visualize most of the pelvic region. However, its field of view is more limited and may not capture the entire levator muscle group. In addition, it may distort the anatomy of distal structures based on pressure exerted on the probe [5, 6]. This led us to explore the use of endovaginal ultrasound, which affords improved visualization of the levator muscle group while maintaining low cost and high availability. Studies comparing the efficacy of MRI and endovaginal ultrasound for diagnosing cancers and other disorders have been published [7,8,9]. Unfortunately, the existing quantitative data focuses on the increased spatial resolution of endovaginal ultrasound and does not compare the effects of insertion of the endovaginal probe on the pelvic organs [10].
Dynamic and static MRI and ultrasound have been used to define the range of motion and shape of the pelvic floor musculature and organs in both symptomatic and asymptomatic individuals [11,12,13,14]. In recent years, ultrasound has become a more commonly used tool in the diagnosis of pelvic floor disorders [6, 15,16,17,18]. The majority of research has focused on comparing the diagnostic efficacy of endovaginal ultrasound and MRI for endometriosis and endometrial cancer [19,20,21]. These studies compare the two modalities’ capability to diagnose specific diseases and not the effect that the insertion of the probe has on the pelvic organs themselves. The two most used ultrasound techniques are translabial/transperineal and endovaginal [6, 22]. A common concern concerning endovaginal ultrasound is the effect of the vaginal probe on the shape and position of the pelvic floor and pelvic viscera. The thought is that this effect can potentially decrease the reliability of this modality. However, there is little quantitative data to justify this claim. A better understanding of the measurements for which this modality is reliable will improve the research and clinical interpretations of the data obtained from endovaginal pelvic floor ultrasound.
The three organs (urethra, vagina, and rectum) that are inside the levator bowl are the most likely to be affected by the insertion of the probe. Additionally, the probe may also affect the levator muscles themselves. Therefore, this study aimed to quantify the difference in the position of urethra, vagina, rectum, and levator muscles between MRI and endovaginal ultrasound. As a secondary comparison, this study compared the surface-to-surface displacement difference between the urethra and rectum between the two modalities. We hypothesized that the majority of the variation in the location of the organs in endovaginal ultrasound would be in the anterior/posterior direction due to the insertion of the probe.
Methods
This is a cross-sectional study including 20 asymptomatic, nulliparous volunteers at Northshore University HealthSystem between the months of September and December 2020. Subjects were all healthcare workers recruited within our institution. Institutional review board approval was received for this study (EH20-133).
All subjects underwent a comprehensive evaluation including a thorough history, symptom assessment including the Pelvic Floor Distress inventory (PFDI-20) questionnaire, pelvic exam including Pelvic Organ Prolapse Quantification (POP-Q) staging, and dynamic MR defecography. Subjects that had a score of zero on the PFDI-20 questionnaire and stage 0 on the POP-Q exam were included in this study. Once included, all women underwent an MRI and endovaginal ultrasound. We chose to conduct this study on healthy asymptomatic individuals in an attempt to limit any potential confounding variables that could arise due to pre-existing medical conditions (i.e., POP and SUI).
MRI technique
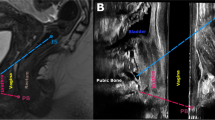
All subjects were imaged in the supine position using a closed-configuration 1.5 T magnet and a Synergy body phased-array coil. No bowel preparation was conducted. The subjects were instructed to empty their bladder 3 h before the MRI to yield a moderately full bladder. Static multiplanar images of the pelvis were acquired for anatomic evaluation using a 4-mm slice thickness with a 0-mm gap, for sagittal and axial T2-weighted sequences (echo time, 105 ms: repetition time, 3000 ms). The pelvic floor was visualized in three planes (axial, coronal, sagittal, T1 and T2).
Endovaginal ultrasound technique
All ultrasound studies were performed in the office setting using a BK Medical 3000 (Peabody, MA) system with X14L4 12 MHz transducers. Patients were scanned in the dorsal lithotomy position with hips flexed and abducted. Patients were asked to present after voiding. Beyond that, no further preparation was required, and no rectal or vaginal contrast was used. The ultrasound probe was inserted endovaginally and used to collect a radial volume surrounding the vagina. To avoid excessive pressure on surrounding structures that could distort the anatomy, the probe was inserted into the vagina in a neutral position minimizing pressure on vaginal walls. Ultrasound volumes were digitally stored for further analysis.
Image overlay and comparison
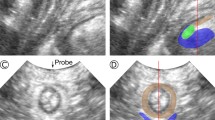
Both image volumes were loaded into 3DSlicer (v. 4.11) and the urethra, vagina, rectum, pubic bone, and levator musculature were segmented on both MRI and ultrasound [23]. Due to the distortion of the vagina caused by the ultrasound probe and its proximity to the probe, the vagina could not be easily segmented in ultrasound. Instead, the cylindrical interior edge of the image volume assumed to represent the surface of the probe was segmented on ultrasound in a position that represented the midline of the vagina. Thus, the midline of this cylinder was used to compare with the vaginal midline obtained from MRI for each subject. For ease of communication, these will both be referred to as the “vagina” throughout this manuscript. Using 3DSlicer’s transform tool, the ultrasound volume was translated and rotated until the curvature of the posterior pubic bone from both volumes were aligned [23]. The pubic bone was chosen as the reference landmark because it was easily identifiable in both modalities and would not be displaced by the insertion of the probe (Figs. 1 and 2). In addition, it has a 3D shape such that image volumes can be registered accurately in the sagittal, coronal, and axial planes. The MRI image volume was cropped such that any portion of a segmented organ that was outside of the ultrasound’s field of view was removed. This was done so that only regions that were observable in both modalities were compared. Next, each shape was individually imported into Blender (v. 3.0.1) where the volume of the urethra and rectum were measured using Blender’s 3D printing toolkit (note: this value matches the corresponding value provided from 3DSlicer) [23]. Again, as stated above, the volume of the vagina was not included here because only the midlines were compared.
Midsagittal representation of the ultrasound volume overlayed on the MRI volume. Organs of interest are labeled using text labels, and the posterior curve of the pubic bone was added to the image to make it easier to intercept. Note the representation of the pubic bone is actually the pubic symphysis because we are in the midsagittal plane
Shows the alignment of the organs between the two modalities MRI (solid) and ultrasound (wireframe) from two views; A sagittal and B frontal. The two modalities were aligned by the pubic bone (green) by attempting to match the curvature of the pubic bone as closely as possible. In the sagittal view, the pubic bone has been trimmed to better show the urethra (yellow) from the side. The segmentation of the rectum on ultrasound was colored red
With the modalities co-registered, the segmented geometries of the urethra and rectum were broken into thirds along the longitudinal axis of the organ. This allowed us to compare the proximal, middle, and distal components of each organ independently. For each of these regions, changes in anatomical position were measured in two ways. The first was comparing the surface-to-surface distance between the organs (proximal, middle, distal). This was used to elucidate how the probe distorted the 3D geometry of the pelvic organs. The second method was the displacement between the centroids of each of the three organ segments (proximal, middle, and distal) along the three anatomical axes. In addition, the magnitude of the vector between these centroids was calculated and compared. This measurement provided information on how the organs were displaced. It should be noted that the same methods were used when comparing the vaginal midlines; however, the analysis of the surface-to-surface distances were excluded because the 3D geometry was not obtained for ultrasound.
Because of the complicated, non-cylindrical shape of the levator muscles and because the thickness of the levator muscles was not always discernable on ultrasound, a different approach was employed to quantify distortions and displacements compared to the aforementioned organs. First, a line consisting of 100 vertices that was oriented in the midsagittal plane was projected posteriorly onto the inner surface of the levator muscles for both the MRI and ultrasound geometries. The resulting curve provided a trace of the inner posterior surface of the levator muscles in the midsagittal plane. Like the other organs, the geometry was split into thirds along the proximal to distal direction. Using the projected trace in the proximal, middle, and distal regions, the distances between corresponding points were used to quantified between MRI and ultrasound. This measurement is essentially providing a quantification of the difference in position of the levator plate at three levels.
Normality of all variables was checked using a Shapiro–Wilk test for normality due to this test’s robustness to small sample sizes. Volume of the urethra and rectum were also compared. All statistical analyses were conducted using SPSS (IBM SPSS Statistics for Macintosh, Version 28.0. IBM Corp.,Armonk, NY, USA).
Results
Twenty women (age: 29.7 ± 8.2 years, body mass index: 24.3 ± 4.5 kg/m2) were initially recruited for this study. One volunteer became pregnant during the study and was excluded. Both measurement modalities captured the full 3D urethral and rectal geometry as observed within the image volumes bounded by those defined by ultrasound. The surface-to-surface differences and centroidal displacements are summarized in Tables 1 and 2 respectively. The measured volume of the urethra was not statistically different between modalities [4.5 ± 1.3 cm3 (MRI) vs 4.3 ± 1.2 cm3 (endovaginal ultrasound), p = 0.667]; however, the volume of the rectum was significantly different between modalities [13.5 ± 4.8 cm3 (MRI) vs 6.6 ± 1.8 cm3 (endovaginal ultrasound), p < 0.001].
Surface-to-surface distance
The largest amount of surface-to-surface distance was observed in the proximal region for both organs. The maximum surface-to-surface deviation of the urethra was approximately 33% higher in the proximal region when compared to the middle and distal segment. The deviation of the surface of the rectum was 20% higher on the proximal surface compared to the middle and distal segment. On average, the surface of the urethra deviated 3.2 mm while the surface of the rectum deviated 5.5 mm for ultrasound relative to MRI. Figure 3 shows an example of the surface comparisons, with a colormap illustrating the deviations, and Table 1 summarizes the surface-to-surface comparison.
Colormap illustrating the differences between the volumes segmented via MRI (solid) and ultrasound (wireframe). The colormap on the solid MRI shape represents the distance between the two surfaces (red = further distance, green = smaller distance). Frames are as follows in the format organ, view. A Urethra, sagittal; B urethra, front; C Rectum, sagittal; D Rectum, front; E Levators, sagittal; F Levators, front
Centroid distance
Across all three organs, the majority of the deviation was in the anterior direction for geometries obtained from ultrasound versus those from MRI. Overall, the rectum deviated by 25% more than the urethra. On average, along the length of the organs the centers of the urethra, vagina, and rectum shifted 2.6 mm anteriorly, 2.0 mm posteriorly, and 2.3 mm anteriorly respectively (Table 2). For the urethra, its distal segment experienced the largest magnitude of anterior/posterior deviation (3.6 ± 3.7 mm) followed by the proximal segment (2.1 ± 2.8 mm). The vagina was displaced posteriorly along the anterior/posterior axis (0.0 ± 6.1, millimeters, distal; 3.5 ± 5.2 mm posterior, middle; and 2.4 ± 5.1 mm posterior, proximal). The proximal segment of the rectum was displaced the most of the three rectal segments (3.8 ± 5.3 mm, anterior) followed by the distal segment (2.0 ± 3.6 mm, anterior). Figure 2 shows an overlay of the surfaces of the shape from both MRI and ultrasound.
Pelvic floor comparison
For each subject, the trace defining the midline of the levator plate in the mid-sagittal plane was more anterior for ultrasound compared to MRI. On average, across the entire length of the trace, the ultrasound segmentation was 8.0 ± 2.9 mm anterior. The most deviation was observed in the distal segment of the pelvic floor (10.8 ± 6.1 mm). On average, the middle and proximal segments were shifted anteriorly by approximately the same amount (6.7 ± 3.6, middle; 6.7 ± 4.4, proximal). Differences are summarized in Table 3.
Discussion
While it has often been assumed that placing a probe in the vagina probably distorts the anatomy, this study quantified the distortion and displacement of the pelvic viscera and levator muscles in nulliparous, asymptomatic controls. It showed that endovaginal ultrasound was associated with a slight shift of the urethra and rectum (~ 2-3 mm) toward the pubic symphysis. The shift was more prominent for the distal portion of urethra and proximal portion of rectum. The fact that the rectum shifted anteriorly was unexpected, since the volume of the vagina that is occupied by the probe would be more likely to result in a posterior shift. However, it was also noted that the levator ani muscles were closer to pubic bone in the midsagittal plane, by ~ 8 mm, when visualized via ultrasound compared to MRI. Thus, there appears to be two contributing factors to the distortions and displacements of the pelvic viscera in this study: 1) the probe in the vagina, and 2) a change in muscle tone between the two modalities.
Understanding the effect of the endovaginal probe on pelvic viscera displacement is a point that must be considered when utilizing endovaginal ultrasound. The current study showed that probe effect is consistent with a minimal anterior (toward pubic bone) displacement of pelvic organs that enhances the reliability of this cheap, practical, and office-based imaging modality compared to pelvic MRI.
The volumes of the urethra and rectum were intended to be used as a check to ensure the same portions of the organs were captured by both modalities. Indeed, the volumes were similar for the urethra. However, the volume of the rectum was significantly larger on MRI even after exclusion of regions outside of the ultrasound’s FOV. There could be multiple reasons for this finding. One of the most likely is that the rectum must store contents while the urethra does not, making the volume of the rectum more variable than the urethra. Another potential explanation is that some of the rectal tissue was pushed superiorly, above the ultrasound’s field of view. This limitation likely did not affect the urethra as it is a smaller organ and has the weight of the bladder resisting the upward force of the probe and potential contraction of the levators.
The anterior motion that we observed is consistent with prior literature, that has shown a trend of measurements of hiatal area on ultrasound being smaller than the same measures taken on MRI in asymptomatic, nulliparous volunteers [24,25,26]. However, there are potentially confounding variables that are related to patient position between the two modalities. While our imaging positions were similar (dorsal lithotomy and supine), there are studies that have shown a significant difference in measures of the bladder neck taken on ultrasound between the two positions [27]. These differences were reported to be largest at rest, which is the state in which all of the analyses for this study were conducted [27, 28].
A major limitation of this study was that the vaginal geometry could not be segmented reliably via ultrasound. Rather, a proxy (i.e., the probe’s midline) was used for comparisons to measures in MRI, which were based on the vaginal geometry. This probably explains why the vagina in ultrasound was observed to shift posteriorly when all other organs and muscle were observed to do the opposite. Thus, it remains unclear if the probe’s midline is a good proxy for the vagina or not. Another major limitation of this study was its small sample size (n = 19). Additionally, this study only included patients who were asymptomatic and nulliparous. Future studies may look to include symptomatic patients with stress urinary incontinence and/or pelvic organ prolapse, as both of these conditions have been associated with changes in pelvic organ support. A final potential source of bias in this study is volume alignment as this may directly affect the outcome measures of the study. However, because all of the alignment was based on the curvature of pubic bone, a structure that should not be different regardless of patient position, this potential bias was minimized. Future work should aim to include attempts to correlate measures that can be conducted on ultrasound with the amount of organ displacement seen in this study. Once this is achieved, more specific clinical implications may be determined.
In conclusion, we quantified the effect of the insertion of the endovaginal ultrasound probe on the pelvic organs by comparing the same organs on MRI. This study directly quantified this difference using methodologies that to our knowledge have not been used before. These findings enable for a better understanding of how various pelvic floor imaging techniques relate to one another. We believe this to be a promising first step that enables us to better understand the potential of endovaginal ultrasound, allowing for better interpretation of clinical and research findings based on this modality.
Abbreviations
- MRI:
-
Magnetic resonance imaging
- POP:
-
Pelvic organ prolapse
- SUI:
-
Stress urinary incontinence
References:
Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA . 2008;300(11):1311–6.
Wilson L, Brown JS, Shind GP, Luca KO, Subak LL. Annual direct cost of urinary incontinence. Obstet Gynecol. 2001;98(3):398–406.
Sung VW, Washington B, Raker CA. Costs of ambulatory care related to female pelvic floor disorders in the United States. Am J Obstet Gynecol. 2010;202(5):483.e1-483.e4.
Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in U.S. women: 2010 to 2050. Obstet Gynecol. 2009;114(6):1278–83.
Ahmad AN, Hainsworth A, Williams AB, Schizas AMP. A review of functional pelvic floor imaging modalities and their effectiveness. Clin Imaging. 2015;39:559–65.
Dietz HP. Pelvic floor ultrasound: a review. Am J Obstet Gynecol. 2010;202:321–34.
Moloney F, Ryan D, Twomey M, Hewitt M, Barry J. Comparison of MRI and high-resolution transvaginal sonography for the local staging of cervical cancer. J Clin Ultrasound. 2016;44(2):78–84.
Noventa M, Scioscia M, Schincariol M, Cavallin F, Pontrelli G, Virgilio B, et al. Imaging modalities for diagnosis of deep pelvic endometriosis: comparison between trans-vaginal sonography, rectal endoscopy sonography and magnetic resonance imaging. A head-to-head meta-analysis. Diagnostics. 2019;9:225.
Abrao MS, Gonçalves MODC, Dias JA, Podgaec S, Chamie LP, Blasbalg R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum Reprod. 2007;22(12):3092–7.
Santoro GA, Wieczorek AP, Stankiewicz A, Woźniak MM, Bogusiewicz M, Rechberger T. High-resolution three-dimensional endovaginal ultrasonography in the assessment of pelvic floor anatomy: a preliminary study. Int Urogynecol J. 2009;20(10):1213–22.
Peter Dietz H, Lanzarone V. Levator trauma after vaginal delivery. Obstet Gynecol. 2005;106:707–12.
Dietz HP, Steensma AB, Vancaillie TG. Levator function in nulliparous women. Int Urogynecol J. 2003;14(1):24–6.
Martin LC, Chill HH, Routzong MR, Abramowitch SD, Rostaminia G. Quantifying the physiologic motions of the pelvic viscera during evacuation in nulligravid asymptomatic women. Int Urogynecol J. 2023;4(2):535–43.
DeLancey J, Kearney R, Chou Q, Speights S, Binno S. The appearance of levator ani muscle abnormalities. Obstet Gynecol. 2003;101(1):46–53.
Beer-Gabel M, Teshler M, Barzilai N, Lurie Y, Malnick S, Bass D, et al. Dynamic transperineal ultrasound in the diagnosis of pelvic floor disorders pilot study. Dis Colon Rectum. 2002;45:239–48.
Beer-Gabel M, Carter D. Comparison of dynamic transperineal ultrasound and defecography for the evaluation of pelvic floor disorders. Int J Colorectal Dis. 2015;30(6):835–41.
Israel GM, Malguria N, McCarthy S, Copel J, Weinreb J. MRI vs. ultrasound for suspected appendicitis during pregnancy. J Magn Reson Imaging. 2008;28(2):428–33.
Constantinou CE. Dynamics of female pelvic floor function using urodynamics, ultrasound and magnetic resonance imaging (MRI). Eur J Obstet Gynecol Reproduct Biol. 2009;144(SUPPL 1):S159-65.
Palmér M, Åkesson Å, Marcickiewicz J, Blank E, Hogström L, Torle M, et al. Accuracy of transvaginal ultrasound versus MRI in the PreOperative Diagnostics of low-grade Endometrial Cancer (PODEC) study: a prospective multicentre study. Clin Radiol. 2023;78(1):70–9.
Bauşic A, Coroleucă C, Coroleucă C, Comandaşu D, Matasariu R, Manu A, et al. Transvaginal ultrasound vs magnetic resonance imaging (MRI) value in endometriosis diagnosis. Diagnostics. 2022;12:1767.
Moura APC, Ribeiro HSAA, Bernardo WM, Simões R, Torres US, D’Ippolito G, et al. Accuracy of transvaginal sonography versus magnetic resonance imaging in the diagnosis of rectosigmoid endometriosis: systematic review and meta-analysis. PLoS ONE. 2019;14:e0221499.
Fletcher J. Magnetic resonance imaging of anatomic and dynamic defects of the pelvic floor in defecatory disorders. Am J Gastroenterol. 2003;98(2):399–411.
Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, et al. 3D slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging. 2012;30(9):1323–41.
Nardos R, Thurmond A, Holland A, Gregory WT. Pelvic floor levator hiatus measurements: MRI versus ultrasound. Female Pelvic Med Reconstr Surg. 2014;20(4):216–21.
Kruger JA, Heap SW, Murphy BA, Dietz HP. Pelvic floor function in nulliparous women using three-dimensional ultrasound and magnetic resonance imaging. Obstet Gynecol. 2008;111:631–8.
van Delft KWM, Sultan AH, Thakar R, Shobeiri SA, Kluivers KB. Agreement between palpation and transperineal and endovaginal ultrasound in the diagnosis of levator ani avulsion. Int Urogynecol J. 2015;26(1):33–9.
Den CG, Lin L, Gardner JD, Yeh NH, Wu GS. Dynamic displacement changes of the bladder neck with the patient supine and standing. J Urol. 1998;159:754–7.
Dietz HP, Clarke B. The influence of posture on perineal ultrasound imaging parameters. Int Urogynecol J. 2001;12:104–6.
Funding
This study was partially funded by NSF 2053851.
Author information
Authors and Affiliations
Contributions
Chill: Project development, data collection, data analysis, manuscript writing and editing.
Martin: Project development, data collection, data analysis, manuscript writing and editing.
Abramowitch: Data collection, data analysis, manuscript editing.
Rostaminia: Project development, data collection, data analysis, manuscript writing and editing.
Corresponding author
Ethics declarations
Conflict of interest
Author Chill has nothing to report.
Author Martin reports Renovia Inc. for investigator-initiated funding for a project unrelated to this work.
Author Abramowitch reports Renovia Inc. for investigator-initiated funding for a project unrelated to this work.
Author Rostaminia has nothing to report.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chill, H.H., Martin, L.C., Abramowitch, S.D. et al. Quantifying the effect of an endo-vaginal probe on position of the pelvic floor viscera and muscles. Int Urogynecol J 34, 2399–2406 (2023). https://doi.org/10.1007/s00192-023-05557-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-023-05557-7