Abstract
Introduction and hypothesis
To analyze the immunochemical and urodynamic outcomes after partial versus complete excision of transvaginal polypropylene mesh (PPM) from pelvic walls of rats.
Methods
Forty-eight female Sprague-Dawley (SD) rats were randomly distributed into seven groups: control, mesh total removal 60 days (M-T 60D), mesh total removal 180 days (M-T 180D), mesh partial removal 60 days (M-H 60D), mesh partial removal 180 days (M-H 180D), sham 60 days (Sham 60D), and sham 180 days (Sham 180D). In the mesh groups, PPM was inserted and partially (0.3 × 0.3 cm) or completely removed 30 days later. In the Sham group, the space between the vagina and bladder was dissected without placing or removing the synthetic mesh at day 1 and day 30 later. Urodynamic studies, immunochemical analysis, and Western blot were done at days 60 and 180.
Results
The M-T 60D voiding pressure was significantly decreased compared to the Sham 60D and M-H 60D. The voiding interval of M-T 60D was significantly shorter than that of M-H 60D. In the M-T 60D and M-T 180D groups, the leak point pressure was significantly less than in their corresponding sham groups. IL-1 and TNF-α were significantly more intense in M-T 60D compared to M-H 60D and Sham 60D. NGF was significantly greater in M-T 60D compared to Sham 60D. There were no significant differences in MMP-2 and CD-31s throughout the group.
Conclusion
Total mesh excision incites a host inflammatory response and transitory lower urinary tract dysfunction. Despite the good outcomes after total excision, the invasiveness and surgical risk associated with repeated procedures should not be underestimateded.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Twenty-nine percent of women who undergo primary repair for pelvic organ prolapse (POP) experience a recurrence requiring a second surgery [1]. Although there have only been a few long-term studies, Lo et al. found repair using polypropylene transvaginal mesh has a lower risk of recurrence compared to native tissue repair after 3 years [2, 3]. Polypropylene mesh (PPM) is a nonabsorbable synthetic graft material, causes tissue ingrowth due to host-tissue interaction, and provides permanent support [4]. However, complications such as exposures, extrusion, dyspareunia, and pelvic pain specific to mesh use led to serious consequent actions from the FDA. In 2016, the agency reclassified surgical mesh for transvaginal repair of POP into class III (high risk), and subsequently, as of April 16, 2019, the FDA ordered two manufacturers to stop selling and distributing their products [5].
Even with the FDA’s ban of transvaginal mesh use, women will continue to seek medical assistance for associated complications and concerns. Warembourg et al. reported 7% of all pelvic surgeries performed in their tertiary referral center were for mesh-related complications. The reintervention rate for mesh-related complications after POP repair was 2.8% [6]. Seventeen percent of all mesh removals were attributed to pain without underlying causes such as exposure or erosion [7]. Short- and medium-term follow-up studies show that most complications occur within the first year. However, long-term follow-up studies show that there is a high rate of infection after 2 years, and complications can even occur 8 years after implantation [8]. Hence, the FDA advises women to continue their routine follow-up care after receiving transvaginal mesh for POP even if they are not experiencing any complications or symptoms.
Management of mesh-related complications includes tropical vaginal estrogen cream use, physical therapy, analgesic pain management, and inpatient or under anesthesia mesh excision [9]. Previous animal studies showed that implantation of synthetic mesh triggers an inflammatory reaction caused by macrophages and fibroblasts [10,11,12]. Likewise, Elmer et al. found that even after 1 year of surgery, synthetic mesh continues to elicit a foreign body response in human vaginal submucosal tissues [13]. Currently, there is no consensus regarding whether partial or complete removal of mesh is superior. No studies have as of yet been done to understand the effects of mesh excisions. The aim of this study is to analyze the immunochemical and urodynamic outcomes of partial versus complete excision of transvaginal PPM from the pelvic walls of rats.
Methodology
All experimental protocols and procedures were approved by Chang Gung Memorial Hospital’s Institutional Animal Care and Use Committee (no. 2018032801) and funded by the Chang Gung Memorial Hospital (CMRPG2H0281).
Animal model design
Forty-eight female Sprague-Dawley (SD) rats were used and randomly assigned evenly into seven groups: control, sham 60 days (Sham 60D), sham 180 days (Sham 180D), mesh total removal 60 days (M-T 60D), mesh total removal 180 days (M-T 180D), mesh partial removal 60 days (M-H 60D), and mesh partial removal 180 days (M-H 180D).
In the control group, no surgery was performed. Urodynamic study was performed, and afterward the rats were killed for immunochemical study. In the study groups (total and partial mesh removal), synthetic mesh was implanted between the vagina and bladder of the SD rats. Thirty days later, the mesh was removed completely (mesh total removal group) or partially (0.3 × 0.3 cm in the mesh partial removal group). Urodynamic study was performed on day 60 (M-T 60D and M-H 60D) or day 180 (M-T 180D and M-H 180D), and the rats were killed for immunochemical study. The Sham groups underwent a similar protocol with the space between the vagina and bladder opened without inserting or removing the synthetic mesh.
All SD rats underwent suprapubic tube (SPT) implantation into the bladder 2 days before conscious cystometry (CMG) and leak-point pressure (LPP) testing. After the study, all rats were killed. Their urogenital tissues (vagina, bladder, and urethra) were harvested and analyzed for immunohemical studies and Western blot analysis.
Surgical procedure
All surgeries were carried out under isoflurane anesthesia in an animal laboratory. Preoperative prophylactic antibiotics with cefazolin were administered, and surgeries were performed by the authors. The rat vaginas were exposed with a Lonestar retractor; 0.5–1.0 cc normal saline was used for hydrodissection at the anterior vaginal wall, and a 1-cm midline incision was made. The space between the bladder and vagina was dissected and opened. A 0.7 × 1.0-cm polypropylene mesh with a density of 26 g/m2 was inserted for the M-T and M-H groups. The sham groups underwent similar surgeries except no mesh was implanted. The vaginal mucosa was closed with Polyglactin 5/0 suture (Vicryl).
At day 30, a second surgery was performed for removal of the mesh in the study groups. Preoperative prophylactic antibiotics with Cefazolin were administered. Hydrodissection with 0.5–1.0 cc normal saline was performed, and a 1-cm midline incision of the anterior wall was done. The space between the vagina and bladder was opened. Complete and partial (0.3 × 0.3 cm in size) removal of the polypropylene mesh was done for the M-T (complete removal) and M-H (partial removal) groups, respectively. The sham groups underwent similar surgeries with opening of the space between the vagina and bladder. The vaginal mucosa was closed with Polyglactin 5/0 suture.
CMG measurement
SPT was placed 2 days prior to the cystometry study as described previously by Lin et al. [14]. Two days after the implantation, the rats were placed in a specialized metabolic cages (Med Associates Inc.) for 70–80 min as previously described by Lin et al. [15]. The bladder catheter was connected to a syringe pump and pressure transducer. Bladder pressures were referenced to the air pressure at the level of the bladder. Pressure and force transducer signals were amplified, recorded, and digitized for analysis. The bladder was filled at a rate of 5 ml/h with room temperature normal saline through the bladder catheter while the bladder pressure was recorded. A beaker was placed underneath the cage to collect urine, and the change in weight of the collected urine was recorded. The saline infusion was continued until rhythmic bladder micturition contractions stabilized (typically after 15–30 min). Data from five micturition cycles were collected. Voided volume (VV) was defined as the expelled volume during micturition. Peak voiding pressure (VP) was measured at the peak of the detrusor contraction. The interval between two successive contractions in each micturition cycle was defined as the intercontration interval (PI).
LPP measurement
LPP was measured as previously described by Lin et al. [14]. The rats were anesthetized with urethane (1 g/kg). Credé’s maneuver was applied to empty the bladder manually. Room temperature normal saline at the rate of 10 ml/h was infused through the bladder catheter. The average bladder capacity of each rat was determined after three to five voiding cycles. The bladder was then filled to half-capacity, and vertical pressure was applied to the rat’s abdomen with one finger. The pressure was gently increased until urine leakage occurred. The pressure at which the urine leak occurred was measured as the LPP. Five measurements were performed, and the mean LPP was obtained.
Immunochemical study
The SD rats were killed via isoflurance overdose. Excision of the implanted mesh and/or the surrounding vagina and bladder wall tissues was performed at 60 days and 180 days for Sham 60D, M-T 60D, M-H 60D and Sham 180D, M-T 180D, and M-H 180D, respectively. The harvested tissues were immediately fixed in 4% formaldehyde for 4 h and dehydrated by a series or graded ethanol solutions before being embedded in paraffin. The tissues were then sectioned onto glass slides. Immunochemistry was performed on formalin-fixed, paraffin-embedded tissue sections as previously decribed [16]. Tissue slides were deparaffinized with xylene and washed in serial dilutions of ethanol. Three percent hydrogen peroxidase (H2O2) was used to block endogenous peroxidase activity, after which appropriately diluted primary antibodies [rabbit anti-NGF antibody (1:750; anti-NGF/ TA300799/ OriGene), rabbit anti-CD31 polyclonal antibody (1:200; PA5–24411/ Invitrogen), rabbit MMP-2 polyclonal antibody (1:500; TA330021/ OriGene), rabbit anti-IL1-beta polyclonal antibody (1:200; TA336742/ OriGene), and rabbit anti-TNF-alpha polyclonal antibody (1:300; PA5–19810/ Thermo)] were applied. The slides were washed with phosphate-buffered saline (PBS) at each step. Appropriately diluted biotinylated secondary antibody (1:200; SIG-A0545/ Sigma) was applied, followed by chromogenic detection using DAB as the substrate. The slides were counterstained in hematoxylin and dehydrated with ethanol and xylene prior to mounting and examination via an optical microscope.
Western blot analysis
The samples were homogenized in a lysis buffer (PRO-PREPTM solution, iNtRON Biotechnology) and incubated for 20 min on ice to induce cell lysis [17]. The lysis was centrifuged at 13,000 rpm (4 °C) for 10 min and the supernatant transferred to a fresh 1.5-ml tube. The protein content of the supernatant was estimated by the Bradford method. The samples (30 μg per lane) were mixed with sample buffer containing 10% mercaptoethanol (Sigma). The mixtures of lysates and sample buffers were heated at 100 °C for 10 min and applied to a 10% sodium dodecyl sulfate polyacrylamide gel for electrophoresis. The proteins were electrophoretically transferred onto nylon membranes and nonspecific bindings blocked for an hour at room temperature with 10% (w/v) milk. After repeated washing with TBS containing 0.1% (v/v) Tween 20 (TBST), the membranes were incubated overnight at 4 °C with the antibody at 1:10,000 dilution (anti-NGF/TA300799/OriGene; anti-IL1 antibody/TA336742/OriGene; anti-MMP2 antibody/TA336592/OriGene; anti-TNF antibody/PA5–19810/Thermo). After rinsing in TBST three times, each of 10-min duration, the membranes were incubated with goat anti-rabbit lgG horseradish peroxidase conjugate antibody (SIG-A0545, Sigma, 1:10000). The membrane was then incubated in chemiluminescence reagent for 5 min and exposed to high-performance chemiluminescence film. The film was developed and used to measure optical density. The optical density of the band was quantified by the UN-SCAN-ITTM gel and graph digitizing software.
Outcome measures
The outcomes measured were the density of the inflammatory reaction produced by interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), nerve growth factor (NGF), matrix metalloproteinases (MMPs), and CD-31 (surface antigen) around the surgical site/area of implants and their association with the functional urodynamic investigation of the SD rats.
Statistical analysis
Study groups included explant of partial or total mesh, sham, and control. Descriptive statistics were used in analysis of the results of NGF, IL-1, TNF-α, MMP-2, CD-31, and urodynamic parameters, with all data expressed as mean ± standard deviation (SD). The differences among groups and pair-wise comparisons for continuous parametric variables were analyzed by post hoc Sidak test for NGF, IL-1, TNF-α, MMP-2, CD-31, and urodynamic parameters. P < 0.05 was considered statistically significant. All statistical methods were performed using SPSS commercial software, version 17 (SAS Institute, Cary, NC).
Results
Forty-eight female SD rats (12.8 ± 1.0 weeks old and weighing 399.3 ± 24.2 g) were used. One rat in the M-T 180D group did not survive the study. No other complications were observed in the study of the other rats. A total of 47 SD rat results were analyzed.
Urodynamic parameters
The UD parameter results are shown in Tables 1 and 2 and Fig. 1. The M-T 60D voiding pressure was significantly decreased compared to the Sham 60D and M-H 60D, but no difference was noted in M-T 180D and M-H 180D compared to Sham 180D. The voiding interval of M-T 60D was significantly shorter than that of M-H 60D, but no difference was noted compared to Sham 60D. There was no significant difference in voiding volumes among the groups. In the M-T 60D and M-T 180D groups, the leak point pressure was significantly less than in their corresponding sham groups. The leak point pressure of M-T 60D was also significantly less than that of M-H 60D. There were no significant differences between the partially explanted groups (M-H 60D and M-H 180D) and sham.
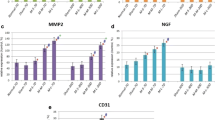
Magnitude of change in immunochemical evaluation and urodynamic parameters. IL-1, TNF-α, MMP-2, NGF, and CD31 on Day 60 and Day 180 after transvaginal mesh explanted in SD rats. VP (voiding pressure), VV (voiding volume), VI (voiding interval), and LPP (leak point pressure) on Day 60 and Day 180 after transvaginal mesh was explanted in SD rats. Control, no surgery was performed; Sham, vaginal dissection alone was done (no mesh); M-H, mesh implanted, removed partially; M-T, mesh implanted, removed totally. * Statistically significant when compared to sham. #Statistically significant when compared to Sham M-H
Immunochemical analysis
IL-1 and TNF-α were significantly more intense in M-T 60D compared to M-H 60D and Sham 60D, but declined after 180 days (M-T 180D), as shown in Fig. 2 and Table 2. There were no significant differences in MMP-2 and CD-31s throughout the group. NGF was significantly greater in M-T 60D compared to Sham 60D but decreased after 180 days (M-T 180D). Compared to the sham groups, there were no significant differences in the partially explanted groups (M-H 60D and M-H 180D).
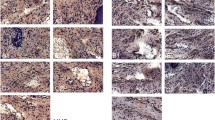
Immunochemical staining. IL-1, MMP-2, TNF-α, NGF, and CD-31 of urogenital tissues at Days 60 and 180 after mesh explantation surgery (×100 magnification) A1 Day 60 after mesh was partially explanted in the M-H group; A2 day 180 after mesh was partially explanted in the M-H group; B1 day 60 after mesh was totally explanted in the M-T group; B2 day 180 after mesh was totally explanted in the M-T group; S1 day 60 after surgery alone in the sham group; S2 day 180 after surgery alone in the sham group; C1 control group. *Brown spots signify antibody staining
Our findings from the immunochemical analysis were corroborated by the Western blot results, with darker and thicker lines as shown in Fig. 3.
Western blot analysis in IL-1, TNF-α, MMP-2, NGF, and CD31 on Day 60 and Day 180 after mesh explanting surgery in SD rats a. Control; b Sham 60, day 60 after surgery alone; c M-H 60, day 60 after mesh was partially explanted; d M-T 60, day 60 after mesh was totally explanted; e Sham 180, day 180 after surgery alone; f M-H 180, day 180 after mesh was partially explanted; g M-T 180, day 180 after mesh was totally explanted
Discussion
Histopathological analysis of transvaginal sling tape excised for clinical indications showed inflammation, fibrosis, and foreign body reactions [18, 19]. To our knowledge, no studies are available evaluating the effect of mesh excision on tissue healing. The process of healing occurs as a cascade of events that consists of four arbitrary phases: hemostasis, inflammation, proliferation, and wound remodeling [20]. Our previous animal studies showed that once synthetic mesh has been implanted into the tissue of the vagina, the migration of macrophages and fibroblasts triggers the release of inflammatory cytokines such as IL-1, MMP-2, TNF-α, CD-31, and NGF. The ideal time to evaluate the effects on tissue is 30 days after a procedure when the tissue has stabilized [10,11,12]. Hence, in the present study, immunohistochemical and urodynamic studies were performed 60 and 180 days after mesh extraction.
The results of this study showed that inflammatory mediators (IL-1 and TNF) were significantly increased in the complete mesh removal group at 60 days (M-T 60D). The inflammatory response is related to the extensiveness of the procedure. As the healing process continues, no significant difference was found within each group (180 days).
MMP-2 plays a role in the tissue recovery phase, including cell proliferation, migration, angiogenesis, and apoptosis [17, 20]. Factors that influence tissue remodeling include the extensiveness of the surgery and implantation of a foreign body [11]. The present study revealed no significant difference between the sham and study groups at 60 and 180 days, which may be attributed to the longer follow-up time period.
The detrimental effects of complete removal of the implanted mesh were seen in the urodynamic study. This may be attributed to the extensive surgery resulting in a greater inflammatory response and requiring a longer time for recovery. M-T 60D had a significantly shorter voiding interval compared to M-H 60D and decreased voiding pressure compared to Sham 60D and M-H 60D. The extensiveness of the procedure was also related to impaired urethral function. LPP was significantly lower and correlated to greater intensity in the NGF in MT groups. NGF, a neurotrophin that mediates apoptosis, may contribute to lower urinary tract symptoms [21].
Tissue integration and angiogenesis is a continuous process. Our previous study showed that mesh enhances the angiogenesis process. However, in the present study, there were no significant differences in the CD-31 marker (a marker expressed by inflammatory cells that usually indicates angiogenesis) [16] throughout the study groups, which may indicate that angiogenesis takes place before day 60 and returns to baseline thereafter.
The mechanism of mesh implant-related complications is not fully understood. Possible causes include the surgical technique, experience, host condition, infection, menopause, and type of mesh used. Mesh exposure, pain, and the patient’s preference due to concerns regarding the FDA’s warnings are reasons for mesh removal. Nevertheless, whether to carry out complete or partial removal of mesh is still controversial. Some authors prefer complete removal of mesh to prevent repeat exposure in the event of an underlying infection or when signs of mesh rejection (self-dissecting from the surrounding tissue) are found [8]. Excessive tension from shrinkage of the mesh’s main body against the serrated arms causes vaginal contracture. Relief of the tension by dividing the central graft from the arms and excising all areas of the mesh contraction leads to an 88% reduction in vaginal pain and 64% reduction in dyspareunia. Further improvement was seen in patients who underwent removal of the entire accessible mesh, but with increased risk of visceral injury and hemorrhage and difficulty in reapproximation of the vaginal epithelium [22]. Contrarily, in patients who present with vaginal pain, 51% of patients continue to exhibit persistent pain symptoms after removal, and those whose accessible mesh was completely removed were not more likely to have improvement of symptoms [23]. POP recurrence is found in 29% and 5% of patients with complete and partial excision of mesh, respectively [24]. Nonetheless, there is a failure rate of 8% requiring repeat excision for partial excision of the mesh [25].
In the present study, the number of control SD rats was reduced in compliance with the IACUC’s recommendation. We found that in rats, extensive, complete removal of implanted mesh is associated with a detrimental effect on bladder and urethral function. Although most complications associated with pelvic mesh excision surgery are minor, serious complications may occur [26]. Proper patient counseling is essential and surgical experience essential. Unless the entire mesh is easily accessible (as seen in infectious or mesh rejection cases), the complicated mesh should be removed and limited to the involved portion.
Limitations and strengths
The strengths of our study include experimental groups with procedures carried out under a controlled environment and specifically designed to simulate TVM placement in female POP surgeries. Sterile mesh was used in the study, and no post-procedure infections occurred. This may be considered a limitation as it may be inadequate to fully elucidate the complexity of the entire wound healing process.
Conclusion
In conclusion, our results suggest that total explantation of mesh incites a direct host inflammatory response and lower urinary tract dysfunction. Low leak point pressure, shortened voiding intervals, and low voiding pressure were found at day 60. However, these reactions were transitory. After the recovery process, no differences were seen in the inflammatory response and lower urinary tract dysfunction by day 180. Despite the good outcomes observed, the invasiveness and surgical risk associated with repeated procedures should not be underestimated.
References
Abbott S, Unger CA, Evans JM, Jallad K, Mishra K, Karram MM, et al. Evaluation and management of complications from synthetic mesh after pelvic reconstructive surgery: a multicenter study. Am J Obstet Gynecol. 2014;210(2):163 e1-8.
Lo TS, Al-Kharabsheh AM, Tan YL, Pue LB, Hsieh WC, Uy-Patrimonio MC. Single incision anterior apical mesh and sacrospinous ligament fixation in pelvic prolapse surgery at 36 months follow-up. Taiwan J Obstet Gynecol. 2017;56(6):793–800.
Lo TS, Pue LB, Tan YL, Wu PY. Long-term outcomes of synthetic transobturator nonabsorbable anterior mesh versus anterior colporrhaphy in symptomatic, advanced pelvic organ prolapse surgery. Int Urogynecol J. 2014;25(2):257–64.
Wolff GF, Winters JC, Krlin RM. Mesh excision: is total mesh excision necessary? Curr Urol Rep. 2016;17(4):34.
Administration USFD. Urogynecologic Surgical Mesh Implants 2019 [Available from: https://www.fda.gov/medical-devices/implants-and-prosthetics/urogynecologic-surgical-mesh-implants.
Warembourg S, Labaki M, de Tayrac R, Costa P, Fatton B. Reoperations for mesh-related complications after pelvic organ prolapse repair: 8-year experience at a tertiary referral center. Int Urogynecol J. 2017;28(8):1139–51.
Viereck V, Rautenberg O, Kociszewski J, Grothey S, Welter J, Eberhard J. Midurethral sling incision: indications and outcomes. Int Urogynecol J. 2013;24(4):645–53.
Marcus-Braun N, von Theobald P. Mesh removal following transvaginal mesh placement: a case series of 104 operations. Int Urogynecol J. 2010;21(4):423–30.
Lo TS, Tan YL, Cortes EF, Wu PY, Pue LB, Al-Kharabsheh A. Clinical outcomes of mesh exposure/extrusion: presentation, timing and management. Aust N Z J Obstet Gynaecol. 2015;55(3):284–90.
Lo TS, Lin YH, Chu HC, Cortes EF, Pue LB, Tan YL, et al. Association of urodynamics and lower urogenital tract nerve growth factor after synthetic vaginal mesh implantation on a rat model. J Obstet Gynaecol Res. 2017;43(1):173–8.
Lo TS, Lin YH, Uy-Patrimonio MC, Chu HC, Hsieh WC, Chua S. Dissecting of the paravesical space associated with lower urinary tract dysfunction—a rat model. Sci Rep. 2020;10(1):1718.
Lo TS, Lin YH, Yusoff FM, Chu HC, Hsieh WC, Uy-Patrimonio MC. The immunohistochemical and urodynamic evaluation towards the collagen-coated and non-coated polypropylene meshes implanted in the pelvic wall of the rats. Sci Rep. 2016;6:38960.
Elmer C, Blomgren B, Falconer C, Zhang A, Altman D. Histological inflammatory response to transvaginal polypropylene mesh for pelvic reconstructive surgery. J Urol. 2009;181(3):1189–95.
Lin YH, Liu G, Daneshgari F. A mouse model of simulated birth trauma induced stress urinary incontinence. Neurourol Urodyn. 2008;27(4):353–8.
Lin YH, Liu G, Kavran M, Altuntas CZ, Gasbarro G, Tuohy VK, et al. Lower urinary tract phenotype of experimental autoimmune cystitis in mouse: a potential animal model for interstitial cystitis. BJU Int. 2008;102(11):1724–30.
Velnar TBT, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanism. J Inter Med Res. 2009;37(5):1528–42.
La Fleur M, Underwood JL, Rappolee DA, Werb Z. Basement membrane and repair of injury to peripheral nerve: defining a potential role for macrophages, matrix metalloproteinases, and tissue inhibitor of metalloproteinases-1. J Exp Med. 1996;184(6):2311–26.
Hill AJ, Unger CA, Solomon ER, Brainard JA, Barber MD. Histopathology of excised midurethral sling mesh. Int Urogynecol J. 2015;26(4):591–5.
Li L, Wang X, Park JY, Chen H, Wang Y, Zheng W. Pathological findings in explanted vaginal mesh. Hum Pathol. 2017;69:46–54.
Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294(5548):1945–8.
Coelho A, Wolf-Johnston AS, Shinde S, Cruz CD, Cruz F, Avelino A, et al. Urinary bladder inflammation induces changes in urothelial nerve growth factor and TRPV1 channels. Br J Pharmacol. 2015;172(7):1691–9.
Feiner B, Maher C. Vaginal mesh contraction: definition, clinical presentation, and management. Obstet Gynecol. 2010;115(2 Pt 1):325–30.
Crosby EC, Abernethy M, Berger MB, DeLancey JO, Fenner DE, Morgan DM. Symptom resolution after operative management of complications from transvaginal mesh. Obstet Gynecol. 2014;123(1):134–9.
Tijdink MM, Vierhout ME, Heesakkers JP, Withagen MI. Surgical management of mesh-related complications after prior pelvic floor reconstructive surgery with mesh. Int Urogynecol J. 2011;22(11):1395–404.
Marks BK, Goldman HB. Controversies in the management of mesh-based complications: a urology perspective. Urol Clin North Am. 2012;39(3):419–28.
Rac G, Greiman A, Rabley A, Tipton TJ, Chiles LR, Freilich DA, et al. Analysis of complications of pelvic mesh excision surgery using the Clavien-Dindo classification system. J Urol. 2017;198(3):638–43.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial disclaimer
This study was supported by Chang Gung University Hospital research grant CMRPG2H0281.
Conflict of interest
The authors claim no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lo, TS., Lin, YH., Huang, TX. et al. Immunochemical and urodynamic outcomes after polypropylene mesh explant from the pelvic wall of rats. Int Urogynecol J 33, 1839–1848 (2022). https://doi.org/10.1007/s00192-021-04842-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-021-04842-7