Abstract
Introduction and hypothesis
Tapes for stress urinary incontinence (SUI) and meshes for pelvic organ prolapse can result in postoperative complications, such as urethral (UP) or bladder (BP) perforations. Martius fat pad (MFP) is an historic procedure, widely used to treat lower urinary tract (LUT) fistulae. We report our experience with the insertion of the biological small intestinal submucosa (SIS) xenograft as an alternative to MFP in these prosthetic complications.
Methods
We conducted a retrospective, monocentric study which included all patients who underwent SIS insertion during surgical removal of tape/vaginal mesh for UP or BP from 2011 to 2019. Preoperative assessment was based on history, symptoms, physical examination and urethrocystoscopy. Primary outcome was successful repair defined as absence of any LUT defect. Secondary outcomes were complications, LUT symptoms, pain and additional SUI surgical procedures.
Results
Thirty-eight patients were included. Twenty-six had a UP and eight a BP. In four cases, perforation involved both the bladder neck and urethra. All LUT defects were cured. Six postoperative complications were reported (five of grade ≤ 2 and one of grade 3b according to the Clavien-Dindo classification). At the mean follow-up of 37.2 (range 6–98) months, 14 patients (36.8%) presenting a postoperative SUI underwent a SUI surgical procedure and 1 patient had a laparoscopic sacrocolpopexy for cystocele recurrence.
Conclusion
Absorbable SIS xenograft is an effective and safe graft for the management of lower urinary tract tape and mesh perforations. The cost has to be balanced with the good results, short operative time and no fat pad complications as in MFP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prostheses have been widely used in stress urinary incontinence (SUI) and pelvic organ prolapse (POP) repair. However, several regulatory agencies have issued health warnings due to an increase in complications [1]. Recently, many national health agencies have prohibited the use of vaginal meshes for POP. In the UK and Ireland, as of July 2018, a period of high restriction with extra safety measures concerning use of tapes for SUI has been instituted.
It is difficult to determine the exact incidence of tape/mesh complications (TMC) because of a lack of a systematic registration of mesh-related complications (underreporting) and lack of studies with adequate length of follow-up. Furthermore, many patients do not have a regular follow-up or are not managed for their TMC until they have been referred to a tertiary center. To standardize the assessment of TMC, the International Urogynecological Association and International Continence Society have published a joint classification (IUGA/ICS C) [2]. Using this classification, Miklos et al. have reported 506 cases of tape/mesh removal (TMR), mostly for pain [3]. Urethral (UP) and bladder (BP) perforations are rarely reported, though their management is a challenging surgical procedure as the inflammation and infection at the mesh-tissue interface and the size of the lower urinary tract (LUT) defect can lead to failure and postoperative fistula [4, 5]. The most frequent surgical procedure performed in the literature to treat LUT defects is Martius fat pad (MFP), described by Heinrich Martius in 1928, to optimize the healing and to minimize the risk of de novo SUI [6]. Nonetheless, there are limits to MFP, as it has been described in native tissue defects without any mesh. In cases of TMC with surrounding inflammatory reaction and vaginal contraction, MFP seems to be an inappropriate choice [5]. We advocate for considering the use of the small intestinal submucosa (SIS) xenograft. Biological scaffolds composed of naturally extracellular matrix have received significant attention for their potential therapeutic applications [7]. Among them, porcine SIS, discovered in 1987, is composed of > 90% collagen with a high expression of type 1 collagen, associated with glycoprotein, glycosaminoglycan and growth factors. Safety and efficacy of this xenograft used for the repair of many tissues including musculoskeletal, cardiovascular, urogenital and integumentary systems have been shown in both preclinical animal and human clinical studies. In a rabbit model with a bladder augmentation using SIS, at 12 months Ayyildiz et al. described histological features, such as presence of new vessel formations, nerve regeneration, and collagen and smooth muscle regeneration, which were indistinguishable from the original bladder and without signs of inflammation [8]. SIS is moreover a completely absorbable graft rapidly degraded when implanted in the genitourinary tract. More than 50% of the scaffold and almost 100% of the scaffold were replaced by infiltrating host cells at 28 days and 90 days, respectively [9]. SIS (Surgisis Biodesign®, Cook Medical, Bloomington, IN, USA) was therefore considered the preferred way to achieve LUT defect repair after TMR in our center. We aimed to assess our results with almost 10 years of experience.
Patients and methods
We conducted a retrospective study including all patients who underwent a SIS insertion for UP and BP due to TMC from January 2011 to December 2019. The study was approved by the local ethics committee (Institutional Review Board approval number: IRB00012437). All patients were referred to our tertiary center.
Baseline assessment included history and physical examination, cytobacteriological uroanalysis, uroflowmetry, measurement of post-void residual volume by bladder scan and urethrocystoscopy. The following data were analyzed: type of tape or vaginal mesh, number of previous tape/mesh revision surgeries (operative reports were required), symptoms at presentation and time interval between initial insertion and our revision surgery.
Data were retrospectively collected by chart review from patient hospital records. The patients were followed by three senior surgeons who performed the baseline assessment and the follow-up visits. The indication of SIS insertion, often decided according to intraoperative conditions, was explained and accepted by all the patients, as well as the length of postoperative urinary catheterization and the need for further surgery in case of SUI recurrence.
Surgical procedure
Urine cytobacteriological examination should be sterile or treated with the appropriate antibiotic for at least 48 h before the surgical procedure.
Intraoperative sequence
In case of stone adhering to the mesh, we first performed an endoscopic lithotripsy. In BP, bilateral intraoperative ureteral stenting was performed to avoid ureteral injury during bladder neck and trigonal dissection.
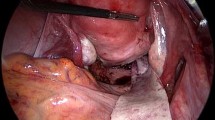
We used a vaginal approach for TMR. In UP, a Hegar dilator was introduced in the urethra. The first step was large dissection to remove the maximal prosthesis length. The second step was the closure of the defect using absorbable suture. The third step was SIS insertion. Surgisis Biodesign® is a four-layer tissue graft. Being paper-thin, it can be folded into three layers after 5 min hydration in sterile saline solution. Its flexibility allows it to be easily slotted into place. It was secured to the surrounding tissue with six stiches of 2–0 polyglactin braided absorbable suture (Fig. 1. Electronic Supplementary Material).
Closure of the vaginal incision was done using 2–0 polyglactin braided absorbable suture. According to the site and size perforation, ureteral stents were left in place in the postoperative period (range 3–5 days) to optimize the LUT defect’s healing. The urethral catheter was maintained at least 7 days in BP and 14 days in UP. A cystourethrogram was performed before removal of the urethral catheter.
Follow-up and outcomes
After urethral catheter removal, patients were routinely assessed at 4–6 weeks, 6 months and then annually, with history, clinical examination, uroflowmetry and post-void residual volume.
The primary outcome was successful repair of the LUT defect, with a normal cystourethrogram and no need for additional surgery for recurrence of the LUT defect during the follow-up.
Secondary outcomes included intraoperative and 30-day complications graduated according to the Clavien-Dindo classification, length of hospital stay, and persistent or de novo symptoms. Voiding symptoms were defined as abnormally slow and/or incomplete micturition with elevated post-void residual volume and/or straining at micturition. Chronic pain was represented by diffuse pelvic or lower abdominal, urethral and vaginal pain. Storage symptoms were assessed according to frequency and urgency with or without urge urinary incontinence (UUI). Recurrent urinary tract infections (UTIs) were defined as ≥ 4 UTIs per year. Change in symptoms (resolved, improved, unchanged) was based on the medical history and patient assessment, collected by the surgeon in the patient hospital record, uroflowmetry, post-void residual volume and number of UTIs. We did not use validated questionnaires. We investigated whether preoperative symptoms (voiding symptoms, chronic pain, dyspareunia, storage symptoms, recurrent UTI) were resolved after TMR. Later complications (SUI, POP recurrence) were collected as well as the need for further surgical procedures. In case of SUI, urodynamic testing was carried out with a minimum of 3 months postoperative delay.
Results
Patients’ baseline characteristics (Table 1 )
Thirty-eight patients were included (Fig. 2). Twenty-six had a UP and eight a BP (6 in the bladder neck, 2 in the trigone). Four patients presented both bladder neck perforation (BNP) and UP. The lesions were classified as 4B/T3—T4/S3 according to the IUGA/ICS C.
Mean age at presentation was 63.8 (range 38–80) years. Median time between tape/mesh insertion and revision surgery in our center was 64 (± 57) months, and mean time was 72 months (range 4 months −19.3 years). Fifteen patients (39.4%) had concomitant operations to the tape/mesh insertion. Eleven patients (28.9%) had previously undergone one or more tape/mesh revision surgeries before being referred to our center. Most common preoperative symptoms were recurrent UTI (28 patients, 73.7%), chronic pelvic pain (28 patients, 73.7%) and UUI (10 patients, 26.3%) or mixed urinary incontinence (MUI) (11 patients, 29%) (Table 2). All these symptoms were associated for 19 patients (50%). In four patients (10.5%), the main symptom was SUI. Seven patients (18.4%) had a stone adhering to the prosthesis of whom three (42.9%) presented macroscopic hematuria and four (57.1%) acute pain at micturition.
Surgical procedure and outcomes
The mean defect’s size was 10.3 (range 6–30) mm (data missing in 5 cases). No intraoperative complication occurred. Ureteral stents were left in place postoperatively in seven patients for a mean period of 4 (range 3–5) days. The reasons were trigonal defect (2 cases), both bladder neck perforation and UP (4 cases) and partial cystectomy in a patient presenting a BNP associated with stapling system inlay through the detrusor (Endofast ®, Allium Ltd., Israël).
Mean length of hospital stay was 4.4 (range 1–10) days. Early complications were reported for six patients (15.8%). Five patients had a grade ≤ 2 complications (Clavien-Dindo classification): three UTIs treated successfully with antibiotics (IUGA/ICS C: 4BT1S3) and one hematoma requiring watchful monitoring (IUGA/ICS C: 7AT1S3). A postoperative tachyarrhythmia occurred in a 72-year-old patient, which medically resolved. In one patient presenting a BNP, a urinary diversion with a double-J stent due to unilateral hyperalgesic renal colic without identified obstruction cause on the CT scan was required (grade 3b on the Clavien-Dindo classification, IUGA/ICS C: 4CT1S5). The double-J stent was removed at 1 month without further complications.
Mean length of urethral catheterization was 13.4 (range 7–30) days: 14 days in case of UP, 9.6 days for BP and 14.5 days for both BNP and UP.
The cystourethrogram was normal, without any urinary fistula, in all patients. The clinical outcomes are provided in Table 2. At 4–6-week postoperative follow-up visits, voiding symptoms and urinary retention were resolved in all cases. Chronic pain was resolved in 26/28 patients (92.9%). Acute per micturition pain disappeared in all cases. Mean follow-up was 37.2 months (range 6–98). During follow-up, recurrent UTIs were cured in 26/28 patients (92.8%). Dyspareunia was resolved in 8/12 patients (66.7%) and persistent for 2 patients (16.7%). No de novo dyspareunia was reported. Fifteen patients (39.5%) presented postoperative SUI (9 persistent, 6 de novo), among which 11 (73.3%) had intrinsic sphincter deficiency (urethral closure pressure ≤ 30 cmH20). Mean urethral closure pressure was 28.5(range 7–50) cmH20. One patient was cured with physiotherapy. Fourteen patients (36.8%) underwent one or more additional SUI surgical procedures (7 retropubic tapes, 6 ACT™ inflatable balloons (Uromedica, Plymouth, MN, USA), 2 artificial urinary sphincters]. Results are provided in Fig. 3. The mean range between TMR and SUI surgery was 17.5 (range 5–73) months. In case of a positive cough stress test, we inserted a retropubic tape: six patients (85.7%) were cured, and one (14.3%) was improved. In case of a negative cough stress test, ACT™ inflatable balloons were inserted with a 66.7% success rate, and a 49-year-old patient had an artificial urinary sphincter. One patient had a laparoscopic sacrocolpopexy for cystocele recurrence 12 months after TMR for BP.
Discussion
UP and BP are likely under-diagnosed and under-reported [10]. Tulokas et al. reported only one UP and one BP after tape in a retrospective cohort of 3531 women [11]. In a recent study on 140 revision surgeries for TMC, 4 UPs (2.9%) and 11 BPs (7.9%) were reported [12]. We reported SIS insertion during tape/mesh removal procedures for 38 cases of urethral or bladder prosthesis perforation. To our knowledge, our series is the largest study assessing SIS insertion in LUT defect repair. Other authors reported use of SIS in LUT defects but not due to TMC. In a pilot study of 23 vesicovaginal fistulae repaired with SIS, a 91.3% success rate was reported [13].
There is a major lack of evidence regarding surgical management of LUT prosthesis perforations. Several authors agreed that surgical procedures for such complications can be complex and challenging [4, 14,15,16,17,18]. Among patients referred to our center, seven (18.5%) were in failure of one or more previous partial TMRs by the vaginal route. We are in step with most authors emphazing that LUT defects due to TMC require complete removal of all of the foreign material (prosthesis, nonabsorbable sutures, staples, anchors) with urethrolysis, urethral reconstruction and bladder excision if necessary in order to avoid reinterventions for failure that can lead to last resort urinary diversion or partial/total cystectomy for the management of a devastated urethra or bladder, even if endoscopic techniques can be chosen in first line [4, 14,15,16,17,18]. In a recent review, endoscopic management of tape/mesh UP (63 cases) and BP (134 cases) was described as an effective minimally invasive technique with minimal morbidity [19]. Nonetheless, if the initial success rate with laser and endoscopic excision was 67% and 80%, respectively, 25% and 17.7% of patients needed one or more additional endoscopic procedures, with 8.3% and 2.2% of patients requiring an open surgical procedure. Three vesicovaginal fistulae and 2 BPs were reported. In our series, five patients had an endoscopic procedure before referral. Furthermore, two open partial resections and one endoscopic approach were previously performed in one patient.
Median time between tape/mesh insertion and revision surgery in our center was 64 (± 57) months, and mean time was 72 months (range 4 months −19.3 years), longer than recent reported data (23 to 34.5 months) [18, 20]. The variety of symptoms including UUI, chronic pelvic or urethral pain, recurrent UTI and voiding symptoms may be one reason why women were not diagnosed for a median of 64 months after the original surgery. For the patient with the latest revision surgery (19.3 years), presenting pain at micturition and dysuria, conservative treatments were attempted (analgesics, anti-depressants, pudendal nerve infiltration) before diagnosis of UP by cystoscopy. LUT perforations following tape/mesh insertion are significant complications that can result in long-lasting patient pain and distress before diagnosis. Patients with unexplained pain, storage or voiding symptoms or recurrent UTI should be thoroughly evaluated with a pelvic examination and cystourethroscopy.
In our study, mean defect size was 10.3 mm, the largest was 30 mm, and all the defects were surrounded by poor quality tissue. Polypropylene implants can cause especially a sustained inflammation, potentially triggered by a subclinical infection [5]. If tissue interposition can be useless in LUT defects surrounded by good quality tissue, most authors emphasized the need for well-vascularized flaps to ensure a successful outcome when the viability of the tissue is in doubt [4, 16, 21, 22]. In their series of urethral and vesicovaginal fistulae, Ockrim et al. reported that all failures occurred in case of extensive defects (≥ 30 mm) and limited native tissue to interpose [22]. The most widely used flap by the vaginal route is MFP [4, 16, 22], with only a few reported cases of LUT prosthesis perforations [10, 20, 23].
We reported few complications with no intraoperative complications, 5 postoperative complications of grade ≤ 2 and only one grade 3b postoperative complication, which was easily resolved. In contrast, patients undergoing MFP are at risk of bleeding (20%), wound infection (5%), hematoma (5%) and lymphorrhea (13%) [24]. One case of MFP necrosis was reported [16]. For Goujon et al., all the patients complained of harvest pain in the 8 weeks postoperatively [23]. Moreover, late complications such as pain or discomfort (5–38%), numbness (5–62%) and labial cosmetic problems (7–21%) can also occur [20, 24,25,26].
Intraoperatively, excessive bulking of the MFP can lead to difficulties in vaginal closure. Therefore, a vaginal flap might be necessary to close the vagina without tension [16]. In our experience, most patients with LUT tape/mesh perforation have severe peri-urethral/bladder scarring as well as vaginal retraction, and the use of such a vaginal flap can be hazardous. Facing these limitations using MFP in tape/mesh urinary perforations management, we advocate that SIS insertion is an easy procedure with a short operative time and low morbidity.
All patients were cured after this one-shot surgical procedure. Management of LUT defects after tape/mesh insertion is not standardized. Only small retrospective cohorts on TMR for LUT defects as well as case reports have been reported in the literature. A wide array of techniques has been published with varying levels of invasiveness, complexity and success [4, 14,15,16,17,18,19,20, 23, 27]. We performed a step-by-step, standardized surgical procedure.
Our results are in contrast to many authors reporting the need of additional reconstructive surgery. For Abbott et al., severe TMC required a median of two (range 1–9) revision surgeries [28]. Five patients (28%) needed between two to five operations to treat the penetrating tape for Goujon et al. [23]. Blaivas et al. reported an overall success in 34 of 47 (72%) patients after a single salvage operation [4].
We reported a high success rate in voiding symptoms and pain resolution, respectively, 100% and 92.8%. Dyspareunia was resolved in 8/12 patients (66.7%). We could not compare our results with other series because many of them have included TMR for both vaginal extrusion and LUT perforation [12, 14]. Blaivas et al. reported resolution of urethral obstruction in all patients after tape excision and MFP; however, pain and dyspareunia were cured or improved only in half of the women [4]. Recurrent UTIs were cured in 26/28 patients (92.8%) in our study, in accordance with the 85.7% (6/7) success rate reported by Frenkl et al. [17].
TMR with SIS insertion also led to good results in UUI resolution with 8/10 (80%) patients cured or improved. Data are somewhat contradictory in the literature: storage symptom improvement occurred only in 5/11 patients (45.4%) for Frenkl et al. whereas Goujon et al. reported a 100% rate resolution (12 patients) [17, 23].
In our study, among the 15 patients with postoperative SUI (39.5%), 14 underwent one or more additional surgical procedure. Large dissection during TMR and tape removal can lead to recurrent SUI. The likelihood of developing SUI after TMR for UP or BP is difficult to assess because most studies reported results including TMR for all types of TMC [12]. SUI recurrence rate was between 12.5 and 66.7% in the few studies focusing on TMR after BP and UP [15, 17,18,19, 23]. Furthermore, many authors used to perform a concomitant preventive SUI surgery (pubovaginal sling) with a 71–87% success rate (patients cured and improved) [4, 27, 29]. A 42–100% SUI recurrence rate was reported with the use of MFP without a pubovaginal sling [20, 23]. Our results are slightly better with a 39.5% SUI recurrence rate (15 patients). We agree with authors recommending that anti-incontinence surgery must be staged after this salvage operation in poor quality tissue [12, 18, 29]. A concomitant pubovaginal sling would add the risk of urethral obstruction. The need for postoperative intermittent catheterization at 1 month varies from 6% to 47% and rates of UUI range from 7% to 20% [16, 29].
In our center, we chose to reassess patients to offer appropriate SUI surgery following complete healing with a delay of at least 3 months following surgery [12, 29]. Indeed, complete SIS integration needs 6 to 12 weeks. Control cystoscopy was normal in all cases, with a restitution ad integrum of the tissue. At vaginal examination, the anterior vaginal wall was non-scarred. Urodynamic testing assessed an intrinsic sphincter deficiency for 11/ 15 women (73.3%). In case of good urethra mobility with a positive cough stress test, a retropubic tape was inserted. With a 85.7% success rate, our results are similar to series of concomitant pubovaginal slings [4, 27, 29], without adding morbidity. Only one complication occurred as a vaginal extrusion requiring the tape’s partial removal. In case of negative cough stress test, we performed ACT™ inflatable balloons or an artificial urinary sphincter. No intraoperative difficulty occurred during SUI surgery, with non-scarred tissue confounding with native tissue. Overall, after the first SUI surgery, 11/14 patients (78.6%) were cured, and 2 (14.3%) were improved. One patient needed an additional SUI surgery.
Limits of the study
We acknowledge limits of our study. First, the impact of this study is limited by its retrospective and descriptive nature. Our retrospective study was not designed to allow pre- and postoperative comparative data using validated functional questionnaires for LUT symptoms and sexuality. Sexuality assessment was particularly impaired, with missing data for 10 patients (26.3%) before revision surgery and for 2 patients (16.7%) during postoperative assessment among the 12 women presenting preoperative dyspareunia. Second, it is difficult to highlight the exact role of SIS in curing or preventing SUI in this small cohort. Among the 15 patients presenting preoperative MUI or SUI, 6 were cured or improved (40%). Results are heterogeneous in the literature with a 0–58% success rate on SUI after tape removal for UP with repair of the urethral defect and MFP [20, 23]. Third, the defect’s size was missing in the operative report for five patients (13.2%); the intraoperative decision for SIS insertion was therefore difficult to assess in these cases. Finally, all included patients being referred to our center for complication management and the number of initially inserted tapes/meshes in the same period was unknown, and we could not estimate the incidence of LUT prosthesis perforation. This limitation was also reported by other authors [12]. It would be of great interest to estimate the incidence of LUT prosthesis perforations as a TMC. A register would help to answer this.
As another limit, our study is a monocentric experience of three senior surgeons. However, in our university center, we performed surgery while teaching interns and residents and we standardized all surgical steps to simplify the procedure’s learning curve. We advocate that SIS insertion is a reproducible surgical procedure.
Management of LUT prosthesis perforations would require guidelines, with evaluation of the incidence and functional impact of these complications.
Prospective registries and/or national health system databases could provide these valuable data.
Conclusion
With a high success rate and low morbidity, absorbable SIS is an effective, feasible and safe xenograft for LUT tape/mesh perforations. Functional results are good, despite an expected risk of postoperative SUI recurrence due to the large dissection and tape removal. The cost has to be balanced with good results, a short operative time and no fat pad complications as in MFP. Comparative studies are needed to implement the optimal strategy in this salvage surgery.
Abbreviations
- SUI:
-
Stress urinary incontinence
- POP:
-
Pelvic organ prolapse
- TMC:
-
Tape/mesh complication
- IUGA/ICS C:
-
International Urogynecological Association/International Continence Society classification
- TMR:
-
Tape/mesh removal
- UP:
-
Urethral perforation
- BP:
-
Bladder perforation
- LUT:
-
Lower urinary tract
- MFP:
-
Martius fat pad
- SIS:
-
Small intestinal submucosa
- UUI:
-
Urge urinary incontinence
- UTI:
-
Urinary tract infections
- BNP:
-
Bladder neck perforation
- MUI:
-
Mixed urinary incontinence
References
Food and Drug Administration. Urogynecologic Surgical Mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse [Internet]. 2011. Available from: https://www.fda.gov/downloads/medicaldevices/safety/alertsandnotices/ucm262760.pdf
Haylen BT, Freeman RM, Swift SE, Cosson M, Davila GW, Deprest J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prostheses (meshes, implants, tapes) and grafts in female pelvic floor surgery. Neurourol Urodyn. 2011;30(1):2–12.
Miklos JR, Chinthakanan O, Moore RD, Mitchell GK, Favors S, Karp DR, et al. The IUGA/ICS classification of synthetic mesh complications in female pelvic floor reconstructive surgery: a multicenter study. Int Urogynecol J. 2016;27(6):933–8.
Blaivas JG, Purohit RS, Weinberger JM, Tsui JF, Chouhan J, Sidhu R, et al. Salvage surgery after failed treatment of synthetic mesh sling complications. J Urol. 2013;190(4):1281–6.
Mangir N, Roman S, Chapple CR, MacNeil S. Complications related to use of mesh implants in surgical treatment of stress urinary incontinence and pelvic organ prolapse: infection or inflammation? World J Urol. 2020;38(1):73–80.
Martius H. Die Operative Wiederherstellungder Vollkommen Fehlenden Harnrohre und des Schiessmuskels Derselbel. Zentralbl Gynakol. 1928;52:480–6.
Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol. 2004;12(3–4):367–77.
Ayyildiz A, Akgül KT, Huri E, Nuhoğlu B, Kiliçoğlu B, Ustün H, et al. Use of porcine small intestinal submucosa in bladder augmentation in rabbit: long-term histological outcome. ANZ J Surg. 2008;78:82–6.
Record RD, Hillegonds D, Simmons C, Tullius R, Rickey FA, Elmore D, et al. In vivo degradation of 14C-labeled small intestinal submucosa (SIS) when used for urinary bladder repair. Biomaterials. 2001;22:2653–9.
Deng D, Rutman M, Raz S, et al. Presentation and management of major complications of midurethral slings: are complications under-reported? Neurourol Urodyn. 2007;26:46–52.
Tulokas S, Rahkola-Soisalo P, Gissler M, Mikkola TS, Mentula MJ. Long-term re-procedure rate after mid-urethral slings for stress urinary incontinence. Int Urogynecol J. 2020;31(4):727–35.
Ismail S, Chartier-Kastler E, Reus C, Cohen J, Seisen T, Phé V. Functional outcomes of synthetic tape and mesh revision surgeries: a monocentric experience. Int Urogynecol J. 2019;30(5):805–13.
Farahat YA, Elbendary MA, Elgamal OM, Tawfik AM, Bastawisy MG, Radwan MH, et al. Application of small intestinal submucosa graft for repair of complicated vesicovaginal fistula: a pilot study. J Urol. 2012;188:861–4.
MacDonald S, Terlecki R, Costantini E, Badlani G. Complications of transvaginal mesh for pelvic organ prolapse and stress urinary incontinence: tips for prevention, recognition and management. Eur Urol Focus. 2016;2(3):260–7.
Amundsen CL, Flynn BJ, Webster GD. Urethral erosion after synthetic and nonsynthetic pubovaginal slings: differences in management and continence outcome. J Urol. 2003;170(1):134–7 discussion 137.
Flisser AJ, Blaivas JG. Outcome of urethral reconstructive surgery in a series of 74 women. J Urol. 2003;169(6):2246–9.
Frenkl TL, Rackley RR, Vasavada SP, HB Goldman. Management of iatrogenic foreign bodies of the bladder and urethra following pelvic floor surgery. Neurourol Urodyn 2008;27(6):491–495.
Padmanabhan P, Hutchinson RC, Reynolds WS, Kaufman M, Scarpero HM, Dmochowski RR. Approach to management of iatrogenic foreign bodies of the lower urinary tract following reconstructive pelvic surgery. J Urol. 2012;187(5):1685–90.
Karim SS, Pietropaolo A, Skolarikos A, Aboumarzouk O, Kallidonis P, Tailly T, de Coninck V, Keller EX, Somani BK. Role of endoscopic management in synthetic sling/mesh erosion following previous incontinence surgery: a systematic review from European Association of Urologists Young Academic Urologists (YAU) and Uro-technology (ESUT) groups. Int Urogynecol J 2020;31(1):45–53.
Malde S, Spilotros M, Wilson A, Pakzad M, Hamid R, Ockrim J, et al. The uses and outcomes of the Martius fat pad in female urology. World J Urol. 2017;35:473–8. https://doi.org/10.1007/s00345-016-1887-2.
Pshak T, Nikolavsky D, Terlecki R. Flynn BJ. Is tissue interposition always necessary in transvaginal repair of benign, recurrent vesicovaginal fistulae? Urology. 2013;82(3):707–12.
Ockrim JL, Greenwell TJ, Foley CL, Wood DN. Shah PJR. A tertiary experience of vesico-vaginal and urethro-vaginal fistula repair: factors predicting success. BJU Int. 2009;103:1122–6.
Goujon E, Jarniat A, Bardet F, Bergogne L, Delorme E. Retrospective study on the management and follow-up of 18 patients with a mid-urethral sling penetrating the urethra or bladder. J Gynecol Obstet Hum Reprod. 2018;47:289–97.
Kasyan G, Tupikina N, Pushkar D. Use of Martius flap in the complex female urethral surgery. Cent Eur J Urol. 2014;67:202–7.
Petrou SP, Jones J, Parra RA. Martius flap harvest site: patient self-perception. J Urol. 2002;167(5):2098–9.
Lee D, Dillon BE, Zimmern PE. Long-term morbidity of Martius labial fat pad graft in vaginal reconstruction surgery. Urology. 2013;82:1261–6.
McCoy O, Vaughan T, Nickles SW, Ashley M, MacLachlan LS, Ginsberg D, et al. Outcomes of autologous fascia Pubovaginal sling for patients with transvaginal mesh related complications requiring mesh removal. J Urol. 2016;196(2):484–9.
Abbott S, Unger CA, Evans JM, Jallad K, Mishra K, Karram MM, et al. Evaluation and management of complications from synthetic mesh after pelvic reconstructive surgery: a multicenter study. Am J Obstet Gynecol. 2014;210(2):163.e1-8.
Shah K, Nikolavsky D, Gilsdorf D, Flynn BJ. Surgical management of lower urinary mesh perforation after mid-urethral polypropylene mesh sling: mesh excision, urinary tract reconstruction and concomitant pubovaginal sling with autologous rectus fascia. Int Uro- gynecol J. 2013;24:2111–7.
Acknowledgements
Authors wish to acknowledge Elodie Menechal, secretary in functional urology, for her technical assistance in data collection, Tarek Ghoneim, MD, for English proofreading and Caroline Pettenati, MD, for manuscript comments.
Funding
This study did not receive funding.
Author information
Authors and Affiliations
Contributions
F Cour: Project development, Data collection, Data analysis, Manuscript writing.
P Munier: Data collection, Manuscript writing.
K Kaulanjan: Data collection.
A Vidart: Project development.
PO Bosset: Project development.
Y Neuzillet: Manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
Florence Cour, Pierre Munier and Kevin Kaulanjan declare that they have no conflict of interest.
Adrien Vidart is a consultant for Boston Scientific.
Pierre-Olivier Bosset is a consultant for Janssen and da Vinci.
Yann Neuzillet has accepted paid travel expenses and has received a speaker honorarium from Astellas, Merck Sharp and Dohme and Ipsen; he is a consultant for AstraZeneca, Bayer, Bristol-Myers Squibb, Sanofi.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cour, F., Munier, P., Kaulanjan, K. et al. Small intestinal submucosa xenograft to manage lower urinary tract prostheses perforation: a new path?. Int Urogynecol J 33, 627–635 (2022). https://doi.org/10.1007/s00192-021-04771-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-021-04771-5