Abstract
Introduction and hypothesis
Pelvic organ prolapse (POP) in women is associated with deficiency of elastic fibers, and fibulin-5 is known to be a critical protein in the synthesis of elastin. The purpose of this study is to investigate the related pathway for the synthesis of elastin via fibulin-5 using fibulin-5 knockout mice.
Methods
Fibulin-5 knockout mice were generated using the CRISPR/Cas9 system, and vaginal dilatation was used to mimic vaginal delivery. We divided the mice into three groups: Fbln5+/+ mice immediately after dilatation (Fbln5+/+ day0), Fbln5+/+ mice 3 days after dilatation (Fbln5+/+ day3) and Fbln5−/− mice 3 days after dilatation (Fbln5−/− day3). Proteins related to elastogenesis in the vaginal wall were measured by liquid chromatography mass spectrometry (LC-MS/MS) analysis, and differences in the expression of these proteins between the Fbln5−/− mice and the Fbln5+/+ mice were analyzed using western blotting.
Results
In the LC-MS/MS analysis, protein tyrosine kinase 7 (PTK7) was not detected in the Fbln5−/− day3 group, although the expression increased by > 1.5 times between the Fbln5+/+ day0 and day3 groups. PTK7 and β-catenin are known to act in the Wnt/β-catenin pathway, and both were upregulated after dilatation in the Fbln5+/+ mice, though not in the Fbln5−/− mice.
Conclusion
Our findings suggest that these proteins are involved in elastogenesis via fibulin-5, and the impairment of these proteins might be the underlying cause of POP manifestation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of pelvic organ prolapse (POP) is 19.7% in developing countries, and the disease causes intense health impairment in both developed and developing countries. Moreover, POP greatly affects womens’ social activities as well as both their mental and physical health [1]. Vaginal birth compared with cesarean section increases the risk of POP according to recent epidemiological studies [2, 3]. The etiology of POP is multifunctional with a growing body of evidence indicating that it can be associated with a genetic pre-disposition. Epidemiologic analyses indicate that a family history of POP is a highly significant risk factor [4, 5]. Jung et al. showed that fibulin-5 expression was lower in the uterosacral ligaments of POP patients, and these results suggest the possibility of defects in elastin synthesis [6] or that fibulin-5 can be a candidate protein associated with POP patients, especially after pelvic floor injury [7]. Nakamura et al. reported that fibulin-5 knockout (KO) mice generated by gene targeting exhibited a severely disorganized elastic fiber system throughout the body and had loose skin, severe emphysema and a tortuous aorta with loss of compliance [8]. Moreover, Drewes et al. reported that fibulin-5 KO mice showed a deficiency of elastic fiber synthesis and presented this in their POP animal model [9]. These findings in mice lead us to suggest that the impaired ability to synthesize or repair new elastic fibers due to a genetic defect in elastic fiber synthesis can cause POP in some women.
Elastic fibers are present in most force-bearing soft tissues located in the lungs, skin, large arteries and also the pelvic floor organs. Elastic fibers enable these organs to stretch and bend with recoil and thus sustain physiological functions. The process of elastogenesis involves numerous proteins and other biomolecules, and its complexity is still only partially understood [10]. The purpose of the present study is to investigate the related pathway or mechanism for the synthesis of elastin via fibulin-5 using fibulin-5 KO mice generated by a clustered, regularly interspaced short palindromic repeats (CRISPR/Cas9) system for a POP animal model.
Materials and methods
Mice
We performed all protocols and experimental procedures in compliance with the institutional guidelines of the Institutional Review Board and received approval from the ethical committee of the Institutional Animal Care and Use Committee of Osaka City University. Fourteen-week-old C57BL/6NCrSlc mice were purchased from SLC (Hamamatsu, Japan). All mice were treated with human care according to the Guide for the Care and Use of Laboratory Animals, National Institutes of Health. All mice were housed in a temperature-controlled (24 ± 1 °C) environment, with humidity levels of 55 ± 5% and alternating 12-h light/12-h dark cycles. They had free access to water and a standard rodent diet.
Generation and maintenance of fibulin-5 KO mice
Fibulin-5 KO mice were generated using CRISPR/Cas9 genome editing technology. A single guided RNA (sgRNA) was designed with the sequence of CCAGTGTATCGAGGGCCTTACTC for targeting exon 4 of the fibulin-5 gene. Cas9 mRNA (Thermo Fisher Scientific, Waltham, MA, USA) and sgRNA were microinjected into the cytoplasm of the zygote at the pronuclei stage. The injected zygotes were then transferred to pseudopregnant mice. After confirmation of the DNA sequence by Sanger sequencing, gene-edited male mice were inbred to obtain homozygous KO mice. To examine genotypes of pups, PCR analysis was performed with primers (forward-1 5’ATCCCTGGAACCAACCCACGATTTCAAGG 3′, forward-2 5’ CTCACATCCTACTCAGGCCCATACCCAGC 3′, reverse 5’ CCATGAAATATGTCAATCAGACAGTGGTC 3′) using Go Taq Green mMster Mix (Promega, Madison, WI, USA).
Vaginal dilatation and vaginal wall preparation
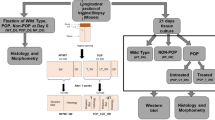
Vaginal mechanical dilatation was performed with a 6-mm-diameter glass rod for 10 s to mimic vaginal delivery (Fig. 1A, B). The mice were divided into three groups: Fbln5+/+ mice immediately after vaginal mechanical dilatation (Fbln5+/+ day0), Fbln5+/+ mice 3 days after dilatation (Fbln5+/+ day3) and Fbln5−/− mice 3 days after dilatation (Fbln5−/− day3). Each group of mice underwent the dilatation described above, and all mice were anesthetized with isoflurane (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) inhalation. All subjects were killed by cervical dislocation, and the rectal vaginal fascia was collected for all analyses described hereafter. The samples were homogenized with a pestle (As one, Osaka, Japan) in a 1.5-ml microtube with lysate, and the pellet was removed. The samples were then used for liquid chromatography mass spectrometry (LC-MS/MS) analysis and western blotting analysis.
Histology and immunohistochemistry
Tissues from the vaginal wall, lung and aorta were fixed with formalin, embedded in paraffin and sectioned at 4 μm. The sections were then stained with hematoxylin and eosin (HE) staining and Elastica van Gieson (EVG) staining. The tissues were observed using a fluorescence digital microscope (BZ-8000; Keyence, Tokyo, Japan).
Mass spectrometry (MS) sample preparation and protein identification
MS samples were desalted and concentrated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 9% polyacrylamide gradient gel, and the resulting gels were stained with Coomassie Brilliant Blue G250 (Sigma-Aldrich, St. Louis, MO, USA). In-gel trypsin digestion (Promega, Madison, WI, USA) was then performed, and the resulting peptides were sequentially extracted from the gel.
The gel-extracted peptides were then dried, dissolved in a solution containing 0.1% trifluoroacetic acid (TFA) and 2% quinoclamine and subjected to nanoLC-MS/MS analysis using an Orbitrap Velos Pro mass spectrometer system (Thermo Fisher Scientific) coupled with an Advance UHPLC (Bruker, Billerica, MA, USA). The peak lists were generated using MSn.exe (Thermo Fisher Scientific) and compared with an in-house-curated target/decoy UniProt Release 2017_05 database (SwissProt database,16,935 entries; European Bioinformatics Institute) using the MASCOT algorithm (version 2.6.2; Matrix Science Inc., Boston, MA, USA).
Scaffold (version Scaffold_4.2.1, Proteome Software Inc., Portland, OR, USA) was used to validate MS/MS-based peptide and protein identification, and proteins were annotated with GO terms from gene_association.goa_uniprot.
Western blot analysis
Proteins were extracted with a radioimmunoprecipitation assay buffer, and the total protein count was quantified using a bicinchoninic acid (BCA) protein assay. Protein samples with loading buffer were separated by 7.5% SDS-PAGE and transferred to polyvinylidene difluoride membranes. After blocking, the membranes were probed with primary antibodies: anti-PTK7, anti-β-catenin (1:1000, 1:1000; Cell Signaling Technology, Danvers, MA, USA) and HRP-coupled secondary antibody purchased from GE Healthcare (Little Chalfont, UK). HRP-coupled anti-β-actin was purchased from Sigma-Aldrich, and β-actin was used as a loading control. We detected proteins by enhanced chemiluminescence (Immobilon Western HRP Substrate; Merck-Millipore) using a Fusion SOLO.7S (Vilber Lourmat, Collégien, France). Data from immunoblots were quantified using ImageQuant TL Analysis Toolbox software (GE Healthcare).
Statistical analysis
All data and means are presented. Comparisons among each group were made by the Student’s t-test. Differences were considered statistically significant at p < 0.05, and the data were analyzed via SPSS 21 (SPSS Inc., Chicago, IL, USA).
Results
Generating fibulin-5 KO mice
Twenty pups were obtained after microinjection; eight of them had gene modification on the fibulin-5 gene. The efficiency of generating fibulin-5 gene edited mice using CRISPR/Cas9 technology (40%, 8/20) was almost the same as previously reported [11].
Phenotype of Fbln5−/− mice
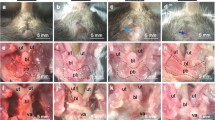
All Fbln5−/− mice showed perineal body prolapse and rectal prolapse until 25 weeks (n = 20) (Fig. 2A, B), and all mice in this group showed lax skin (data not shown). EVG stainings of cross sections of the posterior vaginal wall are shown (Fig. 2C, D). The elastic fibers from Fbln5+/+ mice had formed long and thick strands, but those from Fbln5−/− mice were fragmented and thin. EVG stainings of the cross sections of the descending aorta are shown (Fig. 2E, F). The elastic fibers from Fbln5+/+ mice had also formed long and thick strands, but those from Fbln5−/− mice were fragmented and thickened. Enlarged alveoli were observed in the lungs from Fbln5−/− mice (Fig. 2G, H).
Histological analysis of Fbln5+/+ mice or Fbln5−/− mice at 20 weeks: A Fbln5+/+ mice did not show any pelvic organ prolapse. B Rectal prolapse (arrow a) and perineal body prolapse (arrow b) were observed in Fbln5−/− mice. C, D Elastica van Gieson (EVG) staining from the posterior vaginal wall from Fbln5+/+ or Fbln5−/− mice. In Fblin5+/+ mice, long and thick elastic fibers were observed. In Fbln5−/− mice, the elastic fibers were fragmented and thin. Elastic fibers are indicated with arrows. Bar = 50 μm. E, F Elastica van Gieson (EVG) staining of cross sections of the descending aorta. The elastic fibers of Fbln5−/− mice were fragmented and thickened. Bar = 50 μm. G, H Lung sections stained with hematoxylin and eosin staining from Fbln5+/+ or Fbln5−/− mice. Expanded alveoli were seen in Fblin5−/− mice. Bar = 200 μm
Identification of proteins involved in elastogenesis in vaginal walls of mice
To examine the differences in proteins in the vaginal walls of mice after vaginal mechanical dilatation, a quantitative proteomics analysis of differentially expressed proteins was performed. We performed the vaginal mechanical dilatation with a 6-mm-diameter glass rod for 10 s to mimic vaginal delivery (Fig. 1A, B). The proteins contained in Fbln5+/+ mice immediately after vaginal dilatation (Fbln5+/+ day0), Fbln5+/+ mice 3 days after dilatation (Fbln5+/+ day3) and Fbln5−/− mice 3 days after dilatation (Fbln5−/− day3) were examined. In total, 1932 proteins were detected (Fig. 3A). One hundred thirty proteins of these 1932 proteins were not detected in the Fbln5−/− day0 group. Twenty-six proteins were detected only in the Fbln5+/+ day3 group. Forty-six proteins were not detected in the Fbln5−/− day3 group but were detected in the Fbln5+/+ day0 and Fbln5+/+ day3 groups. Seventeen proteins were not detected in the Fbln5−/− day3 group, but they showed a change in expression > 1.5 times between the Fbln5+/+ day0 and day3 groups (Fig. 3B). The proteins classified in the group were as follows: CPT1A, FBLN5, PTK7, CA12, TOMM34, BDH1, RPLP1, VSNL1, NUP214, DDX46, SLC16A3, CPD, ARHGEF12, AKT1, ARHGEF7, AIFM1 and RBP4 (Table 1). Fibulin-5 was found in that group, so we concluded that some of these proteins interact with fibulin-5. We then focused on PTK7, which is known to exist on the cell membrane and interacts with β-catenin in the Wnt/β-catenin pathway.
Identification and quantitative comparison of proteins involved in the posterior vaginal wall of the Fbln5+/+ control group, after day 3 of vaginal dilatation and Fbln5−/− after day 3 of vaginal dilatation: A Venn diagram showing the number of identified proteins from the mice of the three groups. B Summary of quantitative analysis of the posterior vaginal wall in which proteins are not detected from Fbln5−/− mouse samples
Western blotting analysis
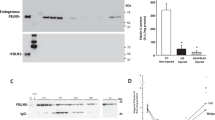
The proteins contained in the posterior vaginal wall from Fbln5+/+ and Fbln5−/− mice immediately after vaginal mechanical dilatation (day0), 3 days after dilatation (day3) and 7 days after dilatation (day7) were examined. PTK7 was significantly upregulated after 7 days of dilatation in Fbln5+/+ mice (P = 0.002), but no significant difference was found in Fbln5−/− mice (P = 0.192) (Fig. 4A, B). β-Catenin was also significantly upregulated after 7 days of dilatation in Fbln5+/+ mice (P = 0.005), but there was no significant difference in Fbln5−/− mice (P = 0.865) (Fig. 4A, B). Moreover, there was no significant difference in PTK7 and β-catenin expressions between Fbln5+/+ and Fbln5−/− mice at day 0 (P = 0.628).
Western blotting analysis of PTK7 and β-catenin in the tissues of the posterior vaginal wall from adult (14 weeks) mice: A Western blot analysis using anti-β-catenin and anti-PTK7 antibodies. Anti-β-actin was used as an internal standard. B Representative immunoblotting and quantification of PTK7 and β-catenin in the tissues of the posterior vaginal wall (n = 3). The unit is relative units/mg protein compared with the mean of Fbln5+/+ day0. β-Actin was used as a loading control. *P < 0.05 compared with vagina at day0; differences were analyzed with Student’s t-test
Discussion
It is well known that the causes of POP are multifactorial and that several environmental factors, vaginal delivery for instance, can cause quantitative and qualitative changes in the connective tissue that supports the lower pelvic organs. The detailed mechanisms of POP caused by pregnancy and parturition are not well known. Analysis from a biochemical perspective can yield better findings as to the causes of POP, and this information may lead us to establish preventive or treatment measures that could eliminate the onset of the disease.
The synthesis of elastin involves a complex pathway, and it is known that fibulin-5 is a critical protein for aiding the assembly of elastic fibers. Additionally, this protein has shown a high binding affinity to tropoelastin [12]. Elastic fibers are made of a central core of elastin surrounded by fibrillin-1 rich microfibrils, and fibulin-5 has been shown to bind to N-terminal fragments of fibrillin-1 to connect cells and elastic fibers [13]. Without fibulin-5, it is reported that elastic fibers are isolated from microfibrils and that tropoelastin simply exists in pieces [14,15,16]. Therefore, it has been proposed that fibulin-5 provides a link between tropoelastin and microfibrils in the pericellular space during elastic fiber assembly. Moreover, fibulin-5 is reported to be related to the regulation of the metabolism of elastic fibers, and a deficiency of the protein can cause POP as well as other diseases such as inguinal hernia, rheumatoid arthritis or chronic obstructive pulmonary disease [9, 15]. The fibulin-5 KO mice generated by Nakamura et al. showed loose skin hanging in folds, severe emphysema and marked aortic tortuosity [15]. Drewes et al. and Chin et al. also showed that Fbln5−/− mice exhibited POP [9, 17]. Additionally, Nakagawa et al. generated fibulin-5 KO mice using a gene-targeting method for the first time, and others have reported using the same method [15]. In this study, we first used a CRISPR/Cas9 system to generate fibulin-5 KO mice, and our mice showed perineal aneurysm and rectal prolapse similar to that in past studies. In previous histological analyses, fibulin-5 KO mice have been reported to show the fragmentation of elastic fibers in the vaginal wall [9, 17]. In this study, fibulin-5 KO mice also showed the fragmentation of elastic fibers in the vaginal wall as well as in the aorta. To generate fibulin-5 KO mice, we used the CRISPR/Cas9 system because this method takes less time to generate subjects, and those mice that it produces have the same phenotype and histologically similar morphology. Furthermore, this method makes it possible to generate KO mice more efficiently, and this is considered very useful in such studies.
LC-MS/MS analysis was performed to find the differences in protein expressions in the vaginal walls between Fbln5+/+ and Fbln5−/− mice. Our examination by the LS-MS/MS method on day0 and day3 after the vaginal mechanical dilatation in the Fbln5−/− and Fbln5+/+ groups yielded 1932 identifiable proteins. Among them, 130 proteins were not detected in Fbln5−/− mice but were detected in Fbln5+/+ mice. Among these 130 proteins, 17 of them (including PTK7) increased by > 1.5 times between the Fbln5+/+ day0 and day3 groups. It is reported that collagen acts as an important structural component, and matrix metalloproteinases (MMPs), known as collagen-degrading enzymes, are upregulated in the vaginal wall of POP patients [18]. In this study, the changes in the expression of collagen were not clearly detected. It is assumed that the MS sample preparation affects the protein identification. PTK7 is a protein related to the construction of the extracellular matrix, and, in this study, the trend of the PTK7 expression level showed a similar pattern to that of fibulin-5. We therefore considered PTK7 to be related to fibulin-5 and focused on PTK7’s role in elastrogenesis.
PTK7 has a potential function as a molecular switch between Wnt signaling pathways and the transmembrane receptor that regulates morphogenetic processes [19]. Based on the dependence of the Wnt signaling effector, Wnt signaling pathways can be characterized by a “canonical pathway” and several “noncanonical pathways.” The canonical Wnt pathway is also known as the “Wnt/β-catenin pathway,” and the noncanonical pathways include the planer cell polarity, c-Jun N-terminal protein kinases, protein kinase C/calcium, receptor-like tyrosine kinase and receptor tyrosine kinase-like orphan receptor pathways [20, 21]. The Wnt/β-catenin pathway is characterized by Wnt binding to its coreceptor complex (that constituted by the low-density lipoprotein-related receptor 5 or 6) and to a member of the ten frizzled family of proteins [22, 23].
Shi et al. reported that activation of Wnt signaling, particularly Wnt/β-catenin signaling, was involved in the pathogenesis of chronic pulmonary diseases such as chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis [24]. Our results showed that PTK7 increased significantly from day0 to day7 in Fbln5+/+ mice after vaginal mechanical dilatation, though it did not change from day0 to day7 in Fbln5−/− mice. This result indicates that PTK7 is involved in the repair of elastic fibers after vaginal mechanical dilatation and that fibulin-5 is a necessary protein for PTK7 expression.
There are some reports on the relationship between fibulin-5 and the Wnt/β-catenin pathway. Naboulsi et al. analyzed quantitative tissue proteomics in 50 patients with hepatocellular carcinoma and 50 patients with non-hepatocellular carcinoma. As a result, they reported that abnormal regulation of the Wnt/β-catenin signaling pathway was associated with fiblin-5 expression; however, the mechanism of action was still unknown [25]. Gao et al. also reported that fibulin-5 protected the extracellular matrix of chondrocytes by inhibiting the Wnt/β-catenin signaling pathway and relieved osteoarthritis [26].
In our study, the β-catenin expression level did not change in Fbln5−/− mice from day0 to day7 after vaginal mechanical dilatation. It did increase significantly, however, in Fbln5+/+ mice, and these results indicate that fibulin-5 promotes the Wnt/β-catenin pathway in the process of repairing elastin. It has been suggested that fibulin-5 acts as an organizer that inhibits the Wnt/β-catenin pathway in both situations of protecting the extracellular matrix in osteoarthritis patients [26] and promoting it in situations of repairing elastin in POP patients. However, the detailed mechanism for controlling this pathway has not yet been clarified.
In conclusion, we succeeded in generating Fbln5 KO mice using the CRISPR/Cas9 system and showed that PTK7 and β-catenin are both involved in elastogenesis via fibulin-5. The pelvic floor supports the vagina and other pelvic organs with a complex dynamic system. Elastic fiber synthesis is critical for supporting normal pelvic organs, and this synthesis is accompanied by a number of complex processes in the vaginal wall after parturition. In elastic fiber synthesis pathways, some remodeling processes of the vaginal wall have been suggested to be involved, although this has not been clearly defined. Our results in Fbln5−/− mice lead us to propose that a deficiency in the restoration and synthesis of elastic fibers via fibulin-5 due to a genetic defect in elastogenesis may lead to POP in some women and that PTK7 and β-catenin involvement is indicated. Clarification of the exact pathway of elastogenesis could enable us to establish new therapies in preventing or ameliorating the clinical symptoms of this disease.
References
Walker GJA, Gunasekera P. Pelvic organ prolapse and incontinence in developing countries: review of prevalence and risk factors. Int Urogynecol J. 2011;22:127–35. https://doi.org/10.1007/s00192-010-1215-0.
Gyhagen M, Bullarbo M, Nielsen T, Milsom I. Prevalence and risk factors for pelvic organ prolapse 20 years after childbirth: a national cohort study in singleton primiparae after vaginal or caesarean delivery. BJOG An Int J Obstet Gynaecol. 2013;120:152–60. https://doi.org/10.1111/1471-0528.12020.
López-López AI, Sanz-Valero J, Gómez-Pérez L, Pastor-Valero M. Pelvic floor: vaginal or caesarean delivery? A review of systematic reviews. Int Urogynecol J. 2020. https://doi.org/10.1007/s00192-020-04550-8.
Chiaffarino F, Chatenoud L, Dindelli M, et al. Reproductive factors, family history, occupation and risk of urogenital prolapse. Eur J Obstet Gynecol Reprod Biol. 1999;82:63–7. https://doi.org/10.1016/S0301-2115(98)00175-4.
Friedman T, Eslick GD, Dietz HP. Risk factors for prolapse recurrence: systematic review and meta-analysis. Int Urogynecol J. 2018;29:13–21. https://doi.org/10.1007/s00192-017-3475-4.
Jung HJ, Jeon MJ, Yim GW, et al. Changes in expression of fibulin-5 and lysyl oxidase-like 1 associated with pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol. 2009;145:117–22. https://doi.org/10.1016/j.ejogrb.2009.03.026.
Khadzhieva MB, Kamoeva SV, Chumachenko AG, et al. Fibulin-5 (FBLN5) gene polymorphism is associated with pelvic organ prolapse. Maturitas. 2014;78:287–92. https://doi.org/10.1016/j.maturitas.2014.05.003.
Hirai M, Ohbayashi T, Horiguchi M, et al. Fibulin-5/DANCE has an elastogenic organizer activity that is abrogated by proteolytic cleavage in vivo. J Cell Biol. 2007;176:1061–71. https://doi.org/10.1083/jcb.200611026.
Drewes PG, Yanagisawa H, Starcher B, et al. Pelvic organ prolapse in fibulin-5 knockout mice: pregnancy-induced changes in elastic fiber homeostasis in mouse vagina. Am J Pathol. 2007;170:578–89. https://doi.org/10.2353/ajpath.2007.060662.
Keeley FW. The synthesis of soluble and insoluble elastin in chicken aorta as a function of development and age. Effect of a high cholesterol diet. Can J Biochem. 1979;57:1273–80. https://doi.org/10.1139/o79-169.
Wang H, Yang H, Shivalila CS, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–8. https://doi.org/10.1016/j.cell.2013.04.025.
Choudhury R, McGovern A, Ridley C, et al. Differential regulation of elastic fiber formation by fibulin-4 and -5. J Biol Chem. 2009;284:24553–67. https://doi.org/10.1074/jbc.M109.019364.
Elahi E, Kalhor R, Banihosseini SS, et al. Homozygous missense mutation in fibulin-5 in an Iranian autosomal recessive cutis laxa pedigree and associated haplotype. J Invest Dermatol. 2006;126:1506–9. https://doi.org/10.1038/sj.jid.5700247.
Zhao BH, Zhou JH. Decreased expression of elastin, fibulin-5 and lysyl oxidase-like 1 in the uterosacral ligaments of postmenopausal women with pelvic organ prolapse. J Obstet Gynaecol Res. 2012;38:925–31. https://doi.org/10.1111/j.1447-0756.2011.01814.x.
Nakamura T, Lozano PR, Ikeda Y, et al. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–5. https://doi.org/10.1038/415171a.
Papke CL, Yanagisawa H. Fibulin-4 and fibulin-5 in elastogenesis and beyond: insights from mouse and human studies. Matrix Biol. 2014;37:142–9.
Chin K, Wieslander C, Shi H, et al. Pelvic organ support in animals with partial loss of fibulin-5 in the vaginal wall. PLoS One. 2016;11:1–16. https://doi.org/10.1371/journal.pone.0152793.
Abramowitch SD, Feola A, Jallah Z, Moalli PA. Tissue mechanics, animal models, and pelvic organ prolapse: a review. Eur J Obstet Gynecol Reprod Biol. 2009;144:S146–58. https://doi.org/10.1016/j.ejogrb.2009.02.022.
Lhoumeau AC, Puppo F, Prébet T, et al. PTK7: a cell polarity receptor with multiple facets. Cell Cycle. 2011;10:1233–6. https://doi.org/10.4161/cc.10.8.15368.
Nusse R. Disarming Wnt. Nature. 2015;519:163–4. https://doi.org/10.1038/nature14208.
Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. https://doi.org/10.4161/org.4.2.5851.
Tamai K, Semenov M, Kato Y, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–5. https://doi.org/10.1038/35035117.
Wehrli M, Dougan ST, Caldwell K, et al. Arrow encodes an LDL-receptor-related protein essential for wingless signalling. Nature. 2000;407:527–30. https://doi.org/10.1038/35035110.
Shi J, Li F, Luo M, et al. Distinct roles of Wnt/ β -catenin signaling in the pathogenesis of chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Mediat Inflamm. 2017;2017:1–16. https://doi.org/10.1155/2017/3520581.
Naboulsi W, Megger DA, Bracht T, et al. Quantitative tissue proteomics analysis reveals Versican as potential biomarker for early-stage hepatocellular carcinoma. J Proteome Res. 2016;15:38–47. https://doi.org/10.1021/acs.jproteome.5b00420.
Gao JB, Lin L, Men XQ, et al. Fibulin-5 protects the extracellular matrix of chondrocytes by inhibiting the Wnt/β-catenin signaling pathway and relieves osteoarthritis. Eur Rev Med Pharmacol Sci. 2020;24:5249–58. https://doi.org/10.26355/eurrev_202005_21307.
Acknowledgements
This work was partly performed in the Cooperative Research Project Program of the Medical Institute of Bioregulation, Kyushu University. We thank the Research Support Platform of Osaka City University Graduate School of Medicine for their technical support. This work was supported by JSPS KAKENHI grant number JP16K11147.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Uemura, R., Tachibana, D., Shiota, M. et al. Upregulation of PTK7 and β-catenin after vaginal mechanical dilatation: an examination of fibulin-5 knockout mice. Int Urogynecol J 32, 2993–2999 (2021). https://doi.org/10.1007/s00192-021-04693-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-021-04693-2