Abstract
Introduction and hypothesis
Chronic perineal pain syndrome due to pudendal nerve impingement is difficult to diagnose and to treat. All the known treatment options leave room for improvement considering the outcome. Early neuromodulation of the pudendal nerve after its surgical release could improve outcomes.
Objectives
The aim of the study was to evaluate the potential beneficial effect of pudendal neuromodulation combined with release surgery using the ENTRAMI technique (endoscopic transgluteal minimally invasive technique).
Study design
This is a single-center prospective descriptive study. Between March 2019 and March 2020, 16 patients (2 males, 14 females) were included. Data were collected at baseline and 1 month after surgery.
Methods
Patients eligible for inclusion had chronic perineal pain for at least 3 months in the area served by the pudendal nerve. We combined pudendal nerve release with neuromodulation.
Results
At 1 month, the numeric pain rating scale (NPRS) dropped from 9.5 at baseline to 3.5 (p = 0.003). Seventy-six percent of patients showed a global impression of change (PGIC) of > 50% at 1 month, and optimal treatment response (PGIC ≥ 90%) was found in 41% of patients.
Limitations
The drawback of our study was that it was not randomized or blinded. The peripheral nerve evaluation lead (PNE) used could only be implanted for 1 month because of infection risk and is also prone to dislocations and technical failures.
Conclusion
Pudendal nerve liberation by the ENTRAMI technique combined with short-term pudendal neuromodulation seems feasible and promising in treating patients with chronic perineal pain.
Clinical trial number: NCT03880786.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic perineal pain syndrome due to pudendal nerve impingement is not just difficult to diagnose, but also to treat. Treatment options for these patients are limited. Besides pharmacological and behavioral treatment, pudendal infiltrations can be offered. These have proven good short-term effects, but lack efficacy at the long term [1]. Pudendal nerve release surgery in case of impingement syndrome is well described in the literature [2,3,4,5]. Recently, a new endoscopic transgluteal approach to pudendal release surgery (the ENTRAMI technique) was described with promising results [6]. Pudendal nerve modulation in case of chronic perineal pain is also a promising technique, and some small studies exist [7, 8]. In general, all the known treatment options for these patients leave room for improvement when considering outcomes. This is probably partially due to the complex “chronic pain syndrome” mechanism. Early neuromodulation of the pudendal nerve after its surgical release can improve outcomes for these patients. The positive effect of neuromodulation on nerve regeneration has been shown in animal studies [9, 10]. The rationale could be that neuromodulation facilitates nerve regeneration and intervenes with the central chronic pain mechanism. The aim of the present study was to evaluate the potential beneficial effect of pudendal neuromodulation on the outcome of pudendal release surgery by the ENTRAMI technique in patients suffering from chronic perineal pain syndrome because of pudendal impingement.
Methods
The ethics committee approved the protocol. Eligible patients gave informed consent after reading written information on the study.
Patients eligible for inclusion had chronic perineal pain for at least 3 months in the area served by the pudendal nerve that was refractory to conservative measurements. Patients met all five of the Nantes criteria for pudendal nerve entrapment syndrome before surgery was proposed [11] (Table 1). Pain was localized in one or more of the sensitive areas innervated by the pudendal nerve and could be unilateral or bilateral. The pain was exacerbated in the seated position, and patients were not awoken by the pain at night. Clinical examination did not reveal a sensory loss in the perineal region. To fulfill the fifth criterion, all patients had a transitory positive diagnostic response to an anesthetic block of the pudendal nerve with a short-term reduction of the pain of at least 3 on a numeric pain rating scale (NPRS-pain scale; score 0–10).
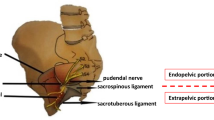
We combined the ENTRAMI technique for endoscopic transgluteal pudendal nerve release with pudendal neuromodulation. The procedure itself is described in a step-by-step manner in two recent articles [6, 12]. The technique we used is the same as described in those two cadaver studies.
The pudendal peripheral nerve evaluation lead (PNE test lead; Medtronic) was placed after release and transposition of the pudendal nerve. The PNE test lead was introduced percutaneously transgluteally and placed under full visual control in contact with the pudendal nerve (Figs. 1 and 2). The PNE lead was only fixated on the skin level. Stimulation was switched on by the patient when the perineal pain appeared postoperatively and this only during daytime. The following stimulation parameters were used: 60 Hz and 210 microsecond amplitude according to the sensory threshold described by the patient. After 4 weeks, the PNE test lead was removed at the outpatient clinical visit. The numeric pain rating scale (NPRS, range 0–10) and Patient Global Impression of Change (PGIC, range 0–100%) were recorded at baseline and at 1 month. The following questionnaires for pelvic health assessment were recorded at baseline and after 1 month of neuromodulation: Wexner Constipation Score (score 0–30), Fecal Incontinence Severity Index (FISI) (score 0–20) and the Urinary Symptom Profile (USP) (score 0–39; concerning symptoms of stress incontinence, overactive bladder, dysuria). Quality of life was also measured by the SF-36 Questionnaire at baseline and after 1 month.
Patient in ventral decubitus, right-sided gluteal region. Bold green arrow: view direction; GM: gluteal muscle, cr: cranial, N: needle position, STL: sacro-tuberous ligament, SSL: sacrospinous ligament cut, PN and *: pudendal nerve, SN: sciatic nerve, PM: piriformis muscle (visible after removal of GM in the figure)
Patients were asked to indicate the sensory location during stimulation immediately after surgery and after 1 month.
Treatment failure is defined as PGIC ≤ 30%, good treatment response as PGIC ≥ 30% and optimal response as PGIC ≥ 90%.
Wilcoxon signed ranks test was used for statistical analysis.
Results
Between March 2019 and March 2020, 16 patients (2 males, 14 females) were included, and they all signed informed consent forms. Average age was 49 (range 30–69) years old. Patients suffered an average of 3 (range 1–9) years before surgery was proposed. Bilateral surgery was done in eight patients. The onset of pain is presented in Table 2. In three patients, PNE leads were removed before the end of the test phase: one male patient experienced a new onset of burning sensation at the glans of the penis, which disappeared gradually after PNE removal; one woman experienced a worsening of pain and burning sensation in her leg, which resolved completely after lead removal; and one female patient wanted to remove the lead after 2 weeks because she experienced no improvement. No other perioperative or early postoperative complications occurred.
Average maximum NPRS at baseline was 9.5 (range 7–10). At 1 month, average maximum NPRS declared by the whole group was 3.5 (range 0–10, p = 0.003). After removal of the PNE, most patients experienced an aggravation of their symptoms but not to the level that they experienced preoperatively (Table 3).
At 1 month, 76% of patients showed a global impression of change of > 50%, and optimal treatment response (PGIC ≥ 90%) was found in 41% of patients. Four out of 16 patients (one patient experienced only improvement on one side) experienced no improvement at all (PGIC < 10%) at 1 month (Fig. 3).
Concerning pelvic health assessment, a global improvement was found, but only symptoms of overactive bladder were significantly improved after 1 month (p = 0.0009).
Quality of life improved in all aspects and was statistically significantly improved for the following aspects: energy, emotional well-being and social functioning (p < 0.05).
Discussion
This pilot study is the first step to combining pudendal release surgery with pudendal neuromodulation to treat patients suffering from chronic perineal pain syndrome by pudendal nerve impingement that is refractory to conservative measures. If this diagnosis is suspected, treatments should be offered in a multidisciplinary setting where all treatments options are available and with health care workers familiar with this rare disease. Besides pharmacological and behavioral treatment, pudendal infiltrations can be offered to the patient. These have proven good short-term effects, but lack efficacy at the long term [1].
Different surgical approaches to pudendal release in case of impingement are described in the literature. Unfortunately, not all studies use the same inclusion criteria, making comparison difficult.
In the open transgluteal approach, which is the only approach validated by a randomized controlled trial, 50% of the patients reported improvement of pain at 3 months, [2]. The endoscopic minimally invasive transgluteal approach (ENTRAMI technique) has shown similar results [13]. Both studies clearly show an improvement of pain over time, with better results reported at 6 and 12 months [14].
In our study, at 1 month, 76% of patients showed a global impression of change of > 50% and optimal treatment response (PGIC ≥ 90%) was found in 41% of patients.
Bautrant et al. reported an immediate postoperative improvement with a trans-ischio-rectal/vaginal approach in 41% of patients. At 1 year, 86% of patients declared an improvement, confirming the tendency that better results are reported at the long term [15].
The laparoscopic approach described by Erdogru et al. reports a reduction in mean VAS score from 5.6 at baseline to 1.5 at 1 month and 2.0 at 12 months [4]. The endoscopic transperineal approach described by Beco found a 50% pain reduction in 41.6% of patients after 2 years [16]. Direct comparison with our results is difficult because of the different inclusion criteria used.
Pudendal neuromodulation in case of chronic perineal pain is also promising. In a comparative pilot study of percutaneous pudendal implantation techniques, Heinze showed a significant decrease of mean pain intensity during stimulation in patients with chronic pelvic pain: mean pain intensity decreased from 80 mm at baseline to 40 mm after 1 month of stimulation [7]. Peters et al. reported short-term “improvement in pain” in all 19 subjects with pudendal neuralgia tested with pudendal neuromodulation [8]. Unfortunately, all the known percutaneous implantation techniques seem very unwieldly, and despite using an optimized placement technique, neither the precise location nor the trajectory of the electrode can be guaranteed [7]. Furthermore, one could question the efficacy of neuromodulation if impingement is still present.
The advantage of the technique used here is the possibility to combine the pudendal nerve release and transposition with the positioning of an electrode under full visual control in close contact with the pudendal nerve [6]. The rationale could be that the surgical act of nerve liberation is necessary in case of impingement and that neuromodulation helps with nerve regeneration after its liberation.
The combination of pudendal nerve neuromodulation with nerve liberation by a minimally invasive transgluteal approach seems to lead to better pain control during the stimulation period. After 1 month of stimulation, a good treatment response (PGIC ≥ 50%) was found in 76% of patients and optimal treatment response (PGIC ≥ 90%) in 41% of patients.
Despite the combination of both techniques, 4 out of 16 patients (1 patient experienced only improvement on one side) experienced no improvement (PGIC < 10%) at 1 month. Failures are hard to explain. All patients had a work-up to exclude central nervous system lesions or other causes that might explain their symptoms: patients had an MRI of the pelvis and lumbosacral region and/or anal sphincter EMG evaluation or a work-up by a neurologist. The patients included in our study had very clear inclusion criteria, and all suffered from perineal pain syndrome because of nerve impingement. All patients also had a positive response to pain after pudendal nerve infiltration. Sensory response of pudendal nerve stimulation was located in the pudendal area or diffusely spread out toward the gluteal region, with a trend toward more lateral sensation in the non-responders (Fig. 4), which could be explained by an immediate postoperative dislocation of the PNE lead. After 1 month, patients were asked again where the stimulation was felt, but no changes in location were recorded at that time, suggesting that no dislocations had occurred during that 1-month period. A study protocol using a quadripolar tined lead could improve outcomes since different programs and stimulation parameters could be tested as well as different stimulation locations. Furthermore, risk of dislocation would probably be reduced. If an implantable pulse generator (IPG) is to be implanted, the duration of neuromodulation needs to be determined since the effect of nerve liberation and transposition remains and even improves at the long term [2]. In our study, after removal of the PNE, most patients experienced aggravation of their symptoms but not to the level that they experienced preoperatively.
The drawback of our study is that it is not randomized and not blinded. The PNE electrode used could only be implanted for 1 month because of the infection risk and is also prone to dislocations and technical failures because of its fragility. Since this is a feasibility study on pudendal nerve stimulation in an off-label condition, a temporary electrode without tines was chosen for ethical and economic reasons.
Another limitation is the small sample size and short-term follow-up.
The ENTRAMI technique we used allows full visualization of the pudendal nerve at its origin, so the PNE electrode was placed next to the nerve under full visual control.
Since we combined both procedures, no extra surgical trauma was performed to be able to place the PNE electrode.
In conclusion, pudendal nerve liberation by the ENTRAMI technique combined with short-term pudendal neuromodulation seems feasible and promising in chronic perineal pain patients with clear inclusion criteria. Following the promising results reported in this study, a prospective study soon will be started using permanent electrodes for stimulation.
References
Tricard T, Munier P, Story F, et al. The drug-resistant pudendal neuralgia management: a systematic review. Neurourol Urodyn. 2019;38:13–21.
Robert R, Labat JJ, Bensignor M, et al. Decompression and transposition of the pudendal nerve in pudendal neuralgia: a randomized controlled trial and long-term evaluation. Eur Urol. 2005;47:403–8.
Possover M. Laparoscopic management of endopelvic etiologies of pudendal pain in 134 consecutive patients. J Urol. 2009;181:1732–6.
Erdogru T, Avci E, Akand M. Laparoscopic pudendal nerve decompression and transposition combined with omental flap protection of the nerve (Istanbul technique): technical description and feasibility analysis. Surg Endosc. 2014;28:925–32.
Beco J, Climov D, Bex M. Pudendal nerve decompression in perineology: a case series. BMC Surg. 2004;4:15.
Jottard K, Bonnet P, Bruyninx L, et al. The ENTRAMI technique: endoscopic transgluteal minimal invasive technique for implantation of a pudendal electrode under full visual control: a cadaver study. Neurourol Urodyn. 2019;38:130–4.
Heinze K, Hoermann R, Fritsch H, et al. Comparative pilot study of implantation techniques for pudendal neuromodulation: technical and clinical outcome in first 20 patients with chronic pelvic pain. World J Urol. 2015;33:289–94.
Peters KM, Killinger KA, Jaeger C, et al. Pilot study exploring chronic pudendal neuromodulation as a treatment option for pain associated with pudendal neuralgia. Low Urin Tract Symptoms. 2015;7:138–42.
Zhang X, Xin N, Tong L, et al. Electrical stimulation enhances peripheral nerve regeneration after crush injury in rats. Mol Med Rep. 2013;7:1523–7.
De Groote S, Goudman L, Peeters R, et al. Magnetic resonance imaging exploration of the human brain during 10 kHz spinal cord stimulation for failed back surgery syndrome: a resting state functional magnetic resonance imaging study. Neuromodulation. 2020;23:46–55.
Labat JJ, Riant T, Robert R, et al. Diagnostic criteria for pudendal neuralgia by pudendal nerve entrapment (Nantes criteria). Neurourol Urodyn. 2008;27:306–10.
Ploteau S, Robert R, Bruyninx L, et al. A new endoscopic minimal invasive approach for pudendal nerve and inferior cluneal nerve neurolysis: an anatomical study. Neurourol Urodyn. 2018;37:971–7.
Jottard K, Bruyninx L, Bonnet P, et al. A minimally invasive, endoscopic transgluteal procedure for pudendal nerve and inferior cluneal nerve neurolysis in case of entrapment: 3- and 6-month results. The ENTRAMI technique for neurolysis. Int J Color Dis. 2020;35:361–4.
Jottard K, Bruyninx L, Bonnet P, et al. Endoscopic trans gluteal minimal-invasive approach for nerve liberation (ENTRAMI technique) in case of pudendal and/or cluneal neuralgia by entrapment: one-year follow-up. Neurourol Urodyn. 2020;39:2003–7.
Bautrant E, de Bisschop E, Vaini-Elies V, et al. Modern algorithm for treating pudendal neuralgia: 212 cases and 104 decompressions. J Gynecol Obstet Biol Reprod (Paris). 2003;32:705–12.
Beco J, Seidel L, Albert A. Endoscopic transperineal pudendal nerve decompression: operative pudendoscopy. Surg Endosc. 2018;32:3720–31.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
All authors approved the manuscript and this submission.
The ethics committee of the Centre Hospitalier Universitaire-Universitair verpleegcentrum Brugmann (CHU-UVC Brugmann) approved the protocol (EC number: CE2019/09).
Rights and permissions
About this article
Cite this article
Jottard, K., Bruyninx, L., Bonnet, P. et al. Pilot study: pudendal neuromodulation combined with pudendal nerve release in case of chronic perineal pain syndrome. The ENTRAMI technique: early results. Int Urogynecol J 32, 2765–2770 (2021). https://doi.org/10.1007/s00192-020-04565-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-020-04565-1