Abstract
Introduction and hypothesis
It is assumed changes occur to the biomechanics and viscoelastic response of the levator ani muscle during pregnancy; however, there is limited evidence of this. This study used instrumentation and clinical measures to determine the stiffness and active force capacity of levator ani muscle during pregnancy and post-partum, investigated any associations with delivery outcomes, and explored the biomechanical properties associated with symptoms of pelvic floor dysfunction.
Methods
This was a prospective observational study, with nulliparous women with a singleton low-risk pregnancy. Data were collected at two stages during pregnancy and post-partum. Measurements included the Australian Pelvic Floor Questionnaire, palpation of active force, and elastometry measurements. Post-partum, 3D/4D ultrasound measurements were included. Repeated measures ANOVAs, pairwise comparisons, Pearson correlation coefficients, and Student’s t-tests were used as appropriate.
Results
Fifty-nine women took part in the study. Active force was significantly different over the pregnancy and post-partum, measured with instrumentation (p = 0.002) and palpation (p = 0.006 right, p = 0.029 left). There was no significant change in muscle stiffness during pregnancy. Post-partum muscle stiffness was significantly different between women who gave birth vaginally vs. caesarean section (p = 0.002). Post-partum there were differences in levator hiatal area, symptoms of bladder dysfunction, prolapse symptoms, and sexual dysfunction symptoms.
Conclusions
Active force of the levator ani muscle was significantly reduced during pregnancy and in the post-partum period, while muscle stiffness reduced only in those who had vaginal deliveries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well understood that significant physiological changes occur during pregnancy and delivery, including increased vaginal distensibility, pelvic fascia lengthening, and reduced ligament stiffness [1, 2]. During delivery, computational models estimate stretch ratios of between 2.2 to 4.3 for the levator ani muscles (LAM) [3]. Due to the large forces developed during stretching, avulsion of the LAM from the pubic bone occurs in up to 35% of women [4] increasing their risk of developing pelvic floor dysfunction [5]. Skeletal muscles typically fail at 50% strain [6]. However, it is assumed that maternal hormones facilitate changes to the biomechanical and viscoelastic properties of the LAM, with animal studies reporting delayed vaginal tissue failure in pregnant versus non-pregnant animals [7, 8]. It is reasonable to assume this also occurs in human vaginal tissue as studies involving human leg muscles demonstrate oestrogen levels affect the muscle stiffness of the musculotendinous unit [9, 10]. However, there is limited evidence to date on the muscle stiffness of pelvic floor muscles (PFM) in both gravid and nulligravid women [11, 12]. As both the viscoelastic and contractile properties contribute to muscle stiffness [13], changes in these properties are of interest during pregnancy and post-partum. Previous research regarding active force measures is ambiguous, with some studies showing reduction, while others show no difference [14, 15].
This study used both instrumentation and clinical measures to determine the stiffness and active force capacity of the LAM during pregnancy and post-partum, investigated any potential associations with delivery outcomes, and explored the biomechanical properties associated with symptoms of pelvic floor dysfunction. These findings will contribute to the knowledge base of the currently known physiological and physical effects of pregnancy and delivery on the LAM.
Materials and methods
This was a prospective observational study conducted over a period of 34 months. Participants were recruited via printed and social media, were given a participant information sheet, and were provided written informed consent. Participation criteria included nulliparous women 18 to 45 years of age with a singleton low-risk pregnancy. Exclusion criteria included: if the participant had a lower than conversational level of English; the diagnosis of a high-risk pregnancy [multiple foetuses, morbid obesity (body mass index, BMI) > 37 in Polynesian women, BMI > 35 in European women]; more than two previous miscarriages after 16 weeks’ gestation; pre-existing or the development of medical conditions in the mother [specifically: pre-eclampsia, multiple sclerosis, cerebral palsy, cauda equina syndrome, pudendal neuropathy, autoimmune disease, cancer, sickle cell anaemia, tuberculosis, herpes, AIDS, heart disease, hypertension, kidney disease, Crohn’s disease, ulcerative colitis, or diabetes]; planned elective caesarean section at the time of entry into the study; and the development of medical conditions in the foetus (heart defect, Rh incompatibility, congenital deformity).
Data were collected at three time points (18 to 24 weeks gestation, 35 to 38 weeks gestation, and 13 to 28 weeks post-partum). These time points were chosen to account for the differences in the likely influence of hormonal changes on the tissue’s mechanical response between the second to third trimester. It is also well recognized that the effects of pregnancy are likely to present up to 6 weeks postpartum; therefore, the postnatal measurements were taken at least 12 weeks after delivery. At each time point the self-administered Australian Pelvic Floor Questionnaire (APFQ) [16] and pelvic floor data were collected, which included palpation of active force on the left and right sides of the LAM at 3 and 9 o’clock using the Modified Oxford Scale (MOS) [17] and elastometry measurement of active force and muscle stiffness. Post-partum, 3D/4D ultrasound measurements were included. Delivery details were obtained from medical records.
Participants voided immediately prior to their positioning on a plinth in a bent-knee, semi-supine position, with their feet placed flat symmetrically, shoulder-width apart. Participants followed standardized instructions for maximum voluntary contraction (MVC) and relaxation of the PFM. After confirmation of a correct PFM contraction, manual palpation assessed active force during one MVC, held for 3s duration.
The elastometer (developed at the Auckland Bioengineering Institute) was used for all instrumented muscle stiffness and active force measurements [11]. The elastometer speculum (pre-set at 30 mm aperture) was positioned 35 mm into the vagina (opening in the coronal direction), with insertion following the natural angle of the vagina. Next, ten sequential step phases of the speculum opening were affected, ranging from 30 mm to 50 mm in 2 mm increments, while the speculum hand piece continuously reported aperture and force to a laptop computer running a customized LabVIEW interface. Each step phase comprised relaxation for 3 s, MVC for 3 s, and relaxation for 3 s. One cycle was defined as commencement of opening the elastometer from 30 mm to closing of the instrument once it had reached the maximum aperture of 50 mm. Two cycles of data were collected per participant, with cycle one considered a pre-conditioning and familiarization cycle. Cycle two was used for all analysis of muscle stiffness and force. No EMG recordings were completed at any stage of the assessment. However, all participants were encouraged to remain as relaxed as possible during the assessment, and the software interface provided real-time visualization of force data and demonstrated reduction of force levels at each appropriate section of the step phase cycle.
Muscle stiffness was calculated from the slope of the force displacement curve, which was formed from the averaged force and displacement measures from the final 1 s of relaxation time, per step phase, between apertures 40 mm to 50 mm. An assumption was made that the stiffness values obtained from the force and displacement values represented the LAM stiffness, as animal studies have found other pelvic tissues (vaginal walls, fascia, ligaments) have higher compliance and make a negligible contribution to stiffness [7] . Three force values were calculated from the raw data at each aperture: total force (the complete force the muscles can generate, inclusive of active and passive); passive force (the inherent force of the muscle in its relaxed state); and active force (the total force minus the passive force, representing the force generated by active, voluntary contraction).
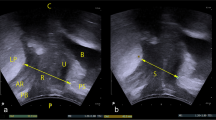
All post-partum assessments included transperineal ultrasound imaging using the GE Voluson i Portable Ultrasound System (GE Healthcare, Auckland, New Zealand). A 4-MHz to 8-MHz electronic curved array volume transducer (acquisition angle of 85°, covered in protective membrane) was positioned over the perineum in the mid-sagittal line. Previously published protocols were used [4] with static image capture of rest and maximum Valsalva used for the analysis of bladder neck descent. The cineloop function was used to capture MVC and maximum Valsalva [18]. Hiatal dimensions (area, anterior-posterior and coronal measurements) were measured of the levator hiatus at rest, MVC, and Valsalva. Tomographic imaging was used to determine the presence of LAM avulsion, with measures of > 2.5 mm on all three central images confirming avulsion [19]. Data were analysed using GE 4D View software (version 18) (GE Healthcare Austria GmbH and Co OG, Tiefenbach, Austria). Two assessors reviewed ultrasound data (blinded and unblinded).
Statistical analyses
Repeated measures ANOVAs (analysis of variance) and pairwise comparisons were used to explore changes over the time points. Pearson correlation coefficients were used to assess associations. Student’s t-tests were completed for comparisons of means. A level of significance of p < 0.05 was chosen a priori for all analyses. No power calculation could be completed for this part of a larger study. Statistical analysis was conducted using the statistical package IBM SPSS version 25 (IBM Corp., Armonk, NY) and Microsoft Excel 365.
Ethical approval
This study was approved by the Health and Disability Ethics Committee: reference number LRS/10/07/029 - AM06 (September 2015), AM08 (May 2016), and AM10 (September 2016).
Results
The demographics and clinical characteristics of participants are presented in Table 1. No participants reported pain or discomfort during the measurements.
While 59 participants were accepted into the study, at each time point participant numbers fluctuated because of difficulties with completing measurements and questionnaires. These difficulties included: low position of foetus preventing positioning of the elastometer; early onset of labour; development of medical complications of the foetus and/or women; elastometer mechanical failure; availability of ultrasound machine. This in turn affected the participant numbers that could be analysed with the repeated measures ANOVAs. See Fig. 1 for a flowchart of participant numbers
There was no significant change in muscle stiffness during pregnancy. Active force was significantly different over the pregnancy and post-partum, measured with both instrumentation and palpation. Table 2 presents the results of elastometry and palpation measurements for participants who had complete data sets over the three time points.
At 18 to 24 weeks and post-partum, there were significant moderate correlations between active force measured with palpation and elastometer [18 to 24 weeks r (correlation coefficient) = 0.58, p < 0.001*; post-partum right r = 0.45, p = 0.004*; post-partum left r = 0.55, p < 0.001*]. There was no significant correlation between measures at 35 to 38 weeks (r = 0.33, p = 0.073).
Independent samples t-tests determined differences between the muscle properties immediately prior to delivery and post-partum, grouped according to delivery mode. There were no significant statistical differences at 35 to 38 weeks between vaginal deliveries and caesarean sections for either muscle stiffness or force. However, there were statistically significant differences between the delivery modes post-partum, with muscle stiffness reduced in women who gave birth vaginally: 264 N/m (99 N/m, 428 N/m) 95% CI, p = 0.002*, d = 1.1 (Cohen’s d effect size), a large effect size.
Independent-samples t-tests determined that there were statistical differences in ultrasound measurements between delivery modes (Table 3) and between participants with LAM avulsions (13%) compared to those without. Participants with right-sided LAM avulsions had an increased coronal diameter of 6.5 mm (0.18 mm, 1.1 mm) 95% CI during PFM contraction compared to those with an intact LAM. This was statistically significant, p = 0.008*, d = 1.22, a large effect size.
Repeated measures ANOVAs were completed for the 41 participants who completed all APFQs at the three time points. Significant changes were demonstrated in bladder, prolapse, sexual dysfunction and global scores (Table 4).
Discussion
This study found active force of the LAM was significantly reduced during pregnancy and in the post-partum period, while muscle stiffness reduced only in those who had vaginal deliveries. This was supported by the ultrasound findings of increased levator hiatal area in those women who gave birth vaginally, and the increased symptoms of pelvic organ prolapse post-partum. Although participant numbers were low, the results present novel findings related to changes to the LAM properties during pregnancy and in the immediate post-partum period.
Instrumental and clinical palpation demonstrated a significant moderate correlation, with similar percentage reductions in active force. This suggests that the MOS could be used to assess muscle strength to guide patient management. However, it is not as precise as instrument measurements and should be used with caution. This finding is similar to what Ferreira et al. [20] found when comparing palpation and manometry.
The ability of the LAM to generate force may have been affected by the increase in the length of the muscle that occurs during pregnancy and delivery [21, 22], reducing its ability to contract because of the suboptimal sarcomere lengths present in the myofibre [23]. A further factor that may have contributed was the increased loading on the tissues caused by the increasing mass of the women and foetus [24]. Increased BMI levels (> 30) have been associated with increased loading on the pelvic floor structures and pelvic floor dysfunction, with reduction in BMI resulting in reduction of pelvic floor dysfunction in patients undergoing bariatric surgery [25]. These findings reinforce the importance of encouraging pelvic floor muscle training (PFMT) during pregnancy and in the post-partum period. Morphological improvements to the PFM have been shown to occur with PFMT [26] and can prevent and treat urinary incontinence during pregnancy [27].
No significant changes were found between time points for muscle stiffness in this study. This is contrary to the findings of Kruger et al. [11], who reported significant differences in muscle stiffness between the third trimester and post-partum period, with considerably lower stiffness values (36% lower ante-natal; 46% lower post-natal). There were notable differences between the two study cohorts: recruitment of participants in the Kruger et al. study occurred at a tertiary care obstetrics unit, with participants having a higher BMI and lower mean age [11]. In comparison, this study’s participants were recruited via social media, were older, had lower BMIs, and were fit and active. The higher stiffness values in this study may have been due to a combination of these factors, with changes LAM muscle properties shown to occur in women who undertake high levels of fitness [28] and lower stiffness values shown to occur in women with higher BMIs [29].
Prior to delivery, there were no differences in muscle stiffness or active force between those who proceeded to vaginal delivery compared to caesarean delivery, supporting previous work by Kruger et al. [11]. This has also been demonstrated in rats [8]. It appears from these studies that muscle stiffness measures taken antenatally do not give any indication as to an optimal delivery mode for minimizing pelvic floor dysfunction post-natally.
Measuring the LAM in vivo requires assumptions to be made: that the LAM has a higher stiffness value than the surrounding tissues and that the measurements made by the instrument primarily reflects the properties of those muscle fibres. However, the stiffness measurements obtained in this study are of the muscle-tendon unit, with contributions from the overlying and adjoining tissues of the vaginal walls, fascia, and the neurovascular structures. Prolonged loading of the pelvic structures over the duration of the gestation, combined with fetal head engagement in the weeks prior to delivery, could have a similar effect to long-term stretching programmes [30].
While sarcomere composition within the myofibre contributes to muscle stiffness, the connective tissues contribute considerably to the passive component of muscle stiffness. Fibroblasts within the muscle complex and surrounding vaginal supporting fascia respond to tension by remodelling the cytoskeleton and lowering stiffness [23]. The remodelling could help to explain why there was significantly reduced muscle stiffness after vaginal delivery compared to caesarean delivery, while there was no significant difference in active force post-partum. This is supported by the ultrasound results, which showed significantly larger diameters and areas of the levator hiatus following vaginal delivery. Both pelvic organ prolapse and sexual dysfunction APFQ scores were higher post-partum, also indicating that stiffness may play a role in the symptoms of pelvic floor dysfunction.
The strengths of this study are its prospective design, and the collection of both objective and subjective muscle measurements at two stages during pregnancy and post-partum. However, the conclusions of this study are limited by the relatively small numbers of participants who were able to be measured at all points during the study and the assessor could not be blinded because of the study design. A further limitation of the study is the slight adjustment in the position of the assessor’s finger for palpation of active force using the MOS. This was done to align palpation measures with measurements of active force by the elastometer, which may affect the correlation between the two measurements.
Conclusion
The reduction in LAM active force supports previous research that advocates pelvic floor muscle training during pregnancy and post-partum. Although the impact of a reduction in active force on delivery outcomes appears negligible, mode of delivery has a statistically significant effect on muscle stiffness, with vaginal delivery resulting in significantly reduced stiffness and enlarged levator hiatus areas post-partum. The moderate correlation of palpation to instrumental measurements indicates that estimation of active force by palpation alone should be used with caution for patient management. Further studies are needed with more participants to further explore the effects of pregnancy and delivery on the muscle mechanics and vaginal tissues of the pelvic region.
References
Varney H. Normal pregnancy database: adaptations for the mother, development and growth of the embryo and the fetus, and the placenta. In: Varney H, Kriebs JM, Gegor CL, editors. Varney's midwifery. 4th ed. Massachusetts: Jones & Bartlett Learning; 2004. p. 543–69.
Abramowitch S, Easley D. Biomechanical characterization of native pelvic floor organs and tissues. In: Hoyte L, Damaser M, editors. Biomechanics of the female pelvic floor. Amterdam: Elsevier; 2016. p. 109–30.
Yan X, Kruger JA, Li XY, Nielsen PMF, Nash MP. Modeling the second stage of labor. Wiley Interdiscip Rev Syst Biol Med. 2016;8(6):506–16.
Dietz HP, Moegni F, Shek KL. Diagnosis of levator avulsion injury: a comparison of three methods. Ultrasound Obstet Gynecol. 2012;40(6):693–8.
Volløyhaug I, Mørkved S, Salvesen KÅ. Association between pelvic floor muscle trauma and pelvic organ prolapse 20 years after delivery. Int Urogynecol J. 2015;27(1):39–45.
Brooks SV, Zerba E, Faulkner JA. Injury to muscle fibres after single stretches of passive and maximally stimulated muscles in mice. J Physiol. 1995;488(2):459–69.
Rahn DD, Ruff MD, Brown SA, Tibbals HF, Word RA. Biomechanical properties of the vaginal wall: effect of pregnancy, elastic fiber deficiency, and pelvic organ prolapse. Am J Obstet Gynecol. 2008;198(5):590.e1–6.
Lowder LJ, Debes MK, Moon KD, Howden DN, Abramowitch AS, Moalli AP. Biomechanical adaptations of the rat vagina and supportive tissues in pregnancy to accommodate delivery. Obstet Gynecol. 2007;109(1):136–43.
Bryant AL, Crossley KM, Bartold S, Hohmann E, Clark RA. Estrogen-induced effects on the neuro-mechanics of hopping in humans. Eur J Appl Physiol. 2011;111(2):245–52.
Eiling E, Bryant AL, Petersen W, Murphy A, Hohmann E. Effects of menstrual-cycle hormone fluctuations on musculotendinous stiffness and knee joint laxity. Knee Surg Sports Traumatol Arthrosc. 2007;15(2):126–32.
Kruger JA, Budgett SC, Wong V, Nielsen PMF, Nash MP, Smalldridge J, et al. Characterizing levator-ani muscle stiffness pre- and post-childbirth in European and Polynesian women in New Zealand: a pilot study. Acta Obstet Gynecol Scand. 2017;96(10):1234–42.
Morin M, Gravel D, Bourbonnais D, Dumoulin C, Ouellet S, Pilon J-F. Application of a new method in the study of pelvic floor muscle passive properties in continent women. J Electromyogr Kinesiol. 2010;20:795–803.
Fung YC. Biomechanics: mechanical properties of living tissues, vol. 1. 2nd ed. New York: Springer Science & Business Media; 1993.
Tennfjord MK, Hilde G, Stær-Jensen J, Ellström Engh M, Bø K. Dyspareunia and pelvic floor muscle function before and during pregnancy and after childbirth. Int Urogynecol J. 2014;25(9):1227–35.
De Souza CA, Riesco MLG, Da Silva SW, Cotrim AC, Sena EM, Rocha NL, et al. Analysis of pelvic floor musculature function during pregnancy and postpartum: a cohort study. J Clin Nurs. 2010;19(17–18):2424–33.
Baessler K, O'Neill SM, Maher CF, Battistutta D. A validated self-administered female pelvic floor questionnaire. Int Urogynecol J Pelvic Floor Dysfunct. 2010;21(2):163–72.
Laycock J, Jerwood D. Pelvic floor muscle assessment: the PERFECT scheme. Physiotherapy. 2001;87.
Tumbarello JA, Hsu Y, Lewicky-Gaupp C, Rohrer S, DeLancey JOL. Do repetitive Valsalva maneuvers change maximum prolapse on dynamic MRI? Int Urogynecol J. 2010;21(10):1247–51.
Dietz HP, Bernardo MJ, Kirby A, Shek KL. Minimal criteria for the diagnosis of avulsion of the puborectalis muscle by tomographic ultrasound. Int Urogynecol J. 2011;22(6):699–704.
Ferreira CHJ, Barbosa PB, Souza Fd, Antônio FI, Franco MM, Bø K. Inter-rater reliability study of the modified Oxford Grading Scale and the Peritron manometer. Physiotherapy 2011;97(2):132–138.
Shek KL, Kruger JA, Dietz HP. The effect of pregnancy on hiatal dimensions and urethral mobility: an observational study. Int Urogynecol J. 2012;23(11):1561–7.
Svabík K, Shek KL, Dietz HP. How much does the levator hiatus have to stretch during childbirth? BJOG Int J Obstet Gynaecol. 2009;116(12):1657–62.
Riley DA, Van Dyke JM. The effects of active and passive stretching on muscle length. Phys Med Rehabilitat Clin. 2012;23(1):51–7.
Edwards RH, Hill DK, Jones DA, Merton PA. Fatigue of long duration in human skeletal muscle after exercise. J Physiol. 1977;272(3):769–78.
Cuicchi D, Lombardi R, Cariani S, Leuratti L, Lecce F, Cola B. Clinical and instrumental evaluation of pelvic floor disorders before and after bariatric surgery in obese women. Surg Obes Relat Dis. 2013;9(<HT>1</HT>):69–75.
Braekken IH, Majida M, Engh ME, Bo K. Morphological changes after pelvic floor muscle training measured by 3-dimensional ultrasonography: a randomized controlled trial. Obstet Gynecol. 2010;115(2 Pt 1):317–24.
Mørkved S, Bø K. Effect of pelvic floor muscle training during pregnancy and after childbirth on prevention and treatment of urinary incontinence: a systematic review. Br J Sports Med. 2014;48(4):299.
Kruger JA, Dietz HP, Murphy BA. Pelvic floor function in elite nulliparous athletes. Ultrasound Obstet Gynecol. 2007;30(1):81–5.
Anumba DOC, Gillespie S, Jha S, Abdi S, Kruger J, Taberner A, et al. Postnatal pelvic floor muscle stiffness measured by vaginal elastometry in women with obstetric anal sphincter injury: a pilot study. Int Urogynecol J. 2019.
Weppler CH, Magnusson SP. Increasing muscle extensibility: a matter of increasing length or modifying sensation? Phys Ther. 2010;90(3):438–49.
Funding
This work was supported by the University of Auckland Doctoral Scholarship, Auckland Bioengineering Institute, the Maurice and Phyllis Paykel Trust, the MedTech Centre of Research Excellence, funded by the Tertiary Education Commission of New Zealand, and Physiotherapy New Zealand.
Author information
Authors and Affiliations
Contributions
MJ Davidson: Project development, data collection, manuscript writing.
PMF Nielsen: Project development, manuscript writing.
AJ Taberner: Project development, manuscript writing.
JA Kruger: Project development, manuscript writing.
Corresponding author
Ethics declarations
Financial disclaimer/conflict of interest statement
This work was supported by the University of Auckland Doctoral Scholarship, Auckland Bioengineering Institute, the Maurice and Phyllis Paykel Trust, the MedTech Centre of Research Excellence, funded by the Tertiary Education Commission of New Zealand, and Physiotherapy New Zealand. No conflicts of interest related to this manuscript.
The abstract describing this research was accepted for podium presentation at the International Continence Society Gothenburg Conference in October 2019.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Davidson, M.J., Nielsen, P.M.F., Taberner, A.J. et al. Change in levator ani muscle stiffness and active force during pregnancy and post-partum. Int Urogynecol J 31, 2345–2351 (2020). https://doi.org/10.1007/s00192-020-04493-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-020-04493-0