Abstract
Introduction and hypothesis
The aim of this study was to identify which factors are associated with anatomic and symptomatic prolapse recurrence in the anterior compartment 1 year after traditional anterior vaginal repair. Our study hypothesis was that major defects in pelvic floor support structures before surgery are associated with higher recurrence rates.
Methods
This was a prospective multicenter study including women with symptomatic anterior compartment prolapse who underwent primary vaginal surgery. Prolapse examination was performed using the Pelvic Organ Prolapse Quantification (POP-Q) system, prolapse symptoms were described using the Pelvic Floor Distress Inventory short form (PFDI-20), and levator ani avulsion and hiatal area were identified by translabial 3D ultrasonography.
Results
During the inclusion period, 455 patients were recruited and 442 (97.1%) attended the 1-year follow-up. In three cases, ultrasound data were not available, and the remaining 439 formed the study group. Anatomic and symptomatic recurrence rates were 45.1% and 6.8%, respectively. Levator avulsion increased the risk of anatomic (OR: 1.96) and symptomatic (OR: 2.60) recurrence; abnormal distensibility of the levator hiatal area increased the risk of anatomic (OR: 2.51) and symptomatic (OR: 2.43) recurrence; advanced prolapse increased the risk of anatomic recurrence: POP-Q stage 3 (OR: 2.34) and POP-Q stage 4 (OR: 5.47).
Conclusions
Major defects in pelvic floor support structures before surgery are associated with higher recurrence rates 1 year after native tissue vaginal repair. Advanced stage of prolapse increases the risk of anatomic recurrence, while levator avulsion and abnormal distensibility of the levator hiatus area increase the risk of both anatomic and symptomatic recurrence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pelvic organ prolapse (POP) is a common condition among parous women, the anterior compartment being the most commonly affected [1]. Women are often not aware of prolapse and treatment is not required, but when they are bothered by prolapse-related symptoms, we should offer conservative, mechanical or surgical interventions. The lifetime risk of POP surgery is about 10–20% [2,3,4], and a wide variety of abdominal and vaginal surgical techniques are available.
The most challenging problem with POP surgery is its high recurrence rate, and seeking to improve outcomes, various different surgical techniques have been introduced over time. The most common procedure for treating the anterior compartment is traditional anterior colporrhaphy, also called anterior vaginal repair, but the wide variety of surgical approaches available reflects the lack of consensus on the optimal treatment [5]. The risk factors involved in prolapse recurrence after surgery have also been analyzed to provide appropriate preoperative counseling and offer the best treatment, but they are not as well documented as those for the development of POP [6]. Vergelt et al. [6] only found advanced preoperative stage as a risk factor for POP recurrence in their systematic review focused on recurrence after native tissue repair including different types of surgery in the three compartments. In a more recent review and meta-analysis, Friedman et al. [7] evaluated the risk factors for recurrence of prolapse after any type of POP surgery including those with mesh or graft augmentation. They concluded that levator avulsion, advanced prolapse stage and family history of POP were significant risk factors for prolapse recurrence in this heterogeneous group of surgical techniques.
The pathophysiology of POP may vary considerably from one compartment to another [7]. Specifically, levator avulsion is more closely associated with anterior and apical prolapse, while obesity is strongly associated with posterior prolapse [8, 9]. Similarly, predictors of POP recurrence after surgery may differ, not affecting all compartments in the same way. Hence, the factors involved in POP recurrence should be assessed separately for the different compartments. Few studies have focused on the identification of risk factors for prolapse recurrence after anterior vaginal repair. Levator avulsion was first proposed as a risk factor for cystocele recurrence after anterior colporrhaphy in 2010 at an average follow-up of 4.6 years [10]. It is particularly important to identify these predictors given that anterior colporrhaphy is the type of surgery associated with the highest recurrence rates among traditional pelvic floor reconstructive procedures [5, 11,12,13].
The aim of this study was to identify which demographic or clinical factors were associated with prolapse recurrence in the anterior compartment 1 year after traditional anterior vaginal repair. Our study hypothesis was that major defects in pelvic floor support structures before surgery are associated with higher recurrence rates.
Material and methods
A prospective multicenter study was undertaken to evaluate the factors involved in prolapse recurrence 1 year after surgery. The study group was selected from all women with symptomatic anterior compartment prolapse that were scheduled for primary surgery between May 2015 and September 2017 in the Pelvic Floor Units of two Public Health Hospitals in Spain: Donostia University Hospital and Insular Maternal and Child University Hospital Complex. Women who did not, in the end, undergo surgery were excluded. Other exclusion criteria were history of any POP surgery, Pelvic Organ Prolapse Quantification (POP-Q) stage < 2 in the anterior compartment, and the indication of tissue augmentation with a biologic graft or synthetic mesh during prolapse surgery, as well as patients not being able to complete the questionnaires.
Pelvic examination was performed in all cases during maximal strain with the woman in the lithotomy position and by the same experienced gynecologists, using the POP-Q system validated by the International Continence Society (ICS) [14]. Prolapse symptoms were identified using the specific questions of the validated Spanish version of the Pelvic Floor Distress Inventory short form (PFDI-20) [15].
Levator ani avulsion and hiatal area were identified by translabial 3D ultrasonography using a GE Voluson E8 system with a 2–9-MHz ultrasound transducer, performed with the woman in a supine position with an empty bladder as described elsewhere [16]. Complete avulsion was diagnosed if all three central slices showed abnormal insertion of the puborectalis muscle on the inferior pubic ramus on tomographic ultrasound imaging [17]. Levator hiatal area during Valsalva was measured at the plane of minimal hiatal dimensions, and a hiatal area of > 25 cm2 was defined as abnormal distensibility of the levator hiatus [18].
All women underwent traditional anterior colporrhaphy. A midline incision was made in the anterior vaginal wall, and a dissection was performed laterally to the level of the arcus tendinous fascia pelvis. We use polyglactin sutures to reduce the prolapse. Anterior colporrhaphy was combined with other prolapse procedures in the apical or posterior compartment or concomitant stress urinary incontinence surgery as indicated.
The primary outcomes were both anatomic and symptomatic recurrence. Anterior anatomic recurrence was defined as POP-Q stage 2 or higher, that is, if the point Ba was ≥ −1. Anterior symptomatic recurrence was defined as the appearance and/or feeling of a vaginal bulge in the vaginal area (women answering “yes” to question 3 of the PFDI-20: “Do you usually have a bulge or something falling out that you can see or feel in your vaginal area?”) among women who had been identified as having anterior anatomic recurrence. Furthermore, women who had been re-treated for recurrent prolapse (use of a pessary or re-operation in the anterior compartment) over the 1-year follow-up period were considered to have experienced both anatomic and symptomatic recurrence.
To investigate the risk factors associated with recurrent prolapse, we analyzed the following variables: age, body mass index (BMI), number of vaginal deliveries, menopausal status, abdominal hernias, chronic constipation, heavy lifting, family history of POP, preoperative POP-Q stage, levator avulsion, and hiatal area.
All the women included in the present study were fully informed before enrollment and gave written consent. The study protocol was approved by the Euskadi Clinical Research Ethics Committee (04/2015).
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 22.0. The potential associations of clinical and demographic characteristics with anatomic and symptomatic POP recurrence were explored by comparison of percentages (chi-squared and Fisher’s test). Continuous variables (age, BMI, and hiatal hiatus) were categorized for the analysis. The threshold for statistical significance was set at p = 0.05. Multiple logistic regression models were used to investigate independent associations between anatomic and symptomatic POP recurrence and the variables described above.
Results
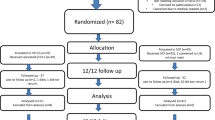
During the inclusion period, we recruited 455 patients with symptomatic anterior compartment prolapse who underwent primary vaginal surgery. One year after surgery, 442 (97.1%) attended the follow-up appointment. In 3 cases, ultrasound data were not available; the remaining 439 women formed the study group.
The mean age of the women in the study group was 63.0 (SD: 9.7; range: 37–86) years, and their mean BMI was 29.7 (SD: 5.4; range: 16.8–49.5) kg/m2. Most women were parous (91.1%). The surgery involved only the anterior compartment in 185 cases (42.1%), two compartments in 203 (46.3%), and all three compartments in 51 (11.6%). Vaginal hysterectomy was performed in 217 (49.4%), posterior colporrhaphy in 89 (20.3%), and urinary incontinence surgery in 75 (17.1%) women. Complete avulsion was diagnosed in 186 (42.4%) women, and the hiatal area was >25 cm2 in 151 (34.4%).
One year after surgery, anatomic recurrence was identified in 198 (45.1%) women and symptomatic recurrence in 30 (6.8%). Four women had a re-operation for anterior compartment recurrence over the 1-year follow-up period, and none of them was using a pessary. The percentage of symptomatic women among those who had had anatomic recurrence was 15.2% (Table 1). Other cutoff points for anatomic recurrence and the percentage of women with symptoms are also shown in Table 1. Among the women who answered yes to question 3 of the PFDI-20, a prolapse occurred in the posterior compartment in 4 cases and in the apical compartment in 1, while in 17 cases no significant prolapse (POP-Q ≥ 2) was identified in any of the 3 compartments.
The results of the univariate and multivariate analysis to explore the association of anatomic recurrence with a range of variables are presented in Table 2. The multiple logistic regression model was built with the variables that reached statistical significance. This analysis indicated that preoperative POP-Q stages 3 (OR: 2.34) and 4 (OR: 5.47), levator avulsion (OR: 1.96), and hiatal area > 25 cm2 (OR: 2.51) were all independent risk factors for anatomic recurrence.
The results of the univariate and multivariate analysis to explore the association of symptomatic recurrence with the same variables are shown in Table 3. In the multiple logistic regression model, we also included the POP-Q stage because it was close to statistical significance in the univariate analysis. This model indicated that levator avulsion and hiatal area > 25 cm2 were independent risk factors for symptomatic recurrence, increasing the risk 2.60- and 2.43-fold, respectively.
We did not find any statistical associations of anatomic or symptomatic recurrence with age, BMI, vaginal delivery, menopausal status, chronic constipation, abdominal hernia, heavy lifting, or family history of POP.
Discussion
Our multicenter prospective study showed anatomic and symptomatic recurrence rates of 45.1% and 6.8%, respectively, 1 year after traditional anterior vaginal repair. The re-operation rate in the anterior compartment was 0.9%. In addition, we have identified an independent association between various factors that indicate major defects in pelvic floor support structures and both anatomic and symptomatic recurrence. Specifically, levator avulsion increased the risk of anatomic (OR: 1.96) and symptomatic (OR: 2.60) recurrence; abnormal distensibility of the levator hiatal area increased the risk of anatomic (OR: 2.51) and symptomatic (OR: 2.43) recurrence; advanced prolapse increased the risk of anatomic recurrence: POP-Q stage 3 (OR: 2.34) and POP-Q stage 4 (OR: 5.47). Although an association of levator avulsion, POP-Q stage, and hiatal area with anatomic recurrence has been described previously, to our knowledge, this is the first study demonstrating an independent association of levator avulsion and abnormal hiatal area with symptomatic failure. We believe that these data have implications when counseling patients about surgery.
Our anatomic recurrence rate is high but consistent with figures reported by other authors that used the same definition of anatomic failure. Maher et al. [5], in a Cochrane review published in 2016, found a recurrent anterior compartment prolapse rate after native tissue repair ranging from 27% to 55%. Longitudinal cohort studies with longer series, including both native tissue repair and the use of meshes [19, 20] or only native tissue repair [21], have also indicated high rates of anatomic recurrence, ranging from 42% to 55%. Currently, the definition used seems too strict because it classifies women who in other circumstances are considered normal as having experienced treatment failure, and the use of the hymen as a threshold for anatomic success seems a more reasonable approach [22]. Applying this cutoff point (Ba > 0), our recurrence rate falls to 10.9%. Chmielewski et al. [23] reported similar results after native tissue repair (11%) in their reanalysis of the randomized trial performed in 2001 by Weber et al. [24].
Nonetheless, defining success after surgical treatment remains controversial, and a standardized method has yet to be established. We chose a cutoff point of −1 for our analysis of risk factors involved in recurrence, because with a lower cutoff, a significant percentage of women with prolapse symptoms would have been left out of the analysis. The clinical relevance of this definition is unclear, and longer follow-ups are needed to assess the course of this group of patients.
The symptomatic recurrence rate is less well documented in the scientific literature, and there are also discrepancies in its definition. Prolapse awareness seems the most appropriate variable, regardless of the degree of bother, a variable that is even more subjective. The problem is that when we are assessing the results of surgery in only one compartment, unless we perform a pelvic examination, we will not be able to rule out that the symptoms are secondary to prolapse in either of the other two compartments. This was what led us to introduce anatomic recurrence in the definition of symptomatic failure. Thus, our symptomatic recurrence rate was only 6.8%, although 11.8% of the women answered yes to the “vagina bulge” question of the PFDI-20. The Cochrane review found figures of awareness of prolapse ranging from 7% to 30% up to 3 years after native tissue repair was performed [5], and in the study of Rodrigo et al. [19], 26% of women complained of recurrent symptoms of prolapse, without specifying the origin of these symptoms. Weemhoff et al. [20] reported a subjective recurrence rate of 5.1% among women who had anatomic recurrence in the anterior compartment, which is quite similar to our results. Reporting the presence of symptoms without an anatomic assessment can lead us to overestimate the rate of symptom recurrence because failure in other compartments may also be involved. In our study, symptoms were reported by five women in whom a posterior or an apical prolapse was identified and by 17 with no evident prolapse (POP-Q < 2) in any compartment.
The most important finding in our study is the identification of an independent association of both anatomic and symptomatic recurrence with levator avulsion and enlarged hiatal area. POP-Q stage was also identified as an independent risk factor for anatomic recurrence, and we report the specific increased risk for each POP-Q stage. The discrepancy between subjective and objective recurrence may be due to the substantial time lag between anatomic recurrence and the patient noticing the symptoms.
Preoperative POP-Q stage is an established risk factor for anatomic recurrence in POP surgery [7, 8]. The meta-analysis performed by Friedman et al. [7] indicated that POP stage 3–4 increased the risk of recurrence 2.11-fold. Studies focused on the anterior compartment have also found this association but none of them have reported the level of risk separately for each POP stage. Weemhoff et al. [20] found a two-fold increase in risk of prolapse recurrence for POP stage 3–4, although these data are questionable because the preoperative staging was performed according to the Baden-Walker classification. Vergelt et al. [21] reported that preoperative POP-Q stage 3–4 increased the risk of anatomic recurrence by a factor of 3.47. Our study, with more cases than the previous ones, has allowed us to establish the risk of recurrence for each of the preoperative stages. Specifically, stage 3 increased the risk of anatomic failure 2.34-fold, and stage 4 increased the risk 5.47-fold. These results define the influence of preoperative POP-Q stage on the risk of prolapse more accurately and allow for better counseling before surgery. As we have pointed out above, the symptomatic recurrence figures published in the literature are much lower, hindering the evaluation of the preoperative POP stage as a risk factor not only for anterior prolapse recurrence but also for all POP surgery. None of the studies included in the recent systematic review, mentioned above, report results concerning the influence of preoperative stage on symptomatic recurrence. In our study, focused on the anterior compartment, the comparison between stage 2 and 3 was limited by the small number of cases, but the difference nearly reached statistical significance (2.2% vs. 8.4%; p = 0.058). The mechanism by which severe anterior prolapse favors prolapse recurrence after surgery is unknown. We believe that an advanced stage of prolapse before surgery reflects major tissue damage to pelvic floor structures and consequently a greater likelihood of failure after traditional tissue repair.
Levator avulsion is another well-established factor for anatomic recurrence. Friedman el al. [7], in their meta-analysis, reported a 2.76-fold higher risk. Studies performed in the anterior compartment have yielded similar results: Rodrigo et al. [19] found a 1.93- and a 2.94-fold higher risk in their multivariate analysis on clinical examination and ultrasound imaging, respectively, although the preoperative stage of POP was not included; Weemhoff et al. [20] reported a 2.3-fold higher risk, without considering the effect of hiatal area. Our results show a 1.96-fold higher risk of anatomic recurrence adjusting for both preoperative stage and hiatal area. Nevertheless, the most interesting finding regarding levator avulsion was its independent association with symptomatic recurrence, increasing the risk by a factor of 2.60 in the multivariate model. Neither of the two aforementioned studies demonstrated such a relationship. Once again, the small number of cases limited their analysis.
Regarding the association between hiatal area and prolapse recurrence, our results are also quite innovative. Friedman el al. [7] reported a 1.06-fold higher risk in their meta-analysis, including only results from Rodrigo et al. [19] (OR: 1.04) and Vergeldt et al. [21] (OR: 1.06). Both studies were focused on the anterior compartment, and these results were obtained without using a cutoff point for excessive distensibility. We were able to demonstrate a relationship of an excessive hiatal area (> 25 cm2) with anatomic and symptomatic recurrence in a multivariate model adjusted for potential confounders. To our knowledge, this is the first study reporting this independent association not only for anatomic but also for symptomatic recurrence.
Family history, as in our previous results in this field [11], was not associated with prolapse recurrence. In contrast, Weemhoff et al. [20] found family history to be associated with a 2.4-fold higher risk of anatomic recurrence. In their study, patients with history of POP surgery and those with mesh augmentation were included, and these could be considered confounding factors. Another limitation of their study is that only 63.7% of women completed the follow-up.
Age, BMI, vaginal delivery, menopausal status, chronic constipation, abdominal hernia, or heavy lifting were not significantly associated with POP recurrence, confirming the findings of other authors [6, 7, 19,20,21].
The main strengths of our study are the multicenter prospective design and the high follow-up rate (97.1%) 1 year after surgery. Furthermore, the same type of surgery in the anterior compartment (native tissue vaginal repair) was performed in all women. Another main distinguishing characteristic is that only women with primary prolapse surgery were included in the study, avoiding the potential bias of previous prolapse surgery in the risk factor analysis.
One of the limitations of the present study is that, in nearly two-thirds of women, anterior compartment surgery was performed in conjunction with other prolapse or anti-incontinence procedures. This reflects the “real world” of prolapse surgery: therefore, our results may be applicable to routine clinical practice. Similarly, the relatively short follow-up period could be considered a limitation, but it has been reported that cystocele recurrence after anterior colporrhaphy seems to be a relatively early phenomenon, with maximum prevalence reached at 18–24 months [25]. Our previous retrospective study in this field [11] showed an even lower anatomic recurrence rate in the anterior compartment 5 years after prolapse surgery, and in a long-term follow-up study, the highest risk of undergoing reoperation for POP was highest within the first year [26]. Finally, due to the small number of cases with symptomatic recurrence, the evaluation of important factors such as preoperative POP-Q stage was also limited.
Despite these limitations, the study confirms our hypothesis that major defects in pelvic floor support structures before surgery are associated with higher recurrence rates. An advanced stage of prolapse increases the risk of anatomic recurrence, while levator avulsion and abnormal distensibility of the levator hiatus area increase the risk of both anatomic and symptomatic recurrence 1 year after native tissue vaginal repair. The preoperative identification of these factors may indicate a higher risk of recurrence, and this should be considered in pretreatment counseling of women assessed for this condition.
References
Hendrix S, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the women’s health initiative: gravity and gravidity. Am J Obstet Gynecol. 2002;186:1160–6.
Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–6.
Smith FJ, Holman CD, Moorin RE, Tsokos N. Lifetime risk of undergoing surgery for pelvic organ prolapse. Obstet Gynecol. 2010;116:1096–100.
Wu JM, Matthews CA, Conover MM, Pate V, Funk MJ. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol. 2014;123(6):1201–6.
Maher C, Feiner B, Baessler K, Christmann-Schmid C, Haya N, Brown J. Surgery for women with anterior compartment prolapse. Cochrane Database Syst Rev. 2016;11:CD004014.
Vergelt TF, Weemhoff M, IntHout J, Kluivers KB. Risk factors for pelvic organ prolapse and its recurrence: a systematic review. Int Urogynecol J. 2015;26:1559–73.
Friedman T, Eslick GD, Dietz HP. Risk factors for prolapse recurrence: systematic review and meta-analysis: systematic review and meta-analysis. Int Urogynecol J. 2019;29(1):13–21.
Dietz HP, Simpson JM. Levator trauma is associated with pelvic organ prolapse. BJOG. 2008;115:979–84.
Young N, Atan IK, Rojas RG, Dietz HP. Obesity: how much does it matter for female pelvic organ prolapse? Int Urogynecol J. 2008;29(8):1129–34.
Dietz HP, Chantarasorn V, Shek KL. Levator avulsion is a risk factor for cystocele recurrence. Ultrasound Obstet Gynecol. 2010;36:76–80.
Diez-Itza I, Aizpitarte I, Becerro A. Risk factors for the recurrence of pelvic organ prolapse after vaginal surgery: a review at 5 years after surgery. Int Urogynecol J. 2007;18:1317–24.
Miedel A, Tegerstedt G, Mörlin B, Hammarström M. A 5-year prospective follow-up study of vaginal surgery for pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(12):1593–601.
Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet. 2007;369:1027–38.
Bump RC, Mattiason A, Bo K, Brubaker LP, DeLancey JO, Klarskov P, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–7.
Sánchez-Sánchez B, Torres-Lacomba M, Yuste-Sánchez MJ, Navarro-Brazález B, Pacheco-da-Costa S, Gutiérrez-Ortega C, et al. Cultural adaptation and validation of the pelvic floor distress inventory short form (PFDI-20) and pelvic floor impact questionnaire short form (PFIQ-7) Spanish versions. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):281–5.
Dietz H, Shek K, Clarke B. Biometry of the pubovisceral and levator hiatus by three-dimensional pelvic floor ultrasound. Ultrasound Obstet Gynecol. 2005;25:580–5.
Dietz HP, Bernardo MJ, Kirby A, Shek KL. Minimal criteria for the diagnosis of avulsion of the puborectalis muscle by tomographic ultrasound. Int Urogynecol J. 2011;22:699–704.
Dietz HP, Shek C, De Leon J, Steensma AB. Ballooning of the levator hiatus. Ultrasound Obstet Gynecol. 2008;31:676–80.
Rodrigo N, Wong V, Shek KL, Martin A, Dietz HP. The use of 3-dimensional ultrasound of the pelvic floor to predict recurrence risk after pelvic reconstructive surgery. Aust N Z J Obstet Gynaecol. 2014;54(3):206–11.
Weemhoff M, Vergeldt TF, Notten KJ, Serrooyen J, Kampschoer P, Roumen F. Avulsion of puborectalis muscle and other risk factors for cystocele recurrence: a 2-year follow-up study. Int Urogynecol J. 2012;23(1):65–71.
Vergeldt TF, Notten KJ, Weemhoff M, van Kuijk SM, Mulder FE, Beets-Tan RG, et al. Levator hiatal area as a risk factor for cystocele recurrence after surgery: a prospective study. BJOG. 2015;122(8):1130–7.
Barber MD, Brubaker L, Nygaard I, Wheeler TL II, et al. Defining success after surgery for pelvic organ prolapse. Obstet Gynecol. 2009;114:600–9.
Chmielewski L, Walters MD, Weber AM, Barber MD. Reanalysis of a randomized trial of 3 techniques of anterior colporrhaphy using clinically relevant definitions of success. Am J Obstet Gynecol. 2011;205:69.e1–8.
Weber AM, Walters MD, Piedmonte MR, Ballard LA. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299–306.
Dietz HP, Hankins KJ, Wong V. The natural history of cystocele recurrence. Int Urogynecol J. 2014;25:1053–7.
Lowestein E, Moller LA, Laigaard J, Gimbel H. Reoperation for pelvic organ prolapse: a Danish cohort study with 15-20 years' follow-up. Int Urogynecol J. 2018;29:119–24.
Author information
Authors and Affiliations
Contributions
• I Diez-Itza: Project development, data collection, data analysis, manuscript writing.
• M Avila: Data collection.
• S Uranga: Data collection.
• M Belar: Data collection.
• A Lekuona: Project development.
• A Martin: Project development, data collection.
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Diez-Itza, I., Avila, M., Uranga, S. et al. Factors involved in prolapse recurrence one year after anterior vaginal repair. Int Urogynecol J 31, 2027–2034 (2020). https://doi.org/10.1007/s00192-020-04468-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-020-04468-1