Abstract
Introduction and hypothesis
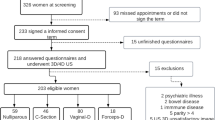
The objective was to determine the mean morphometric characteristics of the rectovaginal septum (RVS) and its variations in correlation with the number of pregnancies, method of delivery, parity, and estrogenic exposure.
Methods
An observational, cross-sectional, retrospective, descriptive, and comparative study was carried out. Pelvic MRI of Hispanic women (≥15 years of age) from the northeast of Mexico were obtained. Age and obstetric and gynecological history were registered and the sample women were categorized by their variables. Length and thickness measurements were standardized.
Results
A total of 102 MRI studies were included, with a mean age of 41; 24.5% were nulligravida, the rest primi- or multigravida. Vaginal delivery was the most common type (49.35%), 16.88% had a cesarean section, and 31.17% had mixed delivery. 74.5% of the women were premenopausal. The mean RVS length was 73.2 ± 15.3 mm, with a thickness of 2.8 ± 1.7, 2.2 ± 1.2, and 2.5 ± 1.3 mm for the upper, middle, and lower thirds respectively. There were tendencies to increase the length of the RVS, and the thickness of the upper and middle thirds in the non-pregnancy and the at-least-one-pregnancy groups; to increase the length and middle-third thickness in those with mixed delivery, and increased upper- and lower-third thickness in those with only a cesarean section. Multiparous women with vaginal delivery had significantly longer and thicker RVS than primiparous. Premenopausal women had significantly longer RVS with a tendency to lose thickness toward postmenopause.
Conclusions
The morphology of RVS can be modified by different factors such as age, number of pregnancies, number of births, and estrogenic exposure. This structure should be evaluated and taken into account in preoperative management and surgical technique planning.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rectovaginal septum (RVS) was first described by Uhlenhuth in 1948 [1]. It is a layer of dense fibro-connective tissue, smooth muscle, and elastic and collagen fibers [1,2,3]. It provides support, innervation (nerve fibers from the inferior hypogastric plexus), and irrigation to the adjacent pelvic structures. It participates in the cycles of continence, defecation, sexual control, and other urinary functions [4,5,6,7]. Its morphology allows for mobility between the rectum and vagina, and aids in limiting the spread of infections or tumors [2, 3, 7,8,9,10].
During surgery, the RVS provides an important anatomical reference [11]. It aids the surgeon as a guide to determine the extent of the dissections and helps with the pelvic nerve-sparing during different types of surgeries such as vaginal reconstruction, anorectoplasty in pediatrics, urogenital sinus surgery, and rectal cancer surgery [2, 5,6,7,8,9,10, 12, 13].
Damage or laceration to the RVS may predispose to the formation of rectocele, enterocele, fistula [6, 8, 10, 14, 15], and could produce constipation, difficulty in defecation [9], fecal incontinence [4, 5, 7] or sexual dysfunction [7, 10, 12, 16].
Currently, there is no consensus regarding the morphology (thickness or length) of the RVS. Some authors report it to be a constant, without any change in relation to age or hormones, whereas others report variance due to parity and advanced age [2, 14, 17]. The aim of this study was to determine the mean morphometric characteristics of the RVS and its variations in correlation with the number of pregnancies, method of delivery, parity, and estrogenic exposure.
Materials and methods
An observational, cross-sectional, retrospective, descriptive, and comparative study was performed. Magnetic resonance images (MRI) were obtained from the database of the Radiology and Imaging Department of the University Hospital “Dr. José Eleuterio Gonzalez,” Monterrey, Mexico.
Pelvic MRIs were obtained from Hispanic women (≥15 years of age) from the northeast of Mexico. Exclusion criteria were: gravid women and women with a history of hysterectomy, rectocele, enterocele, rectovaginal fistula, uterine prolapse, pelvic tumors, previous vaginal surgery, other pelvic surgeries, and other abnormalities or pathological conditions that may affect the normal vaginal anatomy.
Age and obstetric and gynecological history were registered and the population was categorized by the number of pregnancies (nulligravida, 1–3 pregnancies, or ≥ 4 pregnancies), type of delivery (vaginal, cesarean section, or mixed), parity (primiparous or multiparous), and estrogen exposure (premenopause and postmenopause).
All MRIs were performed using General Electric Resonance Magnetic Signa HDx 1.5 Tesla Equipment, in a sagittal T2-weighted sequence (echo time [TE] 102.0 and repetition time [TR] 5,384.0), field of view [FOV] of 24 to 28.9 cm, cutting thickness 3.5 mm, software version 15.0.0947A. The length and thickness of the rectovaginal septum were measured using the Carestream® program (version 12.1.5.6009; Carestream, Rochester, NY, USA).
The studies were assessed by a board-certified radiologist with extensive experience in the pelvic cavity. The measurements were standardized in a mid-sagittal plane by using the posterior fornix and the end of the pouch of Douglas to the perineal body to determine the length of the RVS. To measure its thickness, the RVS was divided into thirds. In the middle of each third, the thickness was measured between the muscular layers of the rectum and the vagina. The width of the RVS was not included owing to the variability of the results and the lack of anatomical reference points (Fig. 1).
A sagittal MRI T2-weighted reconstructed image of the pelvis. Schematics (top row) and MRI slice (bottom row). The standardized top and bottom points are set for length measurements, and the rectovaginal septum traced. This is then divided into thirds to determine the thickness in the middle of each. a Multiparous patient; b primiparous patient; c nulligravid patient
The sample size was calculated based on the number of studies available, to establish the mean in a population, with a 95% confidence and a margin of error of 5%. A total of 96 MRIs were needed, which is the reason why 102 were included. Normality tests were performed using the Kolmogorov–Smirnov test. Central tendency and dispersion measurements were obtained. The comparisons between the different study groups were made using a two-tailed Student’s t test and one-way ANOVA for the parametric data and the Mann–Whitney U and Kruskal–Wallis test for the nonparametric data. Regression model analyses was used to correlate dependent and independent variables. A Pearson correlation coefficient test and a Kappa index of concordance of all measurements were performed. Kappa indexes were 0.80, 0.71, 0.68, and 0.66 for the length and thickness of the upper, middle, and lower thirds of the RVS. A p value of ≤0.05 was considered statistically significant. SPSS Statistics version 20 for Windows 7 (IBM, Armonk, NY, USA) was used for Windows 7.
The study had been previously reviewed and approved by the University’s Ethics and Research Committees with the registration number AH17–00010, certifying that it adheres to the guidelines of the General Health Law on Health Research in Human Beings of our country and the Declaration of Helsinki. None of the MRIs was performed for the purpose of this study.
Results
A total of 102 MRI studies were included, the mean age was 41.03 ± 15.23 years (range 15 to 77). A quarter (n = 25, 24.5%) of the patients were nulligravida, the rest (n = 77, 75.5%) being primi- or multigravida. Of those with previous pregnancies (n = 77, 75.5%), vaginal delivery was the most common type (n = 38, 49.35%); 16.88% (n = 13) had a cesarean section, and 31.17% (n = 24) had a history of both types of delivery. Two patients (n = 2, 2.6%) had a history of miscarriage. Within the women who had a vaginal delivery, 20 (19.6%) were primipara and 42 (41.2%) were multipara. There were 76 (74.5%) premenopausal women and 26 (25.5%) postmenopausal women.
The mean RVS length was 73.2 (± 15.3) mm, with a thickness of 2.8 (±1.7), 2.2 (± 1.2), and 2.5 (±1.3) mm for the upper, middle, and lower thirds respectively.
We made a comparison between the non-pregnancy and the at-least-one-pregnancy group, showing a tendency to increase the length of the RVS, and the thickness of the upper and middle thirds (Table 1). A positive Pearson correlation coefficient was obtained between the number of pregnancies and the length of the RVS (0.291; p = 0.01). Regression analysis suggests significance in an increase in the length of 3.8 mm (p = 0.000) and the upper-third thickness of 0.2 mm (p = 0.031) with each pregnancy (Table 2).
When we compared the different types of delivery, we found a tendency to increase the length and middle-third thickness of the RVS in those who had had mixed deliveries. A similar tendency to increase the upper- and lower-third thickness of the RVS was found in those who had only had a cesarean section (Table 3).
In women with vaginal delivery, multiparous women had a statistically significantly longer RVS than primiparous women (p = 0.011), as well as a tendency towards higher thickness, although the latter was not statistically significant (Table 4). However, regression analysis demonstrated parity to be significant with regard to length (p = 0.013), upper-third thickness (p = 0.031), and middle-third thickness (p = 0.012), increasing these by 8.1, 0.2, and 0.2 mm respectively, with each delivery (Table 2). Regarding the hormonal stage, premenopausal women had statistically significantly longer RVS (p = 0.031) with a tendency to lose thickness (0.3 mm per year; Table 2) toward postmenopause (Table 5).
Discussion
Our study evaluated the RVS through MRI, obtaining an overall mean of its length and thickness in its upper, middle, and lower thirds using a standardized methodology. Data were stratified by the number of pregnancies, types of delivery, parity, and hormonal exposure (premenopausal and postmenopausal), for analysis and correlation with these. The RVS was longer in women with a greater number of pregnancies, those with mixed deliveries, and those who were premenopausal.
The thickness of the RVS had variations depending on where it was measured and the groups. Women with the most pregnancies had the thickest RVS, primarily in the upper third; in women who had a vaginal delivery, the thickest part of the RVS was the lower third especially in primiparous women. These differences could be justified by the healing process secondary to the birthing mechanism. Hormone depletion (postmenopause) correlated with a decrease in RVS length and overall thickness.
Kuhn and Hollyock [14] reported a much shorter RVS length (21 mm), although this could be attributed to the methodology, as their measurements were made during laparoscopic procedures, without specifying how the measurements were undertaken. However, their data also demonstrated a longer RVS length in multiparas than in primiparous women (Table 6).
The length of the RVS (35–60 mm) and thickness (0.1–0.3 mm) ranges reported by Nagata et al. [17] differ from our results. However, the methodology was also different, as measurements were obtained through corpse dissection in a sample of elderly women (mean age 82.4) embalmed with formalin, which could alter the anatomical structures. In their methods, they report dividing the septum into two halves, without mentioning at what level the thickness was measured or the instrument used for the measurements. Similar results were reported from cadaveric 3D endovaginal ultrasound by Shobeiri et al. [18].
Dietz [6] reported a shorter mean length of 45.3 mm and a thickness of 1.16 mm compared with our results. Nonetheless, differences are expected, as the study was carried out in pathological pelvic floors (prolapse, enterocele, rectocele, etc.), using 3D ultrasound, which is operator dependent. Also, they did not mention the anatomical references used for both types of measurements.
Rosenshein et al. [4] classified RVS defects secondary to surgical or obstetric traumas at five levels: type I, perineal body loss without association with fistulas and fecal incontinence; type II, perineal body loss associated with fistulas in the lower third; type III, intact perineal body with fistula in the lower third; type IV fistula in the middle third; and type V fistula in the upper third. Type I was most common in primiparous women, whereas type II predominated in multiparous women. Our results showed a thinner lower third in women who had had vaginal delivery than in nulligravida, and that those who had had multiple vaginal deliveries had a thinner RVS in the lower third compared with primiparous women. This decrease could be attributed to the damage mechanism during vaginal delivery.
Haylen et al. [19] classified the RVS by levels I, II, and III for the upper, middle, and lower thirds respectively, and identified the most common location of defects (prolapse), as level I, level III, followed by level II in frequency. Our results could be correlated with these findings, as we report the upper third (level I) to have the highest overall thickness, followed by levels III and II. This may be due to scar tissue secondary to pregnancy and the vaginal delivery trauma, especially in the primiparous women. This causes the replacement of muscle and elastic fibers with collagen, weakening the upper and lower thirds, predisposing the women to prolapse formation in the posterior wall of the vagina.
Other studies have used MRI to evaluate vaginal anatomy without describing the RVS [20,21,22,23,24]. Huebner et al. were able to demonstrate the presence of the RVS, even in Müllerian agenesis, without detailing the morphometrics [25].
To our knowledge, this is the first study to measure RVS using MRI, which is not a dependent operator and is the gold standard for soft-tissue assessment. The sample consisted of healthy patients, allowing us to obtain parameters of normality when describing their morphometry. However, some limitations include the lack of inter-observer analysis, as all measurements were performed by an expert radiologist from pelvic MRI, without purposefully focusing the imaging study on RVS assessment. Future studies could be conducted prospectively for better RVS visualization and an improved understanding of the female anatomy in correlation with pathological conditions and surgical procedures [26].
Conclusions
The morphology of RVS can be modified by different factors such as age, number of pregnancies, number of births, and estrogenic exposure. There are still many discrepancies regarding the morphology and function of the RVS, although its role is currently becoming more evident in clinical and surgical gynecology. This structure should be evaluated and taken into account in preoperative management and surgical technique planning.
References
Uhlenhuth E, Wolfe WM, Smith EM, Middleton EB. The rectogenital septum. Surg Gynecol Obstet. 1948;86(2):148–63.
Milley PS, Nichols DH. A correlative investigation of the human rectovaginal septum. Anat Rec. 1969;163(3):443–51. https://doi.org/10.1002/ar.1091630307.
Baggish MS, Karram MM (2015) Atlas of pelvic anatomy and gynecologic surgery. Amsterdam: Elsevier.
Rosenshein NB, Genadry RR, Woodruff JD. An anatomic classification of rectovaginal septal defects. Am J Obstet Gynecol. 1980;137(4):439–42. https://doi.org/10.1016/0002-9378(80)91124-2.
Aigner F, Zbar AP, Ludwikowski B, Kreczy A, Kovacs P, Fritsch H. The rectogenital septum: morphology, function, and clinical relevance. Dis Colon Rectum. 2004;47(2):131–40. https://doi.org/10.1007/s10350-003-0031-8.
Dietz HP. Can the rectovaginal septum be visualized by transvaginal three-dimensional ultrasound? Ultrasound Obstet Gynecol. 2011;37(3):348–52. https://doi.org/10.1002/uog.8896.
Dariane C, Moszkowicz D, Peschaud F. Concepts of the rectovaginal septum: implications for function and surgery. Int Urogynecol J. 2016;27(6):839–48. https://doi.org/10.1007/s00192-015-2878-3.
Nichols DH, Milley PS. Surgical significance of the rectovaginal septum. Am J Obstet Gynecol. 1970;108(2):215–20. https://doi.org/10.1016/0002-9378(70)90299-1.
Ludwikowski B, Hayward IO, Fritsch H. Rectovaginal fascia: an important structure in pelvic visceral surgery? About its development, structure, and function. J Pediatr Surg. 2002;37(4):634–8. https://doi.org/10.1053/jpsu.2002.31624.
Zhai LD, Liu J, Li YS, Yuan W, He L. Denonvilliers’ fascia in women and its relationship with the fascia propria of the rectum examined by successive slices of celloidin-embedded pelvic viscera. Dis Colon Rectum. 2009;52(9):1564–71. https://doi.org/10.1007/DCR.0b013e3181a8f75c.
Leffler KS, Thompson JR, Cundiff GW, Buller JL, Burrows LJ, Schön Ybarra MA. Attachment of the rectovaginal septum to the pelvic sidewall. Am J Obstet Gynecol. 2001;185(1):41–3. https://doi.org/10.1067/mob.2001.116366.
Baader B, Herrmann M. Topography of the pelvic autonomic nervous system and its potential impact on surgical intervention in the pelvis. Clin Anat. 2003;16(2):119–30. https://doi.org/10.1002/ca.10105.
Geynisman-Tan J, Kenton K. Surgical updates in the treatment of pelvic organ prolapse. Rambam Maimonides Med J. 2017;8(2):e0017. https://doi.org/10.5041/RMMJ.10294.
Kuhn RJ, Hollyock VE. Observations on the anatomy of the rectovaginal pouch and septum. Obstet Gynecol. 1982;59(4):445–7.
D’Hoore A, Vanbeckevoort D, Penninckx F. Clinical, physiological and radiological assessment of rectovaginal septum reinforcement with mesh for complex rectocele. Br J Surg. 2008;95(10):1264–72. https://doi.org/10.1002/bjs.6322.
Singh N. Development and anatomy: disorders of development. In: Brown L, editor. Pathology of the vulva and vagina. London: Springer. 2013.
Nagata I, Murakami G, Suzuki D, Furuya K, Koyama M, Ohtsuka A. Histological features of the rectovaginal septum in elderly women and a proposal for posterior vaginal defect repair. Int Urogynecol J. 2007;18(8):863–8. https://doi.org/10.1007/s00192-006-0249-9.
Shobeiri SA, White D, Quiroz LH, Nihira MA. Anterior and posterior compartment 3D endovaginal ultrasound anatomy based on direct histologic comparison. Int Urogynecol J. 2012;23(8):1047–53. https://doi.org/10.1007/s00192-012-1721-3.
Haylen BT, Naidoo S, Kerr SJ, Yong CH, Birrell W. Posterior vaginal compartment repairs: where are the main anatomical defects? Int Urogynecol J. 2016;27(5):741–5. https://doi.org/10.1007/s00192-015-2874-7.
Lopez C, Balogun M, Ganesan R, Olliff JF. MRI of vaginal conditions. Clin Radiol. 2005;60(6):648–62. https://doi.org/10.1016/j.crad.2005.02.010.
Paramasivam S, Proietto A, Puvaneswary M. Pelvic anatomy and MRI. Best Pract Res Clin Obstet Gynaecol. 2006;20(1):3–22. https://doi.org/10.1016/j.bpobgyn.2005.09.001.
Hsu Y, Lewicky-Gaupp C, DeLancey JO. Posterior compartment anatomy as seen in magnetic resonance imaging and 3-dimensional reconstruction from asymptomatic nulliparas. Am J Obstet Gynecol. 2008;198(6):651–e1. https://doi.org/10.1016/j.ajog.2007.11.032.
Wasnik AP, Mazza MB, Liu PS. Normal and variant pelvic anatomy on MRI. Magn Reson Imaging Clin N Am. 2011;19(3):547–66. https://doi.org/10.1016/j.mric.2011.05.001.
Salinas-Alvarez Y, Rodriguez-Abarca MA, Quiroga-Garza A, Guzman-Lopez S, Elizondo-Omaña RE, Jacobo-Baca G, et al. Rectovaginal septum and influencing factors in its morphology. FASEB J. 2020;34(S1):1–1. https://doi.org/10.1096/fasebj.2020.34.s1.06241.
Huebner M, Rall K, Brucker SY, Reisenauer C, Siegmann-Luz KC, DeLancey JO. The rectovaginal septum: visible on magnetic resonance images of women with Mayer-Rokitansky-Küster-Hauser syndrome (Müllerian agenesis). Int Urogynecol J. 2014;25(3):323–7. https://doi.org/10.1007/s00192-013-2214-8.
Vázquez-Barragán MÁ, Garza-Báez A, Morales-Avalos R, Martínez-González B, Jacobo-Baca G, Pinales-Razo R, et al. Pelvimetry by reformatted computed tomography in 290 female pelvis: morphometric variations regarding age. Int J Morphol. 2016;34(1):298–304. https://doi.org/10.4067/S0717-95022016000100043.
Acknowledgements
The authors thank Armando Franco for his help in the editing of the figure in this manuscript.
Funding
There was no external funding; resources were provided by the Department of Human Anatomy, UANL.
Author information
Authors and Affiliations
Contributions
M.A. Rodríguez-Abarca: methodology, data collection, validation, writing (review and editing); Edgar Guillermo Hernández-Grimaldo: methodology, data collection, validation, writing (review and editing); D. De la Fuente-Villarreal: methodology, validation, writing (review), supervision, project administration; G. Jacobo-Baca: conceptualization, methodology, validation, writing (review), supervision, project administration; A. Quiroga-Garza: conceptualization, methodology, data collection, validation, writing (review and editing), supervision, project administration; R. Pinales-Razo: methodology, writing (review), supervision, project administration; R.E. Elizondo-Omaña: methodology, writing (review and editing), supervision, project administration; S. Guzman-Lopez: methodology, writing (review), supervision, project administration.
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rodríguez-Abarca, M.A., Hernández-Grimaldo, E.G., De la Fuente-Villarreal, D. et al. Gynecological influencing factors on the rectovaginal septum’s morphology. Int Urogynecol J 32, 1427–1432 (2021). https://doi.org/10.1007/s00192-020-04376-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-020-04376-4