Abstract
Introduction and hypothesis
Although postoperative urinary tract infections (UTIs) after urogynecologic surgery are a common adverse event, there is no standardized postoperative time period used to assess this outcome, and the uropathogens unique to this sub-population of patients have not been well described. Our objective is to describe the timing and uropathogens of postoperative UTI after urogynecologic surgery.
Methods
This retrospective study analyzed postoperative UTI occurring within 90 days following urogynecologic procedures from November 2013 to January 2018 at a single academic institution. Postoperative UTI was defined as any uropathogen growth from standard urine culture. Continuous variables were compared with independent samples t-test and categorical variables with chi-square with Bonferonni corrections as appropriate.
Results
One hundred and two of 1085 (9.4%) patients experienced UTI; 63.7% occurred within 6 weeks and 78.4% within 8 weeks; 36.3% of UTIs occurred at a time period of 6 weeks to 90 days. Most commonly isolated uropathogens were Escherichia coli (47.8%) with an additional 11.2% extended-spectrum beta-lactamase (ESBL) Escherichia coli. Other bacteria included Enterococcus faecalis (10.4%), Klebsiella pneumoniae (9%), and one culture each for ESBL Klebsiella pneumoniae and vancomycin-resistant (VRE) Enterococcus faecium.
Conclusions
More than one third of UTIs after urogynecologic surgery occur between 6 weeks and 90 days postoperatively. A plateau of UTI incidence occurs at 8 weeks, a time period at which 78.4% of all UTIs were captured. Escherichia coli was the most commonly isolated uropathogen, and multi-drug-resistant bacteria were implicated in 12.8% of UTIs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary tract infection (UTI) after urogynecologic surgery is one of the most common postoperative adverse events, ranging from 6.4%–31% [1,2,3]. Surgical teams take steps to reduce this adverse event, including preoperative testing (most commonly by urine dipstick or urinalysis with reflex urine culture), systemic prophylactic antibiotics at the start of surgery, and oral antibiotic coverage during postoperative catheter use. Despite these efforts, postoperative UTIs occur and are associated with increased hospital length of stay and increased patient mortality [4, 5]. UTIs markedly increase the use of systemic antibiotics, which contributes to antibiotic resistance, a global problem that is projected to cost over 10 million lives in 2050 [6]. In addition, individual patients may face collateral adverse effects.
Most contemporary studies use a 30-day to 6-week postoperative period to define postoperative UTI [1, 7,8,9,10,11,12,13], although the time period for a definition of postoperative UTI varies, ranging from 3 weeks to 3 months [14,15,16]. These time frames may be linked to common postoperative events, for example, the 30-day postoperative period is frequently used for documentation of other surgical complications and many urogynecologic surgeons see their patients for their first postoperative visit at 6 weeks. It is possible that the rate of postoperative UTI may be underestimated in studies that limit assessments to events occurring between 30 days and 6 weeks. When the postoperative period is defined as 3 months after surgery, the UTI rate can increase up to 45% [16].
Although Escherichia coli is the most frequently implicated uropathogen [9, 17], other uropathogens have less recognition in the urogynecologic surgical population. To adopt improved prevention strategies for postoperative UTI, further insights into both pathogens and the timeline of events are needed.
Our primary objective is to describe the timing of postoperative UTI within 90 days after urogynecologic surgery. Our secondary objectives are to describe the uropathogens involved and to determine if type of surgery affects rates of postoperative UTI.
Materials and methods
This study was approved by the Biomedical Institutional Review Board at the University of California, San Diego, Human Research Protections Program. We analyzed UTIs following urogynecologic procedures performed at a single academic institution from November 2013 to January 2018. The earliest time point in which data could be accessed from the electronic medical record (EMR) was November 2013. Data were extracted from the EMR for all urogynecologic inpatient surgeries performed by five surgeons, all board-certified in Female Pelvic Medicine and Reconstructive Surgery.
Surgical characteristics were collected including type of surgery performed—pelvic organ prolapse (POP) surgery, stress urinary incontinence (SUI) surgery, both POP and SUI surgery, bladder onabotulinumtoxinA, or other. Surgery for POP included sacrocolpopexy, uterosacral ligament suspension, sacrospinous ligament suspension, colpocleisis, paravaginal repair, or anterior or posterior colporrhaphy. Surgery for SUI included mid-urethral sling, pubovaginal sling, or Burch procedure.
We defined postoperative UTI as a positive urine culture for any uropathogen growth from a standard urine culture within 90 days after surgery. Although the culture criterion of ≥ 100,000 CFUs for UTI diagnosis is traditionally used, this criterion was developed in the 1950s as a predictor of those that would develop urosepsis after kidney surgery [18, 19] and therefore was intended for a different patient population than this cohort. We chose our definition since a lower cutoff of ≥ 102 CFU/ml in the setting of UTI symptoms has been shown to have a high predictive value for UTI [20, 21]. Non-uropathogenic organisms were excluded from our UTI definition, including group B Streptococcus and mixed urethral flora [22]. Additionally, by omitting a presumed diagnosis of UTI (such as UTI symptoms treated with an antibiotic) from our definition, an individual provider’s subjective diagnosis of UTI was avoided. Furthermore, urine cultures from postoperative patients are not sent routinely in the absence of symptoms. Multi-drug-resistant (MDR) bacteria was defined as extended-spectrum beta-lactamase (ESBL) Escherichia coli, ESBL Klebsiella pneumoniae, and vancomycin-resistant Enterococcus faecium (VRE).
Variables including species, colony-forming units (CFUs), and postoperative day of positive urine culture were collected. Statistical analysis was performed with IBM SPSS© version 24. Continuous variables were compared with independent samples t-test and categorical variables with chi-square and Bonferroni corrections as appropriate.
Results
We identified 141 positive urine cultures from 1085 unique surgical encounters during our study period. Seven urine cultures positive for group B Streptococcus were excluded, resulting in 134 urine cultures available for analysis. Approximately one in ten patients experienced at least one postoperative UTI [102/1085 (9.4%)]; some patients experienced more than one event [24 patients (2.2%) experienced > 1 UTI; 6 patients (0.6%) experienced ≥3 UTIs]. Of the six patients that had ≥ 3 UTIs, four patients experienced a relapsing infection with the same uropathogen and two patients experienced a combination of a relapsing infection and reinfection with a different uropathogen. The colony count was ≥ 100,000 CFUs for the majority (61.9%) of positive cultures with 24.6% reporting 50,000–100,000 CFUs and 11.9% reporting 10,000–50,0000 CFUs. Only 1.4% reported < 5,000 CFUs or none recorded.
The average patient age was 60.6 years, with no difference between those with a UTI (60.5) and those without (60.6) (p = 0.895) (Table 1). All patients received perioperative intravenous antibiotic prophylaxis with the vast majority receiving 1–2 g of Cefazolin, unless otherwise dictated based on allergies or recent urine culture-resistance profiles. Indication for surgery varied including POP only (41.4%), SUI only (20.6%), combined POP and SUI (25.9%), bladder onabotulinumtoxinA (3.3%), and other (urethral diverticulectomy, transurethral injections, anal sphincteroplasty) (8.8%). A complete list of the procedure types and corresponding UTI rates can be found in Table 2. Of the surgeries performed for SUI, a vast majority were mid-urethral slings, with pubovaginal sling and Burch procedure contributing 2.9% and 0.6%, respectively. Combined POP and SUI surgery had a significantly higher rate of postoperative UTI compared with POP or SUI surgery alone (12.8% vs. 6.5% and 5.8%, respectively) (p = 0.003) (Table 1).
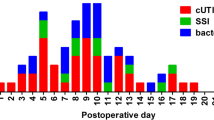
Of the 134 postoperative UTI events, 23.7% occurred in ≤ 2 weeks after surgery, 50% occurred within 30 days, and 63.7% in the first 6 weeks. Over a third (36.3%) occurred between 6 weeks and 90 days postoperatively; 78.4% of events occurred within 8 weeks postoperatively, after which the UTI incidence plateaus (Fig. 1).
Seventeen (12.8%) postoperative UTIs were attributed to MDR bacteria. The most frequently isolated uropathogen was Escherichia coli (47.8%), followed by ESBL Escherichia coli (11.2%). Other uropathogens isolated included Enterococcus faecalis (10.4%), Klebsiella pneumoniae (9%), Proteus mirabilis (5.2%), Enterobacter (3%), and one culture each for ESBL Klebsiella pneumoniae and VRE (Fig. 2).
The six most common uropathogens were relatively evenly distributed over the postoperative period, with the exception of ESBL Escherichia coli. The distribution of the 15 ESBL Escherichia coli cultures was notable for a bimodal pattern—nine positive cultures were present in the first half of the postoperative period and six in the second half. Of the six ESBL Escherichia coli cultures in the second half of the postoperative period, four cultures were seen in patients that had a preceding treatment of a non-MDR UTI in the first half (Fig. 3).
Bar graph demonstrating distribution of the six most common causative bacteria in postoperative UTI up to a time period of 90 days. ESBL Escherichia coli UTIs (red) in the second half of the postoperative period were often preceded by treatment of a non-resistant bacterial UTI in the first half of the postoperative period
Discussion
In this cohort of women undergoing urogynecologic surgery with an overall low rate of postoperative UTI, > 1/3 of UTIs occurred later than the first 6 postoperative weeks. We also found that the incidence of UTI plateaus from approximately 8 weeks to 90 days postoperatively in this cohort. Although 21.6% of UTIs occurred after the 8-week period, given the plateau and appearance of stabilization of the UTI rate after this time period, it is possible that this group represents those patients that progress to frequent or recurrent UTI. This is especially notable in the group of ESBL Escherichia coli UTIs, where a majority of those UTIs diagnosed after 8 weeks were a patient’s second UTI in the postoperative period. The positive urine cultures occurring after the 8-week mark may also represent the cohort’s return to baseline, although we cannot confirm this microbiologically. Our findings support the use of a standard definition of postoperative UTI as occurring within 8 weeks in women undergoing urogynecologic surgery. This definition may help standardize outcomes assessments and inform quality improvement initiatives, leading to a more evidence-based approach to documentation and prevention of postoperative UTIs.
Consistent with previous studies that used standard urine culture, Escherichia coli was the most commonly isolated uropathogen in this cohort [9, 17]. However, previous studies had not included rates of MDR bacteria. In this study, ESBL Escherichia coli was the second most commonly isolated uropathogen, and MDR bacteria comprised 12.8% of all isolates, highlighting the increasing problem with antibiotic resistance. One study of 83 patients undergoing renal transplant surgery showed that the rate of postoperative ESBL Escherichia coli UTIs was 16.8% [23]. It was also shown that the rate of ESBL Escherichia coli UTI increased in patients as their number of postoperative UTIs diagnosed increased. Similar findings were seen in our study—2/3 of the ESBL Escherichia coli UTIs occurring after postoperative week 8 occurred in patients who had preceding treatment of a non-MDR UTI earlier in the postoperative period. It is possible that inadequate treatment of a postoperative UTI or simply exposure to a course of antibiotics leads to subsequent MDR bacterial infection in subsequent UTIs.
Our finding that combined POP and SUI surgery doubled the postoperative UTI rate compared with POP or SUI surgery alone is consistent with the findings of a randomized controlled trial of 337 women who underwent POP surgery or combined POP and SUI surgery, with postoperative UTI rates of 18.3% and 31%, respectively [3]. Although the UTI rates reported in that study are almost triple those found in our cohort, we attribute this to the authors’ expanded UTI definition (positive urinalysis or UTI symptoms resulting in antibiotic treatment). The UTI rate of combined POP and SUI surgery being twice as high as either surgery alone is biologically plausible, as combined surgery is likely to have multiple episodes of urethral and bladder instrumentation with repeat cystourethroscopy.
There was no difference in the ages of women with and without postoperative UTI in this cohort. Given the associated changes in the genitourinary microbiome in menopausal women causing susceptibility to UTI [24], we expected to see more older women with postoperative UTI. A large study of 9022 patients undergoing mid-urethral sling surgery found that age > 65 years was a risk factor for postoperative UTI [13]. It is possible that we were underpowered to show this difference in our study or that there is a distinct or overriding pathophysiology for postoperative UTI after urogynecologic surgery, regardless of age.
Limitations include the retrospective nature of this study and the inherent biases involved with this type of research. Detailed demographics for this retrospective cohort, such as personal history/documentation of recurrent UTI and preoperative positive urine culture, would have allowed us to generate additional testable hypotheses regarding perioperative UTIs. Our definition of UTI—any growth of uropathogen by standard urine culture—deviates from the traditional requirements of ≥ 100,000 CFUs of uropathogen and thus may have overestimated UTI compared with other studies that have used the traditional definition in this patient population. Simultaneously, our definition was based on urine culture without formal assessment of patient symptoms, which excludes patients that would have been clinically diagnosed with a UTI without objective data. The UTI Symptom Assessment questionnaire (UTISA), a validated symptoms assessment tool for UTI, has not been validated for postoperative UTI in this population [25]. Furthermore, it is unlikely that a significant number of postoperative UTIs in this cohort would be classified as asymptomatic bacteriuria [26], as urine cultures are not routinely sent in the asymptomatic postoperative patient that is recovering well. Greater than 10% of the procedures included in this study were for indications other than POP and SUI, and this minority may represent a different patient population. However, this cohort was similar in that all had their procedure in an operating room and not in a clinic setting. Furthermore, given the numerous different procedures performed for both POP and SUI, our study population is quite heterogeneous. Nonetheless, the diversity of procedures is likely representative of a typical academic urogynecologic patient population.
The strengths of this study include the careful documentation of UTI timing with detection of postoperative UTI up to 90 days; the majority of postoperative UTI studies limit the assessment period to 6 weeks after surgery. Our detailed description of uropathogenic species highlights previously unappreciated contributions of MDR bacteria to postoperative UTI in the urogynecologic surgical population. While systemic antibiotics continue to be the mainstay of perioperative UTI prevention and postoperative UTI treatment, these drugs also contribute to growing antibiotic resistance as well. Based on the concerning finding of such a large fraction of MDR UTIs in this cohort, surgical teams are encouraged to conduct further research aimed at reducing the need for postoperative UTI treatment.
Using an expanded time period of 8 weeks after surgery can help to more accurately capture the clinically relevant postoperative UTI events. The time frame of 8 weeks may be an important clinical marker for resilience of the urobiome following likely perturbation from surgical events, such as catheterization and surgical antibiotic prophylaxis. Future perioperative studies are needed to characterize urobiome changes to advance effective postoperative UTI prevention strategies.
Postoperative UTI continues to be a common adverse event and potentially occurs more frequently than previously documented in the literature. Surgical teams have implemented clearly defined measurements and effective prevention strategies for surgical site infection, an event that is fully integrated as a surgical quality metric. Similarly, surgical teams should recognize the impact of perioperative UTI, work toward prevention, and raise awareness of postoperative UTI as an additional, valued quality measure in urogynecologic surgery.
References
Richter HE, et al. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362(22):2066–76.
Albo ME, et al. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007;356(21):2143–55.
Wei JT, et al. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366(25):2358–67.
Mitchell BG, Ferguson JK, Anderson M, Sear J, Barnett A. Length of stay and mortality associated with healthcare-associated urinary tract infections: a multi-state model. J Hosp Infect. 2016;93(1):92–9.
Rosenthal VD, et al. Time-dependent analysis of length of stay and mortality due to urinary tract infections in ten developing countries: INICC findings. J Inf Secur. 2011;62(2):136–41.
de Kraker MEA, Stewardson AJ, Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016;13(11):e1002184.
Chung CP, et al. Incidence and risk factors of postoperative urinary tract infection after uterosacral ligament suspension. Int Urogynecol J. Jul. 2012;23(7):947–50.
Fok CS, et al. Day of surgery urine cultures identify urogynecologic patients at increased risk for postoperative urinary tract infection. J Urol. 2013;189(5):1721–4.
Sutkin G, Alperin M, Meyn L, Wiesenfeld HC, Ellison R, Zyczynski HM. Symptomatic urinary tract infections after surgery for prolapse and/or incontinence. Int Urogynecol J. 2010;21(8):955–61.
Nygaard I, et al. Risk factors for urinary tract infection following incontinence surgery. Int Urogynecol J. 2011;22(10):1255–65.
Gehrich AP, Lustik MB, Mehr AA, Patzwald JR. Risk of postoperative urinary tract infections following midurethral sling operations in women undergoing hysterectomy. Int Urogynecol J. 2016;27(3):483–90.
Thomas-White KJ, et al. Urinary microbes and postoperative urinary tract infection risk in urogynecologic surgical patients. Int Urogynecol J. 2018;29(12):1797–805.
Vigil HR, Mallick R, Nitti VW, Lavallée LT, Breau RH, Hickling DR. Risk factors for urinary tract infection following mid urethral sling surgery. J Urol. 2017;197(5):1268–73.
Rogers RG, et al. A randomized, double-blind, placebo-controlled comparison of the effect of nitrofurantoin monohydrate macrocrystals on the development of urinary tract infections after surgery for pelvic organ prolapse and/or stress urinary incontinence with suprapubic c. Am J Obstet Gynecol. 2004;191(1):182–7.
Dieter AA, et al. Oral antibiotics to prevent postoperative urinary tract infection. Obstet Gynecol. 2014;123(1):96–103.
FitzGerald MP, et al. Pelvic support, pelvic symptoms, and patient satisfaction after colpocleisis. Int Urogynecol J. 2008;19(12):1603–9.
Kow N, Holthaus E, Barber MD. Bacterial uropathogens and antibiotic susceptibility of positive urine cultures in women with pelvic organ prolapse and urinary incontinence. Neurourol Urodyn. 2016;35(1):69–73.
Kass EH. Asymptomatic infections of the urinary tract. J Urol. 2002;167(2):1016–20.
Brubaker L, Wolfe AJ. The new world of the urinary microbiota in women. Am J Obstet Gynecol. 2015;213(5):644–9.
Stamm WE, Counts GW, Running KR, Fihn S, Turck M, Holmes KK. Diagnosis of coliform infection in acutely dysuric women. N Engl J Med. 1982;307(8):463–8.
Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013;369(20):1883–91.
Polin MR, Kawasaki A, Amundsen CL, Weidner AC, Siddiqui NY. Do mixed-flora preoperative urine cultures matter? South Med J. 2017;110(6):426–9.
Pinheiro HS, Mituiassu AM, Carminatti M, Braga AM, Bastos MG. Urinary tract infection caused by extended-spectrum beta-lactamase–producing bacteria in kidney transplant patients. Transplant Proc. 2010;42(2):486–7.
Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329(11):753–6.
Clayson D, Wild D, Doll H, Keating K, Gondek K. Validation of a patient-administered questionnaire to measure the severity and bothersomeness of lower urinary tract symptoms in uncomplicated urinary tract infection (UTI): the UTI symptom assessment questionnaire. BJU Int. Aug. 2005;96(3):350–9.
Nicolle LE, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–54.
Acknowledgements
Kelly Bree, MD (Department of Urology, University of California, San Diego), for assistance with portions of the data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Brubaker receives editorial fees from Female Pelvic Medicine and Reconstructive Surgery, Journal of the American Medical Association, and UpToDate.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jung, C.E., Brubaker, L. Postoperative urinary tract infection after urogynecologic surgery: timing and uropathogens. Int Urogynecol J 31, 1621–1626 (2020). https://doi.org/10.1007/s00192-019-04061-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-019-04061-1