Abstract
Introduction and hypothesis
The beneficial effect of pelvic organ prolapse (POP) surgery on urge urinary incontinence (UI) is well described in the literature, while effect on preoperative stress UI (SUI) is still unclear. The aim of this study was to investigate changes concerning UUI following POP surgery without concomitant anti-incontinence procedures and to identify possible factors influencing the changes.
Methods
We conducted a retrospective study of 678 women with prolapse surgery using native tissue repair during a 3-year period. Patients completed three prolapse questions from the International Consultation on Incontinence–Vaginal Symptoms (ICIQ-VS) questionnaire and the International Consultation on Incontinence Questionnaire–Urinary Incontinence Short Form (ICIQ-UI SF) before undergoing surgery and 3 months postoperatively. Patients who scored >0 on the ICIQ-UI SF before surgery were included in the study.
Results
A total of 379 patients (55.9%) with POP had concomitant UI. At 3 months’ follow-up, 174 patients (46%) became continent compared with 205 patients (54%) with UI. Patients with remaining UI had statistically significant higher mean preoperative ICIQ-UI SF score than patients who became dry. The risk of remaining UI after POP surgery was greater in patients with previous anti-incontinence repair. UI type was not a risk factor for its persistance.
Conclusion
Almost half of the patients with UI before POP surgery became completely dry after prolapse surgery alone. Severity of incontinence and previous anti-incontinence surgery were identified as risk factors for persisting UI after POP surgery. We found a reduction of incontinence after an operation in any of the three compartments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pelvic organ prolapse (POP) is estimated to effect up to 50% of women ≥50 years [1]. Urinary incontinence (UI) is an even more prevalent condition in women [2], and the two conditions often coexist. The relationship is complex and many factors are still unknown [3]. Preoperative concomitant stress UI (SUI) is reported in 40–62.7% of patients with POP [4, 5], while overactive bladder (OAB) symptoms characterized by urgency, frequency, and urge urinary incontinence (UUI) occur in 55–86% of patients with vaginal prolapse [6,7,8,9]. Numerous previous studies report on the beneficial effect of POP repair on symptoms of OAB [4, 6, 10,11,12,13,14,15].
Concomitant anti-incontinence surgery at the time of prolapse repair remains controversial. Some studies have shown a benefit of an anti-incontinence surgery in all women undergoing POP repair [16, 17]. Other studies suggest a one-step procedure (combined prolapse and incontinence surgery) in women with SUI or occult SUI diagnosed preoperatively [16, 18,19,20]. At the same time, a significant proportion of the literature supports a two-step procedure or a delayed approach to avoid unnecessary surgery in a proportion of patients [21,22,23]. The argument is that anti-incontinence surgery could be an unnecessary intervention in almost one third of patients who could be cured after prolapse surgery alone [21].
The main purpose of this study was to investigate changes concerning UI following POP surgery without concomitant anti-incontinence procedures and to identify possible factors influencing the changes.
Methods and materials
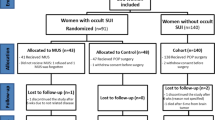
All medical case records and data from the national Danish Urogynecological Database [24] were reviewed for all patients who underwent a prolapse procedure in our department during a 3-year period between January 2012 and January 2015. A total of 678 patients were identified (Fig. 1).
The indication for an operation was a symptomatic genital prolapse and Pelvic Organ Prolapse Quantification (POP-Q) [25] grade ≥2. All patients completed three prolapse questions modified from the International Consultation on Incontinence–Vaginal Symptoms (ICIQ-VS) [26] and the International Consultation on Incontinence–Urinary Incontinence Short Form (ICIQ-UI SF) [27] before surgery and 3 months postoperatively. The three modified questions from the ICIQ-VS used in our evaluation of symptomatic bulge sensation were: 1 Do you feel a lump or bulge come out of your vagina so that you can feel it on the outside or see it on the outside? [never (0), occasionally (1), sometimes (2), most of the time (3), all of the time (4)]. 2 How much does this bother you? [not at all (0), a little (1), some (2), very much (3)]. 3 “How much does this affect your daily life?” [not at all (0), very much (10)] . A total score was calculated by adding scores from each question, with 0 representing an asymptomatic patient and 17 representing a patient with maximum bother.
The ICIQ-UI-SF was used to evaluate the severity of UI and its impact on health-related quality of life (HR-QoL). The form contains three scored items and an unscored self-diagnostic item. A total score for the three items is calculate: 0 indicates a totally continent patient, and a maximum score for worst incontinence is 21. The unscored self-diagnostic item is used to classify the type of incontinence: stress, urge, mixed, or undefined. Undefined UI is described as “leaks when you have finished urinating and are dressed” and “leaks for no obvious reasons”. The Danish version of the ICIQ-UI SF has been translated from English but has not been validated. Patients with UI, defined as a score >0 on the ICIQ-UI SF before surgery were included in this study population, which consisted of 379 incontinent women.
We compared two groups of patients: patients with no UI (N = 174) at 3 months follow-up and patients who remained incontinent after POP surgery (N = 205). Demographic data included age, body mass index (BMI), number of births, previous Cesarean sections, and previous prolapse and incontinence operations. Preoperative evaluation included medical history and physical examination, including POP-Q evaluation. We chose to divide patients into three groups: Group A included all patients with an operation in the anterior compartment with and without surgery in other compartments (anterior colporrhaphy alone or with cervix amputation, vaginal hysterectomy, posterior colporrhaphy, enterocele operation). Group B included all patients with an operation in the middle compartment and no operation in the anterior compartment (cervix amputation, vaginal hysterectomy, vaginal vault suspension alone or with posterior colporrhaphy, enterocele operation). Group C included all patients with an operation only in the posterior compartment (posterior colporrhaphy, enterocele operation).
All patients were operated in our outpatient clinic using native tissue and site-specific repair when indicated. Recurrent cases (N = 107) were in some instances reinforced with a biological graft extracted from porcine small intestinal submucosa (N = 16) (Surgisis). No synthetic meshes were used., and no concomitant incontinence procedures were performed. If a patient was not ready to go home in the evening, she was admitted until the next day. Postoperative clinical follow-up was performed for all patients except those who underwent simple, uncomplicated anterior colporrhaphy surgery, in which case a telephone interview was performed. The primary outcome measure was change in UI on ICIQ-UI SF 3 months postoperatively. Secondarily, we investigated changes in UI according to operations in different compartments.
Statistical analysis
Descriptive statistics were calculated to describe the study population using Excel (Microsoft Office 2013). Categorical data were analyzed using chi-square or Fisher’s exact test if there were five or less patients in one cell. Continuous data not normally distributed were analyzed using a Mann–Whitney, nonpaired, rank-sum test with SPSS (version 24). Confounding variables were compared between patients with no UI and those who were still incontinent at 3 months’ follow-up using parametric or nonparametric statistics, as appropriate. The Ethics Committee, Region Nordjylland, Denmark, approved the study, which was registered at The Health Department, Region Nordjylland (no. 2017–177).
Results
A total of 379 patients (55.9%) were incontinent before surgery and were included in this study. At 3 months’ follow-up, 174 patients (46%) became continent, compared with 205 (54%) with remaining UI. There was no statistical difference between groups concerning age, parity, BMI, previous Cesarean section, hysterectomy, and prolapse surgery (Table 1). A total of 35% of patients who remained incontinent after POP surgery according to the ICIQ-UI SF showed improvement in UI, 6.9% were unchanged, and in 12.1% UI symptoms deteriorated (Fig. 1).
The risk of remaining UI after POP surgery was increased in patients with previous anti-incontinence repair compared with patients without (p = 0.045) (Table 1). UI type was not a risk factor for persisting UI. Of the 379 patients with complaints of UI before surgery, 105 patients (28%) reported UUI, 91 (24%) SUI, 162 (43%) mixed, and 21 (5%) undefined UI. A total of 50% of women with pre-existing UUI and 53% with pre-existing SUI were subjectively cured by POP surgery alone (Fig. 2). Of patients with MUI and undefined UI, 35% and 76%. respectively, were cured (Fig. 2).
When assessing POP compartment treated, patients with operations in the apical compartment were significantly more continent than patients with POP surgery in the anterior or posterior compartment (Fig. 3). After excluding patients with operations in several compartments, however, there was no significant difference between groups (Fig. 4). UI resolved in 81 (44%) of the 183 patients who had anterior repair only and in 59 (43%) with posterior repair only (Fig. 4).
In terms of UI severity, we found a statistically significant difference in preoperative ICIQ-UI SF scores in patients who became continent compared with patients who remained incontinent. Mean ICIQ-UI SF score before surgery among patients who became continent at 3 months’ follow-up was 9.3. Patients with persisting UI at 3 months’ follow-up had a mean ICIQ-UI SF score of 11.2 before surgery, which declined to 8.3 postoperatively.
Degree of bother was not found to be associated with persisting UI. Comparing pre- and postoperative data on the ICIQ-VS score showed improvement in both groups. Patients with no UI after POP surgery had a preoperative mean score of 13.7, declining to 0.46 at 3 months’ follow-up, while patients with persisting UI after prolapse surgery had preoperative mean ICIQ-VS score of 12.97, declining to 1.49 at 3 months’ follow-up.
Discussion
This study found that 379 (55.9%) of 678 patients with POP had concomitant UI. A total of 46% of patients with preoperative UI became completely dry after POP surgery alone, and more women experienced improvement in UI (35%) than worsening (12.1%). This is in accordance with Lensen et al.’s findings, which showed that 44.7% of patients had no complaints of any UI after POP surgery alone. Their study also showed an improvement in UI in 34% of women and a deterioration in 18% after POP repair [4].
In our study, 53% of women with preoperative SUI were subjectively cured by POP surgery alone. The same trend in cure rate was reported in other studies [4, 7, 21]. Digesu et al. stated that it was surprising to find that SUI improved postoperatively despite no continence procedure being performed. Up to 50% of women did not report any SUI 1 year postoperatively [7]. In a study by Borstad et al. [21], 27% of women were cured after prolapse repair alone at 3 months’ follow-up, and after 1 year, 25% of women remained cured of SUI without incontinence surgery. Lensen et al. [4] demonstrated that 39% of women with pre-existing SUI became dry postoperatively. Therefore, they considered it justified not to perform concomitant anti-incontinence surgery and await effects of POP surgery alone. Our study strongly contributes to this consideration.
Previous anti-incontinence surgery was significantly associated with remaining UI after POP surgery in our study. It is difficult to compare our findings with other studies because most studies exclude patients with previous anti-incontinence surgery or did not specifically investigate this factor [4, 6,7,8, 14]. Theoretically, either some patients with a previous anti-incontinence procedure already had support under the urethra and could not be further improved by a prolapse operation, or previously operated patients experienced UI after the incontinence operation that was unchanged by the prolapse operation.
In agreement with other studies [4, 7,8,9,10, 12], we found a beneficial effect of POP repair on UUI. Half of the patients with preoperative UUI were cured by POP surgery alone. OAB symptoms that resolve with prolapse reduction may be derived from myogenic causes that result from outlet obstruction. Smooth-muscle changes induced by relative outlet obstruction lead to increased smooth-muscle excitability and increased ability of activity spread between cells, which promotes uninhibited detrusor contractions [28]. Whether a prolapse is a true causative factor in OAB symptoms remains to be proven [8].
We found that patients with operations in the apical compartment were significantly more continent than patients with POP surgery in anterior or posterior compartments at 3 months’ follow-up. We speculated that a concomitant repair of several compartments could be a source of bias. After excluding patients with operations in several compartments, we found no significant difference between groups and operations in the different compartments. Fletcher et al. [8] investigated persistence of OAB symptoms after anterior vaginal repair and found that 49% had improved UUI after operation. A limitation of their study was that patients had a concomitant repair in apical and posterior compartments. Boer et al. [11] showed that UI symptoms decreased more following anterior compartment operation compared with other compartments. Again, the limitation of their finding was that the vast majority of women underwent surgery in more than one compartment. There is no good evidence of a correlation between compartment involved and presence of OAB symptoms [10]. It is however, somewhat difficult to study this kind of correlation, because often, more than one compartment is involved.
Patients in our study with remaining UI at 3 months’ follow-up had statistically significant higher mean preoperative ICIQ-UI SF scores compared with patients who became dry (11.2 vs 9.3), which suggests that patients with more severe incontinence are less likely to become continent after a prolapse operation. The incidence of subjective de novo UI in our total group of 678 patients was 11% at 3 months’ follow-up, declining to 7% at long-term follow-up. These data have been previously reported and are not within the scope of this paper [29]. Although different techniques to reduce prolapse and identify occult incontinence preoperatively have been described, a gold standard has not been established. Neither the speculum nor the pessary test to reduce the prolapse have acceptable positive predictive values to identify women with occult UI [30]. Until a valid test has been found, we will not investigate for occult SUI in our clinic.
The strength of our study is that the same questionnaire was used both pre- and postoperatively, giving more exact information about changes in UI, no patients were lost to follow-up. Our study is limited somewhat by its retrospective design, but all data were entered into the database when events took place. Another weakness is that a multivariate logistic regression analysis was not performed.
Evidence from this study suggests that >50% of women with subjective complaints of SUI are cured with POP surgery alone. Therefore, the two-step procedure is an appropriate option for managing vaginal prolapse and SUI. A randomized trial by Ploeg et al. [19] comparing transvaginal prolapse repair combined with midurethral sling (MUS) versus prolapse repair alone showed that only 17% of women undergoing POP surgery needed an MUS. This also supports our statement.
In conclusion, almost half of the patients with UI before POP surgery became completely dry after prolapse surgery alone. Previous anti-incontinence surgery was identified as a risk factor for remaining UI after POP surgery. We found reduced incontinence after operation in any of the three compartments.
References
Samuelsson EC, Victor FT, Tibblin G, Svärdsudd KF. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol. 1999;180(2 Pt 1):299–305.
Almousa S, Bandin van Loon A. The prevalence of urinary incontinence in nulliparous adolescent and middle-aged women and the associated risk factors: a systematic review. Maturitas. 2018;107:78–83.
Bump RC, Fantl JA, Hurt WG. The mechanism of urinary continence in women with severe uterovaginal prolapse: results of barrier studies. Obstet Gynecol. 1988;72(3 Pt 1):291–5.
Lensen EJ, Withagen MI, Kluivers KB, Milani AL, Vierhout ME. Urinary incontinence after surgery for pelvic organ prolapse. Neurourol Urodyn. 2013;32(5):455–9.
Bai SW, Jeon MJ, Kim JY, Chung KA, Kim SK, Park KH. Relationship between stress urinary incontinence and pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13(4):256–60.
Ramanah R, Ballester M, Chereau E, Rouzier R, Daraï E. Effects of pelvic organ prolapse repair on urinary symptoms: a comparative study between the laparoscopic and vaginal approach. Neurourol Urodyn. 2012;31(1):126–31.
Digesu GA, Salvatore S, Chaliha C, et al. Do overactive bladder symptoms improve after repair of anterior vaginal wall prolapse? Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1439–43.
Fletcher SG, Haverkorn RM, Yan J, et al. Demographic and urodynamic factors associated with persistent OAB after anterior compartment prolapse repair. Neurourol Urodyn. 2010;29:1414–8.
Foster RTS, Barber MD, Parasio MF, et al. A prospective assessment of overactive bladder symptoms in a cohort of elderly women who underwent transvaginal surgery for advanced pelvic organ prolapse. Am J Obstet Gynecol. 2007;197(82):e1–4.
de Boer TA, Salvatore S, Cardozo L, et al. Pelvic organ prolapse and overactive badder. Neurourol Urodyn. 2010;29:30–9.
de Boer TA, Kluivers KB, Withagen MI, Milani AL, Vierhout ME. Predictive factors for overactive bladder symptoms after pelvic organ prolapse surgery. Int Urogynecol J. 2010;21(9):1143–9.
Nguyen JK, Bahatia NN. Resolution of motor urge incontinence after surgical repair of pelvic organ prolapse. J Urol. 2001;166:2263–6.
Miranne JM, Lopes V, Carberry CL, Sung VW. The effect of pelvic organ prolapse severity on improvement in overactive bladder symptoms after pelvic reconstructive surgery. Int Urogynecol J. 2013;24(8):1303–8.
Kim MS, Lee GH, Na ED, Jang JH, Kim HC. The association of pelvic organ prolapse severity and improvement in overactive bladder symptoms after surgery for pelvic organ prolapse. Obstet Gynecol Sci. 2016;59(3):214–9.
Costantini E, Lazzeri M, Zucchi A, Mearini L, Fragalà E, Del Zingaro M, et al. Urgency, detrusor overactivity and posterior vault prolapse in women who underwent pelvic organ prolapse repair. Urol Int. 2013;90(2):168–73.
Wei JT, Nygaard I, Richter HE, Nager CW, Barber MD, Kenton K, et al. Pelvic floor disorders network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366(25):2358–67.
Brubaker L, Cundiff GW, Fine P, Nygaard I, Richter HE, Visco AG, et al. Pelvic floor disorders network. Abdominal sacrocolpopexy with Burch colposuspension to reduce urinary stress incontinence. N Engl J Med. 2006;354(15):1557–66.
Liapis A, Bakas P, Georgantopoulou C, Creatsas G. The use of the pessary test in preoperative assessment of women with severe genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2011;155(1):110–3.
van der Ploeg JM, van der Steen A, Oude Rengerink K, van der Vaart CH, Roovers JP. Prolapse surgery with or without stress incontinence surgery for pelvic organ prolapse: a systematic review and meta-analysis of randomized trials. BJOG. 2014;121(5):537–47.
Baessler K, Maher C. Pelvic organ prolapse surgery and bladder function. Int Urogynecol J. 2013;24(11):1843–52.
Borstad E, Abdelnoor M, Staff AC, Kulseng-Hanssen S. Surgical strategies for women with pelvic organ prolapse and urinary stress incontinence. Int Urogynecol J. 2010;21(2):179–86.
Ennemoser S, Schönfeld M, von Bodungen V, Dian D, Friese K, Jundt K. Clinical relevance of occult stress urinary incontinence (OSUI) following vaginal prolapse surgery: long-term follow-up. Int Urogynecol J. 2012;23(7):851–855.
Jundt K, Wagner S, von Bodungen V, Friese K, Peschers UM. Occult incontinence in women with pelvic organ prolapse - does it matter? Eur J Med Res. 2010;15(3):112.
Guldberg R, Brostrøm S, Hansen JK, Kærlev L, Gradel KO, Nørgård BM, et al. The Danish Urogynecological database: establishment, completeness and validity. Int Urogynecol J. 2013;24:983–90.
Haylen BT, Maher CF, Barber MD, Camargo S, Dandolu V, Digesu A, et al. An international Urogynecological association (IUGA)/international continence society (ICS) joint report on the terminology for female pelvic organ prolapse (POP). Int Urogynecol J. 2016;27(4):655–84.
Price N, Jackson SR, Avery K, Brookes ST, Abrams P. Development and psychometric evaluation of the ICIQ vaginal symptoms questionnaire: the ICIQ-VS. BJOG. 2006;113(6):700–12.
Abrams P, Avery K, Gardener N, Donovan J. ICIQ advisory board. The international consultation on incontinence modular questionnaire: www.iciq.net. J Urol. 2006;175:1063–6.
Brading AF. A myogenic basis for the overactive bladder. Urology. 1997;50(6A Suppl):57–67. discussion 68-73
Ugianskiene A, Kjærgaard N, Inger Lindquist AS, Larsen T, Glavind K. Retrospective study on de novo postoperative urinary incontinence after pelvic organ prolapse surgery. Eur J Obstet Gynecol Reprod Biol. 2017;219:10–4.
King AB, Goldman HB. Stress incontinence surgery at the time of prolapse surgery: mandatory or forbidden? World J Urol. 2015;33:1257–62.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Ugianskiene, A., Kjærgaard, N., Larsen, T. et al. What happens to urinary incontinence after pelvic organ prolapse surgery?. Int Urogynecol J 30, 1147–1152 (2019). https://doi.org/10.1007/s00192-018-3677-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-018-3677-4