Abstract
Introduction
Rectocele is a common condition, which on imaging is defined by a pocket identified on Valsalva or defecation. Cut-offs of 10 and 20 mm for pocket depth have been described. This study analyses the correlation between rectocele depth and symptoms of bowel dysfunction to define a cut-off for the diagnosis of “significant rectocele” on ultrasound.
Methods
A retrospective study using 564 archived data sets of patients seen at tertiary urogynaecological clinics. Patients underwent a standardised interview including a set of questions regarding bowel function, and translabial 3D/4D ultrasound. Assessments were undertaken supine and after voiding. Rectocele depth was measured on Valsalva.
Results
Out of 564, data on symptoms was missing in 18 and ultrasound volumes in 25, leaving 521. Mean age was 56 years (range 18–86), mean BMI 29 (17–56). Presenting symptoms were prolapse (51 %), constipation (21 %), vaginal digitation (17 %), straining at stool (46 %), incomplete bowel emptying (41 %) and faecal incontinence (10 %). A clinically significant rectocele (ICS POPQ stage ≥2) was found in 48 % (n=250). In 261 women a rectal diverticulum was identified, of an average depth of 17 (SD, 7) mm. On ROC statistics a cut- off of 15 mm in depth provided optimal sensitivities of 66 % for vaginal digitation and 63 % for incomplete emptying, and specificities of 52 and 57 % respectively.
Conclusions
Rectocele depth is associated with symptoms of obstructed defecation. A “clinically significant” rectocele may be defined as a diverticulum of the rectal ampulla of ≥15 mm in depth, although poor test characteristics limit clinical utility of this cut-off.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rectocele, i.e. the formation of a diverticulum through a defect of the rectovaginal septum causing a herniation of the rectal muscularis, mucosa and contents into the vagina, is frequently diagnosed in patients symptomatic for pelvic floor disorders [1, 2]. It is likely to be the most common anatomical cause of symptoms of obstructed defecation such as straining at stool, the sensation of incomplete bowel emptying and vaginal digitation [3], but it also occurs in asymptomatic volunteers [4, 5]. While gynaecologists use the terms “rectocele” and “posterior compartment prolapse” synonymously, this is clearly inappropriate since posterior compartment prolapse may be due to a number of different conditions, not just true (“radiological”) rectocele, but also enterocele, intussusception and perineal hypermobility [6]. The diagnosis of rectocele has to date been hampered by the lack of a simple, cheap investigative method, but recently, translabial 3D/4D ultrasound has been shown to be a valid and repeatable method for assessing anatomical abnormalities of the posterior vaginal compartment in women with pelvic floor dysfunction, whether with or without the use of rectal contrast medium [7, 8]. This methodology seems to correlate moderately with findings on defecation proctography and is cheaper and much better tolerated by patients [9, 10]. While it has been shown in the past that the depth of a rectocele as measured on translabial ultrasound is associated with symptoms of obstructed defecation [11], the cut-offs used for the diagnosis of rectocele (10 mm or 20 mm) are entirely arbitrary. In this study we attempted to define an objective cut-off for the diagnosis “clinically relevant true rectocele” as diagnosed on translabial ultrasound, using symptoms of obstructed defecation as an outcome measure and maximal depth of a diverticulum of the rectal ampulla during a voluntary Valsalva manoeuvre without an attempt to defecate, as imaged in the mid-sagittal plane.
Materials and methods
This is a retrospective study using 564 archived data sets of consecutive patients seen at a tertiary urogynaecological clinic between July 2009 and August 2011. They attended for symptoms of lower urinary tract and/ or pelvic floor dysfunction, including symptoms of pelvic organ prolapse and defecatory dysfunction. Patients underwent an in-house, non-validated standardised interview, which included a set of questions regarding bowel function, a clinical examination using the prolapse quantification system of the International Continence Society (ICS POP-Q) [12] and translabial 3D/4D ultrasound [13]. Assessments were undertaken under the supervision of the senior author (HPD), supine and after voiding, with a cine-loop of ultrasound volume data obtained during the best (that is, the most effective in showing organ descent or rectocele development) of at least three Valsalva manoeuvres archived for later analysis. We did not stipulate any bowel preparation, although patients were encouraged to defecate before the assessment if they felt so inclined. No rectal contrast medium was used. Offline analysis for rectocele was undertaken at a later date by the second author (ZX) after 1 week’s training and achieving acceptable repeatability (see below), using the software GE Kretz 4DView v.10.0 on a desktop PC, to allow blinding against all clinical data. Maximal caudad displacement of the rectal ampulla was determined on maximal Valsalva [14]. Maximal rectocele depth was determined as previously described [15] by assessing the entire Valsalva manoeuvre and selecting the volume showing the rectocele pocket at its deepest, as descending stool may at times flatten the diverticulum at maximal Valsalva. The sonographic assessment of posterior compartment abnormalities seems valid compared with defecation proctography [9], and repeatable even when re-tested after an interval of months [16]. Postprocessing analysis of rectal descent and rectocele depth appears to be sufficiently repeatable for research use [17].
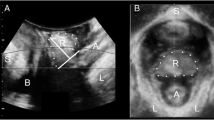
Figure 1a and b show the measurement of rectal descent relative to the inferoposterior margin of the symphysis, while Fig. 1c and d illustrate rectocele depth measurement, both in the midsagittal plane.
Translabial ultrasound images in the midsagittal plane. a and c are images at rest, b and d are obtained on maximal Valsalva. b shows rectocele descent against a reference line placed through the inferoposterior symphyseal margin; d shows rectocele depth measured against a reference line placed through the ventral aspect of the internal anal sphincter. S symphysis pubis, B bladder, V vagina, R rectal ampulla, A anal canal
Intraclass correlations (single measurement, absolute agreement definition) were used to test the repeatability of offline measurements performed by ZX. Power calculations were not performed owing to the retrospective nature of this research and the lack of pilot data. Rectocele depth was then analysed against symptoms of prolapse, faecal incontinence, chronic constipation and symptoms of obstructed defecation such as vaginal digitation, the sensation of incomplete bowel emptying and straining at stool, using Wilcoxon’s U tests. The symptoms most predictive of a sonographic diagnosis of rectocele were then used for receiver operating characteristics analysis to define a cut-off for the diagnosis of “clinically significant rectocele” as diagnosed by translabial ultrasound. Potential confounders such as age, BMI, parity, previous hysterectomy and incontinence or prolapse surgery were tested by univariate analysis; it was planned that those with a significant or near-significant association with symptoms of obstructed defecation would be tested in a multivariate logistic regression. Approval for this retrospective study had been obtained from the local Human Research Ethics Committee (ref. NBMLHD HREC 12–15). Statistical analysis was undertaken using the software Minitab version 13 (Minitab, State College, PA, USA) and SAS V9.2 (SAS institute, Cary, NC, USA).
Results
During the inclusion period, 564 women attended a tertiary urogynaecological clinic for the assessment of symptoms of the lower urinary tract and pelvic floor dysfunction. Out of 564, data on the symptoms of posterior compartment prolapse was missing in 18 patients, and in another 25 ultrasound volumes were either missing or technically unsatisfactory, leaving 521.
The following analysis pertains to these 521 women. The mean age was 56 years (range 18–86), mean BMI was 29 (17–56) kg/m2. Median parity was 2 (0–10), and 92 % (n = 477) were vaginally parous. Twenty-eight percent of our patients (n = 147) had had a hysterectomy, and 26 % (n = 136) reported prior prolapse or incontinence procedures.
The main symptoms relating to posterior compartment abnormalities were those of prolapse (vaginal lump or bulge or a dragging sensation, n = 266, 51 %), chronic constipation (n = 107, 21 %), vaginal digitation (n = 86, 17 %), straining at stool (n = 239, 46 %), the sensation of incomplete bowel emptying (n = 215, 41 %) and faecal incontinence (n = 54, 10 %).
On clinical examination, using the ICS POP-Q system, we found a significant cystocele (POPQ stage ≥2) in 55 % (n = 284), significant uterine prolapse in 9 % (n = 33), significant enterocele in 4 % (n = 22) and a significant rectocele (Ba >≥1) in 48 % (n = 250).
Acceptable repeatability of offline measurements of rectocele descent and depth (ICC > = 0.70) were obtained within 5 days of training. On postprocessing of ultrasound volume data sets obtained on Valsalva manoeuvre, a rectal diverticulum was identified in 261 women (50 %), of an average depth of 17 (SD 7) mm.
Symptoms including prolapse (sensation of a lump or dragging), constipation, vaginal digitation, straining at stool, incomplete bowel emptying and faecal incontinence were tested against rectal position and rectocele depth on univariate analysis (Table 1). It is evident that there were substantial associations between symptoms of chronic constipation and obstructed defecation with rectocele depth (P = 0.03 to P = 0.001), with vaginal digitation and the sensation of incomplete bowel emptying the most significant. Prolapse symptoms were associated only with rectal descent, while symptoms of faecal incontinence showed no association with ultrasound measures.
On testing multiple confounders such as age, BMI, parity, previous hysterectomy and incontinence or prolapse surgery as potential predictors of symptoms of obstructed defecation, we found not a single significant association between these potential predictors and symptoms. Hence, the originally planned multivariate modelling was not undertaken.
We used ROC statistics to determine the best cut-off for clinically significant (that is, likely to be symptomatic) rectocele. The cut-off depth of 15 mm provided optimal sensitivities of 66 % for vaginal digitation and 63 % for incomplete emptying, and specificities of 52 and 57 % respectively. AUCs are 0.61 and 0.614 (Fig. 2).
Receiver operating characteristic (ROC) curves for the association between a vaginal digitation and b incomplete emptying on the one hand and rectocele depth on the other hand (n=261). The illustrated cut-off depth of 15 mm provides optimal sensitivities of 66 % for vaginal digitation and 63 % for incomplete emptying, and specificities of 52 and 57 % respectively. Area under the curve (AUC) values are a 0.61 and b 0.614
Discussion
In this large retrospective study on over 500 women with imaging assessment of the posterior pelvic floor compartment we were able to confirm the previously demonstrated association between symptoms of constipation and obstructed defecation on the one hand, and true rectocele, i.e. a diverticulum of the rectal ampulla, on the other hand [11]. While such symptoms are clearly multifactorial and commonly occur in women who have demonstrably normal anorectal anatomy [15], and while rectoceles are also found in young nulliparous women [18] and asymptomatic volunteers [4], there is a significant association between this particular anatomical abnormality and symptoms of obstructed defecation. Patients intuitively understand an anatomical explanation and in fact sometimes report that they have identified a “pocket” or diverticulum of the rectal ampulla themselves, as well as the obvious means of emptying out this pocket, i.e. vaginal digitation.
While the anatomical abnormality is likely to be due to a defect in the rectovaginal septum [19], and while repair of such a defect is feasible and highly successful [20], there is no consensus on diagnosis and treatment of this simple condition. Progress is hampered by the fact that clinical diagnosis is not commonly taught, not the least because this requires a rectal examination [21]. The identification of a posterior compartment prolapse is not sufficient for this purpose as such may be due to a number of different conditions [12]. Hence, imaging confirmation of a “true rectocele” seems essential for appropriate treatment, and translabial ultrasound is the method of choice, given the inconvenience and expense associated with competing modalities such as magnetic resonance and defecation proctography [10]. Endoanal ultrasound has also been proposed as a diagnostic modality [22], but it is difficult to see how the inherent invasiveness of the method, let alone its inability to monitor organ descent, could make it a viable proposition for clinical practice.
However, as rectocele is such a common diagnosis even in asymptomatic women, it is not surprising that successful obliteration of a “true rectocele” confirmed by imaging does not always result in symptom relief [20]. It therefore seems important to define cut-offs for the diagnosis of “significant” rectocele, i.e. of a rectocele that is likely to cause symptoms, symptoms that could be expected to disappear after successful obliteration of the pocket or diverticulum. The results of this study suggest that a cut-off of 15 mm may be optimal for the diagnosis of “significant rectocele”.
A number of limitations of this study need to be acknowledged. It is a retrospective study in women seen for a variety of symptoms, as to be expected in a urogynaecological clinic. Our findings may not fully apply to other populations, such as in colorectal services. Furthermore, our patients were almost exclusively of Caucasian background, which limits the utility of our conclusions to this ethnic group. In addition, it may have been preferable to obtain quality of life measures focussed on anorectal dysfunction, in the form of dedicated, validated questionnaires [23] or in the form of visual analogue scale data on symptom bother [24]. We are unable to provide such data as we do not use colorectal questionnaires in clinical practice. It is possible that more complex measures of obstructed defecation and its bother would provide stronger associations with anatomical findings, enhancing the validity of ROC statistics and resulting recommendations for cut-offs. Finally, it may well be argued that the ROC statistics reported in this study are too weak to provide reliable cut-offs for clinical practice. The authors are tempted to concur with this view; however, there is an obvious need for such a cut-off to define “significant rectocele” because of implications for surgical management, and we are not aware of any data in the world literature that would provide better information on this issue. Because of sample size requirements it seems unlikely that such data may be provided by other imaging modalities in the foreseeable future.
Conclusion
This large retrospective study has again confirmed that sonographically determined rectocele depth is associated with most of the symptoms of obstructed defecation. The best cut-off for rectocele depth as a predictor of symptoms of obstructed defecation seems to be 15 mm, although the poor performance of rectocele depth in the prediction of symptoms of obstructed defecation limits the utility of such a cut-off.
References
Zbar A, Lienemann A, Fritsch H et al (2003) Rectocele: pathogenesis and surgical management. Int J Colorectal Dis 18:369–384
Kahn MA, Stanton SL (1997) Posterior vaginal wall prolapse and its management. Contemp Rev Obstet Gynecol 9:303–310
Andromanakos N, Skandalakis P, Troupis T, Filippou D (2006) Constipation of anorectal outlet obstruction: pathophysiology, evaluation and management. J Gastroenterol Hepatol 21:638–646
Kenton K, Shott S, Brubaker L (1999) The anatomic and functional variability of rectoceles in women. Int Urogynecol J Pelvic Floor Dysfunct 10(2):96–99
Burrows LJ, Meyn LA, Walters MD, Weber AM (2004) Pelvic symptoms in women with pelvic organ prolapse. Obstet Gynecol 104(5 Pt 1):982–988
Dietz HP (2014) How I do it: Translabial ultrasound in the investigation of posterior compartment vaginal prolapse and defecatory disorders. Tech Coloproctol 18(5):481–494
Beer-Gabel M, Assoulin Y, Amitai M, Bardan E (2008) A comparison of dynamic transperineal ultrasound (DTP-US) with dynamic evacuation proctography (DEP) in the diagnosis of cul de sac hernia (enterocele) in patients with evacuatory dysfunction. Int J Colorectal Dis 23:513–519
Dietz HP, Beer-Gabel M (2012) Ultrasound in the investigation of posterior compartment vaginal prolapse and obstructed defecation. Ultrasound Obstet Gynecol 40:14–27
Steensma AB, Oom DM, Burger CW, Schouten WR (2010) Assessment of posterior compartment prolapse: a comparison of evacuation proctography and 3D transperineal ultrasound. Colorectal Dis 12(6):533–539
Perniola G, Shek C, Chong CC, Chew S, Cartmill J, Dietz HP (2008) Defecation proctography and translabial ultrasound in the investigation of defecatory disorders. Ultrasound Obstet Gynecol 31:567–571
Dietz HP, Korda A (2005) Which bowel symptoms are most strongly associated with a true rectocele? Aust NZ J Obstet Gynaecol 45:505–508
Bump RC, Mattiasson A, Bø K et al (1996) The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 175(1):10–17
Dietz HP (2004) Ultrasound imaging of the pelvic floor: 3D aspects. Ultrasound Obstet Gynecol 23(6):615–625
Dietz HP, Haylen BT, Broome J (2001) Ultrasound in the quantification of female pelvic organ prolapse. Ultrasound Obstet Gynecol 18(5):511–514
Dietz HP, Steensma AB (2005) Posterior compartment prolapse on two- dimensional and three- dimensional pelvic floor ultrasound: the distinction between true rectocele, perineal hypermobility and enterocele. Ultrasound Obstet Gynecol 26:73–77
Tan L, Guzman Rojas R, Dietz H (2014) The repeatability of sonographic measures of functional pelvic floor anatomy. Neurourol Urodyn 33:1058–60
Dietz HP, Guzman Rojas R, Shek KL (2014) Postprocessing of pelvic floor ultrasound data: how repeatable is it? Aust NZ J Obstet Gynaecol 54(6):553–557
Dietz H, Clarke B (2005) The prevalence of rectocele in young nulliparous women. Aust NZ J Obstet Gynaecol 45:391–394
Richardson AC (1993) The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol 36(4):976–983
Guzman Rojas R, Shek KL, Kamisan Atan I, Dietz HP (2013) Defect-specific rectocele repair: medium-term subjective and objective outcomes. Neurourol Urodyn 32(6):868–870
Rachaneni S, Kamisan Atan I, Guzman Rojas RA, Dietz H (2014) Can defects of the rectovaginal septum be palpated? Abstract, Annual Scientific Meeting of the International Urogynecological Association, Washington
Santoro G, Wieczorek AP, Dietz HP et al (2011) State of the art: an integrated approach to pelvic floor ultrasonography. Ultrasound Obstet Gynecol 37:381–396
Barber M, Walters M, Bump R (2005) Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol 193:103–113
Alam P, Kamisan Atan I, Guzman Rojas RA, Mann K, Dietz HP (2014) The ‘bother’ of obstructed defecation. Int Urogynecol J 25(S1):S114–S115
Conflict of interest
H.P. Dietz and K.L. Shek have received unrestricted educational grants from GE Medical.
Contributions
H.P. Dietz: project development, manuscript writing; X. Zhang: data entry, analysis; K.L. Shek: analysis, manuscript editing; R. Guzman Rojas: data entry, analysis, manuscript editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dietz, H.P., Zhang, X., Shek, K.L. et al. How large does a rectocele have to be to cause symptoms? A 3D/4D ultrasound study. Int Urogynecol J 26, 1355–1359 (2015). https://doi.org/10.1007/s00192-015-2709-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-015-2709-6