Abstract
Introduction and hypothesis
We aimed to evaluate the association between obstructive defecatory symptoms in women with levator ani deficiency (LAD), worsened minimum levator hiatus measurements, widened anorectal angle (ARA), and increased levator-plate descent angle (LPDA).
Methods
Using a cross-sectional study design, patients who had undergone 3D endovaginal ultrasound (3D EVUS) imaging of the pelvic floor were sampled and categorized into two groups: those with and those without obstructive defecatory symptoms (ODS) based on their Colorectal and Anal Distress Index (CRADI-8) questionnaire. The levator ani (LA) muscle was scored based on severity of defect. ARA and LPDA were measured and dichotomized (ARA ± 170°; LPDA ± 9°.
Results
One hundred patients were analyzed: 52 asymptomatic and 48 with ODS. The mean (standard deviation ) age was 59 years (SD ±14.97). There was no difference in the distribution of LAD severity between groups (p = 0.1438) or mean minimal levator hiatus (MLH) (p = 0.3326). ARA and LPDA were significantly different in those with ODS compared with their asymptomatic counterparts (p < 0.0001 and 0.0004, respectively) (Table 1). On multivariable logistic regression, ARA and LPDA were included in the final model. Patients with an ARA >170° had seven times the odds of ODS than those with ARA ≤170° [odds ratio (OR) = 7.01, 95 % confidence interval (CI) 2.30–21.35; p = 0.0006). Patients with an LPDA <9° had 3 times the odds of ODS than those with an LPDA ≥9° (OR = 3.30, 95 % CI 1.22, 8.96, p = 0.0190).
Conclusions
This study demonstrates that increased levator plate descent and widened ARA as measured on 3D endovaginal ultrasound imaging are associated with ODS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
While often used synonymously with constipation, the term obstructive defecation refers to a constellation of symptoms that can include straining with bowel movements, need for splinting to initiate and/or complete defecation, and incomplete bowel emptying. Like many other pelvic floor disorders (PFDs), it is seen three times more frequently in women than in men [1, 2] and is not an uncommon complaint among women presenting to primary care, gastroenterology, and gynecology offices. In spite of reported prevalence rates as high as 9 % [3] and their frequent association as both a cause and effect of other pelvic disorders, such as pelvic organ prolapse (POP) and fecal incontinence (FI) [2, 4, 5], options available for the evaluation and treatment of these symptoms remains limited.
Anatomic abnormalities and functional disorders have both been recognized as causes of obstructive defecatory symptoms; a number of studies have been undertaken to evaluate possible associations between abnormalities of the pelvic floor and obstructive defecation. Levator ani avulsion has been thought to be a risk factor for ODS; however, some magnetic resonance imaging (MRI) studies have demonstrated that women with obstructive symptoms often have intact levator ani muscles [5, 6]. Static and dynamic transperineal US imaging in both men [7] and women have suggested perineal descent, intussusception, and rectocele as important etiologic factors in the development of these symptoms [8, 9].
Transperineal US imaging and the newer 3D endoluminal US techniques are highly reliable for evaluating pelvic floor structures but have not been extensively used to evaluate the pelvic floor of women with obstructed defecation. In this study, we aimed to evaluate the association between ODS in women with minimum levator hiatus measurements, anorectal angle (ARA), levator-plate descent angle, and severity of levator ani deficiency (LAD).
Methods
The study was approved by our Institutional Review Board. This was a retrospective analysis of patients with or without ODS as identified by chart review and who had undergone 3D endovaginal US (EVUS) imaging as part of the evaluation for PFDs between January 2010 and November 2012. Charts were reviewed for relevant demographic and medical information, including age, race, body mass index (BMI), parity, menopausal status, smoking history, and history of anal sphincter injury. All patients had documented physical examinations, which included a POP Quantification (POP-Q) examination and had completed the Pelvic Floor Distress Inventory (PFDI-20) on their initial visit [10]. PFDI-20 is a validated questionnaire that measures the degree of bother from PFDs and is a compilation of three independently validated questionnaires: the Urinary Distress Index (UDI-6), POP Distress Index (POPDI), and the Colorectal and Anal Distress Index (CRADI-8). Questions 7–14 are related to symptoms associated with bowel dysfunction and the degree of bother caused by each symptom. Patients with ODS were those who responded in the affirmative to questions 4 and 8 on the PFDI, corresponding to symptoms of obstructive defecation and/or who admitted to using at least one method of splinting to aid with defecation. Based on responses to questions 4 and 8 of the CRADI-8 portion of the PFDI-20, we grouped participants into two groups: those with ODS, and asymptomatic patients (comparison group). Patients in the comparison group were chosen from the database in a random fashion.
All ODS patients who underwent 3D EVUS with good-quality US volumes (ability to visualize pubic bone, levator plate, and superficial transverse perinei muscle on EVUS and the internal and external anal sphincters on EAUS) during the defined time period were included in this study. We excluded patients with a history of sphincteroplasty, pelvic floor irradiation, rectovaginal fistula, and neurologic diseases (including those affecting the central nervous system, such as dementia and multiple sclerosis, as well as spinal cord diseases). The BK Ultraview 8838 probe (Peabody, MA, USA) was used to acquire 3D volumes.
Ultrasound protocols
All USs were performed using the existing protocol at our institution. For each US, the patient was placed in the dorsal lithotomy position, with the hips flexed and abducted. No preparation was required, and the patient was recommended to have a comfortable volume of urine in the bladder. No rectal or vaginal contrast was used. To avoid excessive pressure on surrounding structures that might distort the anatomy, it was important to keep the probe inserted in the vagina in a neutral position. All measurements were taken from images obtained with the patient at rest and in the dorsal lithotomy position. The 3D cube for each compartment was then digitally cataloged for future analysis.
EVUS volume data sets were analyzed in a blinded fashion by an experienced investigator (GR). We previously reported on the excellent interobserver reliability of the 3D EVUS measurements performed in this study [11–13]. The levator ani muscle was divided into three subgroups based on our prior work [14]: puboanalis (PA); puborectalis (PR), and pubovisceralis (PV) (comprised of pubococcygeus and iliococcygeus). Subgroups were evaluated and scored (0 no defect, 1 minimal defect with ≤ 50 % muscle loss, 2 major defect with >50 % muscle loss, 3 total absence of the muscle) on each side based on thickness and detachment from the pubic bone. Each muscle-pair score ranged from zero (no defect), to six (total absence of muscle). For the entire levator ani muscle group, a cumulative score that ranged between 0 and 18 was possible (Fig. 1). Scores were categorized as 0–6 mild, 7–12 moderate, and >13 severe LAD.
Three-dimensional (3D) endovaginal ultrasound (EVUS) image of normal levator ani muscle. Axial image at the level of minimal levator hiatus, each muscle group earns a score of zero on each side. OI obturator internus, PA puboanalis, PR puborectalis, PS pubic symphysis, PV pubovisceralis, R rectum, U urethra, V vagina
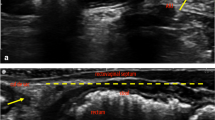
The ARA was measured in the midsagittal plane of the 3D EVUS volume as the angle between anal canal and rectum (Fig. 2). A widened ARA as visualized by EVUS has been defined as ARA ≥170° based on reported literature and our prior study on normative values for ARA in a group of nulliparous women of different ages. The levator-plate descent angle (LPDA) was also measured in the midsagittal plane and measurements dichotomized as <9° (greater levator plate descent) or ≥9° (less levator plate descent) based on our previous study reporting the normative values for LPDA [15]
a The right midsagittal view of the levator-plate descent angle (LPDA) in a healthy patient without levator ani deficiency (LAD). The levator plate position relative to the perineum is shown. The green line signifies the anorectal angle. LPDA relative to the reference line (PLURAL) is 15°. b Right midsagittal view of the levator plate and LPDA in a healthy woman
(Fig. 2).
Statistical methods
Summary statistics were calculated for the patient population. Continuous variables were compared using Student’s t tests; categorical variables were compared using chi-square or Fisher’s exact tests. Univariate logistic regression was used to evaluate the associations between sonographic measurements [ARA, LAD status, LPDA, minimal levator hiatus (MLH)] and the presence of ODS. Any variable that was significant on univariate analysis at p < 0.25 was included in the multivariable analysis. Backward stepwise multivariate logistic regression was used to determine the significant predictors of interest, with only covariates meeting a significance level of p = 0.05 remaining in the model. The model was assessed for the presence of effect modification and confounding, with a predetermined difference of > 20 % between the crude and adjusted odds ratios (OR) being considered evidence of confounding. A two-sided p value of 0.05 was considered significant for all final analyses.
Results
Population
We analyzed 100 patients: 48 with ODS, and 52 asymptomatic. The mean age was 55 years [standard deviation (SD 14.97)], 99 % were Caucasian, median parity was two (range two to six), and mean BMI was 27.93 (SD 6.75), with ODS patients having a slightly higher BMI (mean 29.5, SD 7.7, p = 0.0241). Overall, 65 % were menopausal, 16 % were current or ever smokers, and 80 % had stage ≤2 prolapse, with no differences between groups in stage of prolapse of the posterior compartment. There were 14 patients (19 %) with a history of anal sphincter injury, and 68 % had complaints of anal incontinence. Although there were no differences in the proportion of sphincter injuries between groups, a significantly higher proportion of those with ODS complained of anal incontinence (p < 0.0001) (Table 1).
Table 2 compares the sonographic parameters of interest between groups as noted on univariate analyses. There was no difference in the distribution of LAD severity (p = 0.1438) or mean MLH (p = 0.3326) between groups. The proportion of patients with a widened ARA was significantly greater in the ODS group (p < 0.0001). Similarly, ODS patients also had significantly greater levator plate descent (p = 0.0004) compared with their asymptomatic counterparts.
These relationships held in the multivariable regression model, where ARA and LPDA were included in the final model. Patients with an ARA >170° had 7 times the odds of also having ODS compared with those with an ARA ≤170° [OR = 7.01, 95 % confidence interval (CI) 2.30–21.35; p = 0.0006). Patients with LPDA <9° had three times the odds of being symptomatic compared with those with less descent (OR = 3.30, 95 % CI 1.22–8.96; p = 0.0190) (Table 3). The final model was assessed for both interaction and confounding, and none was identified.
Discussion
This study demonstrates that increased LPDA <9° and widened ARA >170° as measured on 3D EVUS imaging are associated with ODS. Consistent with previous studies, we found no association between ODS and severity of LAD or minimal levator hiatus [16]. Also consistent with prior work, there was no difference in stage of prolapse between groups, although the majority of patients had prolapse of stage ≤2 [17, 18]. This suggests that morphologic changes specific to the anorectum are more important in the presence of obstructive defecation than those in other pelvic floor compartments.
Traditionally, defecography and dynamic MRI have been used to evaluate bowel motility disorders, including symptoms of obstructive defecation [9, 6]. These imaging modalities are limited in use by expense, patient discomfort and embarrassment, and lack of trained personnel needed to perform and interpret them. EVUS is an imaging modality that can be easily performed in an office setting and can reveal abnormalities such as intussusception and sigmoidocele, which are not readily obvious on clinical examination. It has the advantage of allowing the investigator to also obtain other important anatomic information on the posterior compartment structures at the same time. To our knowledge, this is the first study to evaluate posterior pelvic floor compartment measurements obtained with 3D EVUS imaging and analyze their relationships to ODS. Three-dimensional EVUS has been demonstrated to be a reliable technique for imaging the female pelvic floor and is currently used to evaluate a number of different PFDs. As such, it is a reasonable alternative to MRI for evaluating ODS.
We acknowledge the limitations of this study, stemming from its retrospective design. Patients underwent 3D EVUS for any pelvic floor indication and not specifically for ODS. As such, symptomatic patients were defined as those who had affirmative responses to the pertinent questions on the PFDI-20. Degree of bother, frequency of symptoms, and any situational characteristics of the symptoms could not be assessed or accounted for. Furthermore, the majority of patients had little to no prolapse. Posterior compartment prolapse is a proposed risk factor for obstructive defecation, although some studies suggest that it is the result rather than the cause [19, 20]. We are unable to comment on whether, in the presence of advanced prolapse, these two sonographic measures remain significant. However, it is reasonable to assume that given the multifactorial nature of all PFDs, prolapse and widened anorectal angle, or increased levator-plate descent are not mutually exclusive findings in the workup of patients with PFDs and may act together to further exacerbate ODS. It is also possible that the parameters we found significant in this study are early findings in the pathophysiology of POP; however, it is impossible at this time to draw this conclusion.
Anal incontinence was more common in symptomatic patients. This finding agrees with prior studies showing that the prevalence of anal incontinence is increased in women with obstructed defecation [21, 4, 22]. Additionally, while 3D EVUS imaging techniques are standard at our institution, we recognize that a transperineal approach is common throughout many parts of the world. Further studies evaluating the relationship of these parameters and ODS would be a useful contribution to the growing literature on PFDs.
In summary, patients with widened ARA have seven times the odds of ODS as those without a widened ARA, and those with greater levator-plate descent have three times the odds of the same symptoms compared with those without. Given the significantly increased odds seen here, assessing these structures may be warranted in the workup of patients presenting with ODS. However, more studies are needed to understand the clinical significance of these findings in guiding management modalities.
References
Blanchette G (2003) The prevalence of pelvic floor disorders and their relationship to gender, age and mode of delivery. Bjog 110(1):88, author reply 88–89
Kepenekci I, Keskinkilic B, Akinsu F, Cakir P, Elhan AH, Erkek AB, Kuzu MA (2011) Prevalence of pelvic floor disorders in the female population and the impact of age, mode of delivery, and parity. Dis Colon Rectum 54(1):85–94
MacLennan AH, Taylor AW, Wilson DH, Wilson D (2000) The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. BJOG: Int J Obstet Gynaecol 107(12):1460–1470
Lam TJ, Kuik DJ, Felt-Bersma RJ (2012) Anorectal function evaluation and predictive factors for faecal incontinence in 600 patients. Color Dis 14(2):214–223
Lammers K, Futterer JJ, Inthout J, Prokop M, Vierhout ME, Kluivers KB (2013) Correlating signs and symptoms with pubovisceral muscle avulsions on magnetic resonance imaging. Am J Obstet Gynecol 208(2):5
Piloni V, Tosi P, Vernelli M (2013) MR-defecography in obstructed defecation syndrome (ODS): technique, diagnostic criteria and grading. Tech Coloproctol 17(5):501–510
Viscardi A, Ratto C, Parello A (2012) Dynamic transperineal ultrasound in the workup of men with obstructed defecation: a pilot study. Dis Colon Rectum 55(9):976–982
Beer-Gabel M, Teshler M, Barzilai N, Lurie Y, Malnick S, Bass D, Zbar A (2002) Dynamic transperineal ultrasound in the diagnosis of pelvic floor disorders: pilot study. Dis Colon Rectum 45(2):239–245, discussion 245–238
Martellucci J, Naldini G (2011) Clinical relevance of transperineal ultrasound compared with evacuation proctography for the evaluation of patients with obstructed defaecation. Color Dis 13(10):1167–1172
Barber MD, Walters MD, Bump RC (2005) Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol 193(1):103–113. doi:10.1016/j.ajog.2004.12.025
Santoro GA, Wieczorek AP, Shobeiri SA, Mueller ER, Pilat J, Stankiewicz A, Battistella G (2011) Interobserver and interdisciplinary reproducibility of 3D endovaginal ultrasound assessment of pelvic floor anatomy. Int Urogynecol J Pelvic Floor Dysfunct 22:53–59
Shobeiri SA, Leclaire E, Nihira MA, Quiroz LH, O’Donoghue D (2009) Appearance of the levator ani muscle subdivisions in endovaginal three-dimensional ultrasonography. Obstet Gynecol 114:66–72
Rostaminia G, White D, Hegde A, Quiroz LH, Davila GW, Shobeiri SA (2013) Levator Ani Deficiency and Pelvic Organ Prolapse severity. Obstet Gynecol 121:1017–1024. doi:10.1097/AOG.0b013e31828ce97d
Shobeiri SA, Chesson RR, Gasser RF (2008) The internal innervation and morphology of the human female levator ani muscle. Am J Obstet Gynecol 199(6):686.e681–686.e686. doi:10.1016/j.ajog.2008.07.057
Shobeiri SA, Rostaminia G, White D, Quiroz LH (2013) The determinants of minimal levator hiatus and their relationship to the puborectalis muscle and the levator plate. BJOG: Int J Obstet Gynaecol 120(2):205–211
Athanasiadis S, Weyand G, Kuprian A, Kohler A (1995) What is the role of the pubococcygeal and puborectal muscles in patients with obstructive defecation disorders? An electromyography study. Der Chirurg; Zeitschrift fur alle Gebiete der operativen Medizen 66(10):974–981
Saks EK, Harvie HS, Asfaw TS, Arya LA (2010) Clinical significance of obstructive defecatory symptoms in women with pelvic organ prolapse. Int J Gynaecol Obstet: Off Organ Int Fed Gynaecol Obstet 111(3):237–240
Dietz HP (2009) Rectocele or stool quality: what matters more for symptoms of obstructed defecation? Tech Coloproctology 13(4):265–268
Ellis CN, Essani R (2012) Treatment of obstructed defecation. Clin Colon Rectal Surg 25(1):24–33
Hicks CW, Weinstein M, Wakamatsu M, Pulliam S, Savitt L, Bordeianou L (2013) Are rectoceles the cause or the result of obstructed defaecation syndrome? A prospective anorectal physiology study. Colorectal Dis 15(8):993–999
Brown HW, Wexner SD, Segall MM, Brezoczky KL, Lukacz ES (2012) Quality of life impact in women with accidental bowel leakage. Int J Clin Pract 66(11):1109–1116
Pucciani F (2013) Faecal soiling: pathophysiology of postdefaecatory incontinence. Colorectal Dis 15(8):987–992
Financial disclaimers/conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
O’Leary, D., Rostaminia, G., Quiroz, L.H. et al. Sonographic predictors of obstructive defecatory dysfunction. Int Urogynecol J 26, 415–420 (2015). https://doi.org/10.1007/s00192-014-2515-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-014-2515-6