Abstract
Recent advances in cellular porous materials for improved performance in their areas of application require the integral design of their porous structure. In this work, open-cell porous aluminium with pores of spherical geometry and interconnecting windows were fabricated using the replication casting method by infiltrating porous preform formed by packing sodium chloride spheres of two different size ranges (1.4–2.0 mm and 2.0–2.5 mm diameters) with molten aluminium under externally applied differential pressure of between 0.01 and 0.1 MPa. The preform was removed by water dissolution which resulted in the formation of porous materials. The influence of infiltration pressure on the window size and relative density of open-cell porous aluminium manufactured was investigated. The resulting open-cell porous aluminium structures were characterized by using the micro-computed tomography and optical and scanning electron microscopes. The results show that within the range of applied pressure, the relative density of the open-cell porous aluminium structures increases as the infiltration pressure is increased, while the window diameter of the porous samples decreases as the applied pressure is increased. A geometric model based on interaction between poorly wetted sodium chloride particles and molten metal which leads to the formation of windows is proposed for the estimation of window radius of porous metals fabricated by replication casting. The estimated window size based on the geometric model is in good agreement with the measured window diameter of the experimental samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Porous metals are a new class of materials with unique properties which are derived from their porous cellular structures and metallic behaviours and offer an interesting combination of structural and functional properties such as low density, high specific surface area, high energy-absorption capacity, good damping properties, good fluid permeability and tortuosity, and controllable thermal and electrical conductivities [1,2,3,4]. The porous metals (particularly those made of aluminium) are finding an increasing range of applications: as structural components in automotive industries (light weight construction, crash energy absorption, noise control), in aerospace, ship, railway, biomedical, and building industry, and in functional applications for filters, heat exchangers, silencers, flame arresters, and for water purification [2, 5].
There exists a wide range of methods for the production of porous metals [1, 2]. Amongst these, the replication casting process offers a simple and versatile technique for low-cost fabrication of structurally uniform, open-celled porous structures for low melting point metals such as aluminium [6]. Replication casting consists of the following processes. A porous bed of preheated sodium chloride particles is prepared in a metallic die. The porous bed is infiltrated with molten metal under pressure. The resulting composite is allowed to solidify and, thereafter, shaped into the desired shape, and finally, the salt particles are dissolved in water. Sodium chloride (NaCl) is often used as the preform/space holder material for the replication casting of porous aluminium structures [7,8,9]. This is due to its low cost, chemical inertness in contact with aluminium, its relatively high melting point, and ease of dissolution in water [8]. A critical factor in replication casting is the poor wetting of the salt particles by the metal; hence, molten aluminium does not spontaneously penetrate the open pores in the bed of NaCl particles. Therefore, external pressure is applied via suction or highly pressurized gas (argon) in order to overcome the capillary forces due to the non-wetting condition between aluminium and sodium chloride particles and to drive the liquid metal into the pore spaces within the salt bed [9, 10].
The use of replication casting to produce porous aluminium alloy was first proposed by Polonsky et al. [11] in 1961. They fabricated open-cell aluminium by infiltrating molten metal through porous preform made from granular particles. Fabrizio et al. [12] used hydraulic cylinder to provide pressure and produced porous aluminium by replication of NaCl perform. Rodriguez [13] developed replication casting technique that utilized pressurized argon gas to infiltrate magnesium alloy through a leachable preform. A detailed review of the manufacturing of porous aluminium structures using the infiltration casting method is reported in [14]. Matej Vesenjak et al. [15] manufactured porous aluminium by infiltration liquid Al7Si0.6 Mg through NaCl prefom using low-pressure die casting process and determined the dynamic compression behaviour of the aluminium alloy. They reported that mechanical properties increase with the porous materials’ density. The work did not consider the effect of processing variables on the structure of fabricated porous aluminium materials. Despois et al. [16] produced replicated porous aluminium by infiltrating 400 µm NaCl particles packed to 75% relative density with pure aluminium at varying infiltration pressure to vary the relative density of the porous aluminium. The authors reported that as the pressure is increased, the relative density of the porous material increases and that at relatively high infiltration pressure, small finger-like protrusions appear on the strut of the porous structure which lowers the permeability of the porous material. The researchers used relatively small NaCl particle sizes. There is need to extend investigation to lager sodium chloride sizes. Goodall et al. [17] varied the porous aluminium material’s density by densifying the NaCl preform by means of sintering and cold pressing, prior to infiltration. The result showed that preform sintering resulted in limited increase in preform density, while cold pressing gave rise to significant preform densification but resulted in the cracking of NaCl particles which they observed to be replicated in the final structure of the porous material. Furman et al. [18] fabricated porous aluminium structures by pouring 99.95% liquid aluminium over pure sodium chloride preform whose sizes ranged between 0.35 and 1.5 mm and utilizing vacuum suction to achieve infiltration aluminium through beds of sodium chloride particles. They reported that aperture radius (window size) increases with the sodium chloride particle sizes. The authors developed a model for window diameter of porous metal fabricated by replication casting. There is need to apply this model to other replicated porous structures. The relative density of a porous metal is related to nearly all the important properties of the porous material by way of simple scaling relationship and is generally a far better predictor of these properties than any other parameter [1]. Similarly, the fluid dynamic properties of replicated structures such as permeability and pressure drop are determined by the size of pore connecting windows rather than the pore size [2]. It is, therefore, crucial to specify the average size of pore connecting windows and relative density in order to establish the necessary relationship between micro-structural features and macroscopic properties.
A few researchers have till date developed models for estimating the size of windows formed in porous metals manufactured by replication casting. Furman et al. [18] developed a foam structure model which is based on the interaction between molten aluminium and poorly wetted sodium chloride particles which leads to the formation of air pockets or air collars for the estimation of the size of windows formed during replication casting process. They described the shape of formed air collar by Laplace’s equation and thereafter derived a formula for the estimation of window size from the Laplace’s equation. The aim of this study is to investigate the influence of a processing parameter (infiltration pressure) on the key structural parameters (relative density and window diameter) of replicated porous metal and to develop a simple model for the estimation of the window size of porous metal manufactured via the replication casting technique. It is hoped that the findings of this work will contribute to the development of methodologies for the optimization of the structural parameters of these materials.
1.1 Theory
A window is formed at the contact between two sodium chloride beads or space holder’s particles which cannot be penetrated by molten metal due to its high surface tension. The physical model shown in Fig. 1 illustrates the condition at the contact between two sodium chloride beads once a molten alloy has been poured. The figure shows two sodium chloride beads of equal radius (Rp) in contact with each other. Rw represents the radius of window formed in the porous metal after replication casting, while Rc represents capillary radius due to an infiltrating liquid metal. The curvature of the capillary radius is assumed to be circular for simplification. The model also assumed that the only resistance encountered by the infiltrating liquid metal is the one due to capillary action and that the NaCl particles are spherical in shape. A geometric model based on the interaction between poorly wetting liquid metal and contacting sodium chloride beads is proposed for the estimation of the window radius of porous structures produced by replication casting method.
Rc is defined by Young-Laplace equation as follows [19]:
where P is the applied pressure, σLV is the liquid-vapour surface tension, and θ is the angle of contact between the liquid and solid. [σLV = 0.87 N/m [20], θ = 139° [20].
Applying Pythagora’s theorem, the window radius, Rw, can be derived as follows:
2 Experimental
2.1 Specimen manufacture
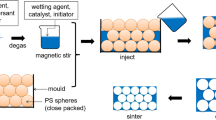
The open-cell porous aluminium materials were fabricated by casting liquid 99.5% aluminium over pack of preheated sodium chloride beads (perform) in a stainless steel mould and then infiltrating the molten aluminium through the pore spaces between the salt beds by vacuum suction. Sodium chloride (NaCl) beads with the following size ranges 1.4–2.0 mm and 2.0–2.5 mm were used as preform for the production of the open-cell porous aluminium samples. The NaCl beads were made using a process described in [21] which consists of the disintegration of a paste containing pre-gelatinized flour, NaCl, and water in heated vegetable oil-followed by the sintering of the formed sodium chloride beads. The salt beads were poured into a 35-mm diameter flanged stainless steel mould with a porous base, tapped a few times to improve packing. The whole arrangement was pre-heated to 600 °C in an electric furnace and, thereafter, part-inserted into a vacuum chamber. Liquid aluminium at 800 °C was poured over the bed of beads, and pressure differential of between 0.01 and 0.1 MPa was applied in five increments to drive the molten aluminium through the pore spaces of the salt beads. Cast samples were machined into suitable sizes for further analyses. The sodium chloride salt beads were dissolved in a water bath maintained at 50 °C for 48 h.
The resulting porous aluminium samples were weighed using an electronic balance, and the densities of the porous metals were determined from the geometry of the samples and their mass. Similarly, the density of loose-filled NaCl beads was determined by measuring the volume of a known mass of each bead size using a measuring cylinder. The stages of the manufacturing process for the porous aluminium samples are shown in Fig. 2.
2.2 Specimen characterization
The structure of the porous samples was investigated using the micro-computed tomography (Scanco AG, μCT40), scanning electron microscope (JEOL 6400), and a Nikon optical microscope, and in all cases combined with image analysis. 3D images of the aluminium foams were obtained using X-ray computed tomography (SCANCO AG µCT 40). In this technique, X-rays from a fixed source pass through a rotated sample and the signals are attenuated and measured by a fixed detector. To conduct the test, samples were placed in appropriate specimen holder (36.9 mm in diameter) and fitted onto the stage of the micro-CT. The X-ray scanner was operated using a medium resolution, 55 kV and 145 µA; voltage, and current density, respectively. A threshold value of 180 was used for the porous aluminium samples.
SEM characterization was performed using a JEOL 6400 scanning electron microscope operating in combination with the LINK ISIS software system. The microscope was operated at an accelerating voltage of 20 kV, a spot size of 3 mm, and a working distance of 39 mm in secondary electron (SE) imaging mode. Prior to SEM operation, the foam samples were sputter coated with platinum in order to increase their electrical conductivities and prevent charge accumulation due to any residual salt particles in the samples. During the sputter coating operation, the coating chamber was de-gassed with argon before a vacuum condition of 2 × 10−2 mbar was achieved and coating was done at a voltage of 2.2 kV for 90 s.
The porous aluminium samples were examined using a Nikon optical microscope which is equipped with a CMEX digital camera (KL1500-T) for image capturing. In this work, the microscope was used in combination with image processing software (Image focus v3.0) to measure the pore and window sizes of the porous samples. Thirty images were captured from each sample with each image having roughly 10 to 30 pores. The average pore/window sizes and standard deviation were calculated and tabulated.
The window and cell sizes of the open-cell porous aluminium samples were measured from 30 images acquired randomly from different sections of a porous sample using the (image focus v.3) image analysis software incorporated in the Nikon optical microscope. The resulting porous aluminium is shown in Fig. 2.
3 Results and discussion
3.1 Structure of porous aluminium
As shown in Fig. 2, the porous aluminium structure consists of cells of near spherical shapes embedded in aluminium matrix, inner windows interconnecting the cells, and struts with unequal thicknesses which demarcate the cells. Figure 3 shows the micro-CT images of bed of NaCl beads (left) and porous aluminium structures resulting from the infiltration of liquid aluminium through beds of NaCl beads (right). The porous aluminium consists of cells which are randomly distributed within the aluminium matrix in a manner that is similar to the random packing of beads within a “bed of beads.” The cells have sizes and shape (near-spherical) which replicate the size and shape of the beads from which the porous aluminium samples are manufactured. The cells are interconnected by “pore windows” which give the porous aluminium samples an open-cellular structure.
3.2 Windows
Table 1 presents the process conditions for the fabrication of porous aluminium samples and the variation of window diameter/relative density with infiltration pressure, while Fig. 4a and b compare the measured (experimental) window diameter with predictions from the geometric model (Eq. 3) and Furman’s model for porous aluminium fabricated using sodium chloride beads with size ranges of 1.4–2.0 mm and 2.0–2.5 mm, respectively. The Furman’s model is expressed in Eq. 4 as follows [18]:
rmin is the window radius, σ is the surface tension of molten metal, P is the vector sum of external pressure, R is the radius of NaCl bead, and θ is the contact angle between the liquid metal and sodium chloride. The estimation of window diameter using the geometric model agrees favourably well with the measured window diameter and showed an average of 9% deviation from the experimental data, while the estimation of window diameter with the Furman’s model showed up to 32% deviation from the experimental values. The discrepancy between the experimentally measured window diameters and calculated diameters using the geometric model could be because the geometric model assumed that the penetrating liquid metal has a circular capillary radius rather than hyperbolic radius for simplification. Moreover, the geometric model also assumed that the only resistance encountered by the liquid metal during infiltration is the one due to capillary action, whereas in reality, aside from opposing capillary forces, the infiltrant encounters other resistances such as viscous resistance, air back pressure, frictional resistance, and solidification resistance [21] which were not considered in the formulation of the model.
As stated earlier, porous metals manufactured by replication casting have open cells with pores interconnected by windows of characteristic size. Windows are formed as a result of the poor wetting properties of the Al-NaCl system, which at a given pressure leaves small regions around the contact points of sodium chloride spheres-unfilled with liquid metal (non-infiltrated zones). These regions are the origin of the pore-connecting windows. The size of these pore connecting windows is a function of the intrinsic properties of the liquid metal, the particle/space holder material and size. the metal-particle interface, and the pressure used to infiltrate the metal into the porous perform [2]. Figure 4 also shows that the window diameter of porous aluminium structures decreases as the infiltration pressure is increased for the same bead size range. This is probably because increase in pressure results in corresponding increase in the infiltration rate of the liquid metal through the capillaries of the NaCl space holder. This increases the liquid metal’s ability to penetrate the pore spaces between contacting beads and leaves smaller gaps (windows) than would be possible if infiltration occurred at lower pressure.
3.3 Relative density
Infiltration of molten metal through a non-wetting liquid metal/solid particle system as is the case with liquid aluminium/NaCl particle system requires the application of external pressure in excess of a minimum value. This threshold pressure P0 (also called capillary pressure) is estimated as with Eq. 5 as follows [22 AAInfiltration Gravity Casting IMP]:
where λ denotes the correcting factor for spherical deviation and surface conditions of the solid particles, γlv is the liquid–vapour surface tension, θ is the contact angle between the liquid metal and solid particle, Vp is the particulate volume fraction, and D is the mean diameter of the particles. For liquid aluminium/NaCl system, γlv = 0.87 N/m, θ = 139 °C, and Vp = 0.58 and 0.57, respectively, for 1.7 mm and 2.25 mm average NaCl particle sizes [20], and assuming λ = 1, the threshold pressure for infiltration was determined to be 0.003 MPa and 0.002 MPa for the 1.7 mm and 2.25 mm average particle sizes, respectively. This implies that infiltration can proceed under the pressure range for the fabrication of the open-cell porous aluminium samples.
The effect of infiltration pressure on the relative density of the porous aluminium samples is presented in Table 1. It can be seen from Table 1 that for porous aluminium samples manufactured from sodium chloride beads of the same bead size range and packing density, the relative density of the materials increases as the infiltration pressure was increased from 0.01 to 0.1 MPa. The samples showed 8.6% increase in relative density as the infiltration pressure is increased within this pressure range. This is probably because the extent of saturation of open pores within the sodium chloride bed increases with pressure; hence, high fraction of liquid metal is infiltrated into the porosity within the bed of NaCl beads at high pressure-resulting in increase in density [16]. This finding is in agreement with that of Despois et al. [16] who reported that for porous aluminium produced by infiltrating liquid aluminium in compacted NaCl preform with 75% packing density, and varying pressure from 0.2 to 15.5 MPa, the relative density of the resulting porous aluminium structures increased with the applied pressure up to the pressure of 12 MPa (corresponding to full infiltration of nearly all open porosity in the preform) and remains constant with further increase in pressure to 15.5 MPa.
4 Conclusions
Open-cell porous aluminium structures have been manufactured by forcing liquid metal through the pore spaces between non-wetting contacting spherical salt beads. Experimental approach was utilized to study the influence of infiltration pressure on the relative density and window diameter of fabricated porous aluminium structures. A geometric model was also formulated for the estimation of the window diameter of open cell porous materials manufactured via the replication casting technique. The following conclusions can be drawn from the results of this study:
-
(1)
A geometric model based on contact between solid particles which are poorly wetted by liquid metal and resulting in the formation of interconnecting window was developed. Prediction of window diameter of open-cell porous aluminium produced by replication casting using the model is in good agreement with measured window sizes for open-cell porous aluminium produced in this study.
-
(2)
At constant bead size and packing density of NaCl beads, the window diameter of porous aluminium structures manufactured by replication casting increases as the pressure of infiltration is reduced.
-
(3)
The relative density of open-cell porous aluminium materials increases as the applied pressure on the liquid metal during infiltration is increased from 0.01 to 0.1 MPa.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Ashby MF, Evans A, Fleck NA, Gibson LJ, Hutchinson JW, Wadley HN (2000) Metal foam — a design guide, Butterworth Heinmann
Durmus FC, Maiorano LP, Molina J (2022) M, Open-cell aluminium foams with bimodal pore size distributions for emerging thermal management applications. Int J Heat Mass Transf 191:p1-15
Srivastava VC, Sahoo KL (2007) Processing, stabilization and applications of metal foams. Mater Sci- Poland 25(3):206
Berchem K, Mohr U, Bleck W (2002) Controlling the degree of pore opening in metal sponges, prepared by infiltration processing method. Mater Sci Eng A 323:52–57
Surace R, Filippies LA, Ludovico AD, Boghetich G (2009) Influence of processing parameters on aluminium foam produce by space holder technique. Mater Des 30:1878–1885
Otaru JA, Morvan HP, Kennedy A (2018) R, Measurement and simulation of pressure drop across replicated porous aluminium in Darcy-Forchheimer regime. Acta Materialla 149:265–273
Marchi S, Mortensen C (2002) Infiltration and replication process for producing metal sponges. In Degischer, HP, editor Handbook of cellular material. Weinheim: Wiley- VCH, p 44
Medik F, Sunar T, Cetin M, Yasar M, Turhan L (2017) Production of open-cell aluminium foam via infiltration method, 1st International Conference of Advanced Materials and Manufacturing Technologies Safranbolu, Karabuk, Turkey pp 25-27
Weber L, Ingram D, Guardia S, Anthanasio-ioannou A, Mortensen A (2017) Fluid flow through replicated microcellular materials in the Darcy-Forchheimer regime. Acta Materialla 126:265–273
Gaillard C, Despois JS, Mortensen A (2004) Processing of NaCl powders of controlled size and shape for the tailoring of aluminium foams. Mater Sci Eng, A 374:250–262
Polonsky L, Lipson S, Marcus H (1961) Light weight cellular metal. Mod Cast 39:51–57
Fabrizio Q, Boschetto A, Rovatti L, Santo L (2011) Replication casting of open-cell AlSi7Mg0.3 foam. Mater Lett 65:2558–2561
Rodriguez G, Figueroa I, Suarez M, Novelo-Peralta O, Alfonso I, Goodall RA (2017) Replication casting device for manufacturing open-cell Mg foam. J Mater Process Technol 243:16–22
Banhart J (2001) Manufacture, characterization and application of cellular metals and metal foams. Prog Mater Sci 46:p559-632
Vesenjak M, Sulong M, Krstulovic-Opare L, Borovinsek M, Mathier V (2015) and Fiedler, T, Dynamic compression of aluminium foam derived from infiltration casting of salt dough. Mech Mater. https://doi.org/10.1016/j.mechmat.2015.10.012
Despois JF, Marmottant A (2007) Salvo, L and Mortensen, A, Influence of the infiltration pressure on the structure and properties of replicated foams. Mater Sci Eng A 426:p68-75
Goodall R, Despois JF, Marmottant A, Salvo L, Mortensen A (2006) The effect of preform processing on replicated aluminium foam structure and mechanical properties. Scripta Materialia 54:2069–2073
Furman EL, Arcady BF, Maxim LC (2013) Permeability of aluminium foams produced by replication casting. Metals 3:49–57
Despois JF, Muller R, Miserez A, Weber L, Rossoll A, Mortensen A (2007) Structural metallic materials by infiltration. Metallic materials with high structural efficiency. Edited by, O. Senkov., D. Miracle and S. Firstov. Vol. 146, Springer Netherlands, WILEY- VCH Verlag GmbH & Co. KGaA, Weinheim 379-390
Nagy V, Vas Laszlo M (2005) Pore characteristic determination with mercury porosimetry in polyester sample yarns. Fibres Textiles Eastern Europe 13:21–26
Asthana R, Singh M, Sobczac N (2005) Infiltration processing of ceramic-metal composite. J Korean Ceram Soc 42(11):703–717
Wang Z, Gao J, Chang K, Meng L, Zhang N, Guo Z (2018) Manufacturing of open-cell aluminium foams via infiltration casting in super-gravity field and mechanical properties. R Soc Chem 8:15933
Author information
Authors and Affiliations
Contributions
All the authors are involved in the development of the alloy, conducting of the research, and writing of the article.
Corresponding author
Ethics declarations
Ethics approval
This work does not include humans and animal, hence does not require ethical approval from any committee.
Consent to participate
This work does not include human and animal, hence does not require consent to participate in the research.
Consent for publication
The authors give the publisher the consent to publish the work.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Njoku, R.E., Boniface, O.O. & Aigbodion, V.S. Influence of infiltration pressure on the window size and relative density of open-cell porous aluminium manufactured by replication casting. Int J Adv Manuf Technol 126, 3015–3021 (2023). https://doi.org/10.1007/s00170-023-11309-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00170-023-11309-0