Abstract
Purpose
The purposes of this systematic review were to (1) identify the commonly used definitions of radiographic KOA progression, (2) summarize the important associative risk factors for disease progression based on findings from the OAI study and (3) summarize findings from radiographic KOA progression prediction modeling studies regarding the characterization of progression and outcomes.
Methods
A systematic review was performed by conducting a literature search of definitions, risk factors and predictive models for radiographic KOA progression that utilized data from the OAI database. Radiographic progression was further characterized into “accelerated KOA” and “typical progression,” as defined by included studies.
Results
Of 314 studies identified, 41 studies were included in the present review. Twenty-eight (28) studies analyzed risk factors associated with KOA progression, and 13 studies created or validated prediction models or risk calculators for progression. Kellgren–Lawrence (KL) grade based on radiographs was most commonly used to characterize KOA progression (50%), followed by joint space width (JSW) narrowing (32%) generally over 48 months. Risk factors with the highest odds ratios (OR) for progression included periarticular bone mineral density (OR 10.40), any knee injury within 1 year (OR 9.22) and baseline bone mineral lesions (OR 7.92). Nine prediction modeling studies utilized both clinical and structural risk factors to inform their models, and combined models outperformed purely clinical or structural models.
Conclusion
The cumulative evidence suggests that combinations of structural and clinical risk factors may be able to predict radiographic KOA progression, particularly in patients with accelerated progression. Clinically relevant and feasible prediction models and risk calculators may provide valuable decision-making support when caring for patients at risk of KOA progression, although standardization in modeling and variable identification does not yet exist.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Knee osteoarthritis (KOA) management is made challenging by the heterogeneity of the disease process and etiology, from the speed of progression to the categorization of disease severity [3]. The increasing incidence of KOA and difficulty in preventing progression presents significant societal and cost burdens for patients and the health system [44].
The focus of this study is to characterize the radiographic progression of KOA. Radiography is a simple and cost-effective method to monitor KOA progression [19]. Previous research has focused on structural progression, utilizing metrics such as joint space width (JSW) narrowing, osteophyte formation and Kellgren–Lawrence (KL) grade [18, 19]. The KL classification is described using anteroposterior (AP) knee radiographs, with a grading classification system based on joint space and bony changes from Grade 0 (no joint space narrowing or reactive changes) to Grade 4 (large osteophytes with marked joint space narrowing, sclerosis and bony deformity) [27]. However, there is no consensus on which metric is best for clinical and research use, particularly for assessing and predicting radiographic disease progression.
The ability to accurately characterize and predict KOA progression may not only inform treatment decisions, but also may improve the design and efficacy of clinical studies by better targeting sample populations. The osteoarthritis initiative (OAI) continues to be a multi-center, longitudinal, prospective observational cohort study with a public database to support the investigation of the natural history of, and risk factors for, KOA onset and progression over 96 months. This makes the OAI database the ideal source for evaluating and predicting KOA progression over a longitudinal timeframe. The most recent review of prognostic factors for radiographic KOA progression was in 2015 [2]. Since then, there have been advances in imaging technology captured by the OAI database and studies utilizing newer methodologies such as deep learning that provide a more updated evidence base for better understanding radiographic KOA progression. Consequently, published studies that have utilized this dataset to investigate KOA progression are the focus of this systematic review. The goals of this review were to (1) identify the commonly used definitions of radiographic KOA progression, (2) summarize the important associative risk factors for disease progression based on findings from the OAI study and (3) summarize findings from radiographic KOA progression prediction modeling studies regarding the characterization of progression and outcomes.
Materials and methods
The OAI is a multi-center, longitudinal observational study on the risk factors for KOA onset and progression sponsored by the National Institutes of Health [37]. Comprehensive imaging, biochemical, clinical and genetic measurements from 4796 men and women ages 45–79 years with or at risk for KOA were collected over 8 years of clinical follow-up [37]. Screening and baseline data collection occurred between February 2004 and May 2006, and patient follow-up in the clinic was recorded up to 96 months after enrollment.
Study design
A systematic electronic literature search was performed in July 2021 to identify articles that studied radiographic KOA progression using the OAI database. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were implemented [30]. PubMed and Embase databases (January 2003–July 2021) were searched for articles including nested-cohort and case–control studies of risk factors, and prediction models for radiographic KOA progression that utilized data from the OAI database, using various combinations of the following key phrases: (osteoarthritis initiative), (knee), (radiograph*), (progress*), (predict*) and (model*). Studies that did not define radiographic progression or had the sole outcome measures of knee replacement, incident radiographic KOA, or symptomatic OA with no mention of radiographic progression were excluded.
Eligible studies fell into two categories: “Associated risk studies,” or studies that did not involve predictive modeling of progression and generally analyzed few specific independent variables as possible correlative risk factors for KOA progression, and “Predictive modeling studies,” or studies that sought to model and predict radiographic KOA progression using various methods and risk factor variables. Two main categories of risk factors of radiographic KOA progression were identified from a preliminary review of these studies: (a) structural factors and (b) clinical factors. Structural factors included imaging-based measurements and signs that can be determined using radiography, magnetic resonance imaging (MRI) and dual-energy X-ray absorptiometry (DXA). Clinical factors included any variable collected in the “AllClinical” files from the OAI database that included subjective symptom questionnaires and functional measurements, as well as comorbidity and other patient-level data [37]. A predefined data extraction protocol was used to systematically extract data from studies (Appendix 1). Each study’s definition of and metrics used to classify radiographic progression were examined. Radiographic progression was further characterized into “accelerated osteoarthritis” (AKOA) or “typical progression” as defined by respective studies as either accelerated (KL0/1 to KL3/4 up to 48 months) or typical (any other increase in KL) to account for the heterogeneity of disease progression (Appendix 2).
The quality of the included studies and risk of bias were assessed. For nested case–control and cohort studies, the Newcastle–Ottawa Scale (NOS) assessments were utilized. For predictive modeling studies, the Prediction model Risk of Bias Assessment Tool (PROBAST) was used to assess risk of bias and applicability of models.
Statistical analysis
The extracted data were pooled, and the frequencies of the various KOA progression definitions and risk factors identified were reported for the associated risk and predictive modeling studies separately. Odds ratios (OR), hazard ratios (HR) and the respective 95% confidence intervals (CI) for associated risk studies were reported when available. OR/HR subcategorizations of 0–1, 1–2 and > 2 were utilized to report and compare the pooled results between studies. The mean and ranges for the predictive performance of the models were analyzed and reported when the area under the curve (AUC), sensitivity and specificity data were available. The general convention of AUC interpretation was utilized, with AUC between 0.7 and 0.8 determined as having fair discrimination or predictive power, and AUC > 0.8 determined as having strong discrimination or predictive power. When studies reported p values, the threshold for statistical significance was set uniformly to p < 0.05. All descriptive statistics were analyzed using IBM SPSS Statistics (IBM Corp. Version 27.0. Armonk, NY. 2020).
Results
There were 314 studies based on the OAI database that were identified using the search protocol (Fig. 1). Of these studies, 41 articles met inclusion criteria with clear definitions of radiographic KOA progression described. There were 28 studies that analyzed various risk factors for progression, with specific definitions of radiographic KOA progression as the outcome variables (Table 1) [4, 5, 8, 9, 12,13,14,15,16,17, 21, 25, 26, 28, 31, 32, 34, 35, 38, 39, 41, 43, 46,47,48,49,50,51]. The 13 other studies out of the 41 total created or validated prediction models, tools, or risk calculators for radiographic KOA progression (Table 2) [1, 7, 10, 11, 18, 20, 22,23,24, 29, 33, 36, 40]. The quality assessments of associated risk and predictive modeling studies are summarized in Tables 3 and 4, respectively.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram of included studies [41]. (Asterisk) Studies that did not define radiographic progression or had the sole outcomes measure of knee replacement, incident radiographic knee osteoarthritis, or symptomatic osteoarthritis with no mention of radiographic progression were excluded. From: Moher et al. [52]
Definition of radiographic progression
The KL grade was most commonly used to characterize radiographic KOA progression. The breakdowns of definitions and characterizations of KOA progression for associated risk studies are summarized in Table 5 and for predictive modeling studies in Table 6.
The studies that did not subclassify based on accelerated versus typical OA progression primarily characterized progression as any increase in KL grade or JSW narrowing greater than 0.7 mm up to 72 months (15%), 48 months (50%), 24 months (20%) and 12 months (15%). Of note, Kawahara et al. categorized the severity of progression with “OA Change” as KL0 and KL1 increasing to KL2 or KL3, “Mild Change” as KL2 increasing to KL3 and “Severe Change” as KL2 and KL3 increasing to KL4 over 24 months [25].
Nine studies defined AKOA progression as an increase to KL3 or KL4 within 48 months [4, 5, 7,8,9, 15, 16, 22, 40]. Foreman et al. further sub-characterized AKOA to 24-month and 48-month groups [16], while Harkey et al. defined AKOA as an increase to KL3 or KL4 from KL0 and KL1 within 12 months [21]. Attur et al. uniquely defined AKOA as JSW narrowing of greater than 0.5 mm within 24 months [1].
Risk factors and outcomes-associated risk studies
Among the associated risk studies for progression, there were 18 studies (64%) that examined structural risk factors. Fourteen studies analyzed MRI measures, including baseline and yearly changes in cartilage volume, meniscal and ligament changes, meniscal extrusion, meniscal root tears and knee effusion volume. One study utilized DXA scans to analyze how static alignment of the knee affects periarticular bone using the proximal tibial plateau periarticular bone mineral density (paBMD) measures, while four studies utilized radiography to analyze coronal tibial slope, femorotibial alignment and angle and JSW narrowing (Table 5) [9, 14, 32, 38, 43]. Pain was the most common clinical risk factor evaluated by these studies.
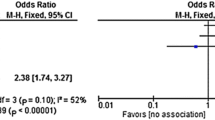
Notably, among associated risk studies on AKOA, any knee injury within the observational period (OR 3.37, 95% CI 1.82–6.25) and within 1 year of accelerated progression (OR 9.22, 95% CI 4.5–18.90) showed the greatest odds ratios for AKOA progression compared to no KOA progression (Table 1) [5]. Degenerative cruciate ligaments, meniscal extrusion and meniscal root tears were found to be significantly associated with AKOA progression with OR > 2, and pain, disability scores, coronal tibial slope and femorotibial angle were moderately associated with AKOA with OR between 1 and 2 [4, 8, 9, 15, 16].
For studies that investigated any radiographic KOA progression, measures of periarticular bone mineral density (OR 10.40, 95% CI 3.50–30.60) and bone marrow lesions (OR 7.92, 95% CI 3.45–18.16) showed the greatest odds for progression, followed by intra-articular corticosteroid injections (HR 4.67, 95% CI 2.92–7.47) [32, 41, 51]. The data for intra-articular corticosteroid injections suggested that the treatment, particularly with continued use, was associated with worsening KOA irrespective of pain.
Risk factors and outcomes: predictive modeling studies
The prediction modeling studies incorporated numerous risk factors to provide the models with enough training variables or to ensure the optimal mix of risk factors within the OAI dataset (Table 6). Nine of the modeling studies (69%) utilized mixtures of both structural and clinical risk factors to maximize the predictive potential of KOA progression over time. This was evidenced by five of these studies comparing the performance of the models using only structural, only clinical, and combined risk factors, and concluding that the combined models outperformed the separate models (p < 0.05) [1, 18, 23, 24, 29]. One study developed an Ambulation Adjusted Score based on the Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores and average hours of daily walking as a clinical tool for monitoring KOA progression, while another constructed a risk score based on the top nine risk variables predicting progression to end-stage KOA identified from all variables in the OAI database—these included the KL Grade, Knee Injury and Osteoarthritis Outcome Score (KOOS) Quality of Life score, OARSI medial joint space narrowing, flexion contracture/hyperextension, knee pain severity in the past 30 days, the WOMAC disability and pain scores, and baseline symptomatic KOA status calculated by the OAI investigators as any baseline symptom in the affected knee, such as pain and stiffness [11, 33]. Of note, Dunn et al. utilized a biomarker, peripheral blood mononuclear cells (PBMC), and analyzed the epigenetic patterns and methylation to create a prediction tool for radiographic KOA progression [10].
Among the 13 prediction modeling studies, the overall mean sensitivity of the models was 70% (range 44–80.5), mean specificity was 80% (range 70–94) and the mean AUC was 0.81 (0.71–0.88) (Table 6).
Discussion
The most important finding of this study was that KL grading was most commonly utilized to define radiographic KOA progression. However, this review demonstrated that there remains wide variability and no consensus on what definition of radiographic KOA progression is best for use in research and clinical practice.
In this review, half of the studies utilized the KL classification to define radiographic KOA progression, roughly one-third utilized JSW narrowing and the rest a combination of both KL and JSW narrowing. In addition, the timeframe of progression was also found to be variable between studies. While most studies defined progression within 48 months, timeframes up to 8 years were also utilized. This variability in classification and timeframe demonstrates the need for standardization within the scientific community in defining KOA progression. This review found KL grading and a timeframe of 48 months to be most commonly used, similar to findings by Bastick et al. in a separate meta-analysis of KOA progression [2].
Of note, as the heterogeneity of the phenotype of KOA is further elucidated, interest in AKOA has added another layer of complexity to classifying radiographic progression. The present review found most studies to define AKOA progression as KL increase from 0/1 to 3/4 within 48 months, with a few utilizing timeframes between 12 and 60 months or JSW narrowing > 0.5 mm. These findings are consistent with a review on AKOA by Driban et al. that defined AKOA as rapid KL increase within 48 months [6]. They also found studies in the literature with variable descriptions of progression, from “fast” to “rapid” and “accelerated”. Definitions ranged from > 0.25 to > 2 mm JSW narrowing within 1 year or KL change greater or equal to 2 within 4–5 years. The definition of AKOA utilizing KL grading and 48 months as the timeframe is offered for standardization of reports.
The findings in this review add to the evidence from meta-analyses by Culvenor et al. and Bastick et al. with updated risk factors and imaging modalities [2, 4]. Bastick et al. reported strong evidence that varus alignment and baseline pain were associated with radiographic KOA progression [2]. In their analysis, conflicting evidence was noted for baseline radiographic or clinical OA severity and past knee injury, while limited evidence was found for other imaging-based risk factors such as meniscal damage, radiographic fractal signature analysis that determines three-dimensionality based on the two-dimensional image and MRI-detected subchondral bone cysts. The more recent studies published after Bastick et al.’s meta-analysis [2] summarized in this present review suggest that there is a trend towards greater association with radiographic KOA progression utilizing imaging-based objective measurements compared to subjective scoring and tools. Further studies investigating these imaging-based risk factors will better inform the strength of the association with radiographic KOA progression.
There were five prediction modeling studies with tools that would be considered to have strong predictive power (AUC > 0.8), with the rest having fair predictive power (AUC > 0.7). These results were comparable, if not better than prediction tools developed outside of the OAI database, further supporting the utility of this database in assessing predictive modeling studies. Runhaar et al. developed a prediction model for early KOA progression using data from the Cohort Hip and Cohort Knee (CHECK) study with an AUC of between 0.746 and 0.764, while Tiulpin et al. developed a multimodal machine learning-based KOA prediction tool that yielded an AUC of 0.79 [42, 45]. Evidence suggests that generally, studies with large patient numbers, diverse yet targeted prediction variables and nonlinear models performed well.
While the OAI database is the largest longitudinal database on KOA, limitations of this review include evaluation of retrospective studies drawing from one patient population with exclusion and inclusion criteria limited to those set by the initial OAI study and having no access to individual patient charts. While internal validation of the risk associations and prediction models is adequate, it is difficult to generalize the results of these studies to the larger population without validation using more datasets drawn from different sample populations. Even so, Riddle et al. validated their clinical prediction rule for estimating the likelihood of developing incident radiographic KOA using both the OAI and the Multicenter Osteoarthritis Study, and found that the AUC was similar in both sets at 0.81 and 0.79, respectively [40]. This shows promise for the generalizability of the OAI database, and further study should focus on the external validity of risk factors and prediction models derived from the OAI database.
Conclusions
Cumulative evidence suggests that combinations of structural and clinical risk factors may be best to predict radiographic KOA progression. To better understand what risk factors to identify, a consensus on the definition of radiographic KOA progression will aid in comparing and designing future studies. While current standardization in modeling and variable inputs do not yet exist, the collection of studies based on the OAI database shows that following KL grade over time may be a valid outcomes proxy that can be utilized to create prediction algorithms, models and tools for radiographic KOA progression.
References
Attur M, Krasnokutsky S, Zhou H, Samuels J, Chang G, Bencardino J, Rosenthal P, Rybak L, Huebner JL, Kraus VB, Abramson SB (2020) The combination of an inflammatory peripheral blood gene expression and imaging biomarkers enhance prediction of radiographic progression in knee osteoarthritis. Arthritis Res Ther 22:208
Bastick AN, Belo JN, Runhaar J, Bierma-Zeinstra SMA (2015) What are the prognostic factors for radiographic progression of knee osteoarthritis? A meta-analysis. Clin Orthop Relat Res 473:2969–2989
Cisternas MG, Murphy L, Sacks JJ, Solomon DH, Pasta DJ, Helmick CG (2016) Alternative methods for defining osteoarthritis and the impact on estimating prevalence in a US population-based survey. Arthritis Care Res (Hoboken) 68:574–580
Davis JE, Harkey MS, Ward RJ, MacKay JW, Lu B, Price LL, Eaton CB, Lo GH, Barbe MF, McAlindon TE, Driban JB (2019) Accelerated knee osteoarthritis is associated with pre-radiographic degeneration of the extensor mechanism and cruciate ligaments: data from the osteoarthritis initiative. BMC Musculoskelet Disord 20:308
Driban JB, Eaton CB, Lo GH, Ward RJ, Lu B, McAlindon TE (2014) Association of knee injuries with accelerated knee osteoarthritis progression: data from the osteoarthritis initiative. Arthritis Care Res (Hoboken) 66:1673–1679
Driban JB, Harkey MS, Barbe MF, Ward RJ, MacKay JW, Davis JE, Lu B, Price LL, Eaton CB, Lo GH, McAlindon TE (2020) Risk factors and the natural history of accelerated knee osteoarthritis: a narrative review. BMC Musculoskelet Disord 21:332
Driban JB, McAlindon TE, Amin M, Price LL, Eaton CB, Davis JE, Lu B, Lo GH, Duryea J, Barbe MF (2018) Risk factors can classify individuals who develop accelerated knee osteoarthritis: data from the osteoarthritis initiative. J Orthop Res 36:876–880
Driban JB, Price LL, Eaton CB, Lu B, Lo GH, Lapane KL, McAlindon TE (2016) Individuals with incident accelerated knee osteoarthritis have greater pain than those with common knee osteoarthritis progression: data from the osteoarthritis initiative. Clin Rheumatol 35:1565–1571
Driban JB, Stout AC, Duryea J, Lo GH, Harvey WF, Price LL, Ward RJ, Eaton CB, Barbe MF, Lu B, McAlindon TE (2016) Coronal tibial slope is associated with accelerated knee osteoarthritis: data from the osteoarthritis initiative. BMC Musculoskelet Disord 17:299
Dunn CM, Nevitt MC, Lynch JA, Jeffries MA (2019) A pilot study of peripheral blood DNA methylation models as predictors of knee osteoarthritis radiographic progression: data from the osteoarthritis initiative (OAI). Sci Rep 9:16880
Dunn R, Greenhouse J, James D, Ohlssen D, Mesenbrink P (2020) Risk scoring for time to end-stage knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthr Cartil 28:1020–1029
Edd SN, Omoumi P, Jolles BM, Favre J (2021) Longitudinal femoral cartilage T2 relaxation time and thickness changes with fast sequential radiographic progression of medial knee osteoarthritis—data from the osteoarthritis initiative (OAI). J Clin Med 10:1294
Everhart JS, Abouljoud MM, Flanigan DC (2019) Role of full-thickness cartilage defects in knee osteoarthritis (OA) incidence and progression: data from the OA initiative. J Orthop Res 37:77–83
Felson D, Niu J, Sack B, Aliabadi P, McCullough C, Nevitt MC (2013) Progression of osteoarthritis as a state of inertia. Ann Rheum Dis 72:924–929
Foreman SC, Liu Y, Nevitt MC, Neumann J, Joseph GB, Lane NE, McCulloch CE, Link TM (2020) Meniscal root tears and extrusion are significantly associated with the development of accelerated knee osteoarthritis: data from the osteoarthritis initiative. Cartilage 13(1_suppl):239S–248S
Foreman SC, Neumann J, Joseph GB, Nevitt MC, McCulloch CE, Lane NE, Link TM (2019) Longitudinal MRI structural findings observed in accelerated knee osteoarthritis: data from the osteoarthritis initiative. Skelet Radiol 48:1949–1959
Fuerst D, Wirth W, Gaisberger M, Hunter DJ, Eckstein F (2021) Superficial cartilage transverse relaxation time is associated with osteoarthritis disease progression—data from the FNIH biomarker study of the osteoarthritis initiative. Arthritis Care Res (Hoboken). https://doi.org/10.1002/acr.24627
Guan B, Liu F, Haj-Mirzaian A, Demehri S, Samsonov A, Neogi T, Guermazi A, Kijowski R (2020) Deep learning risk assessment models for predicting progression of radiographic medial joint space loss over a 48-MONTH follow-up period. Osteoarthr Cartil 28:428–437
Guermazi A, Hayashi D, Roemer FW, Felson DT (2013) Osteoarthritis: a review of strengths and weaknesses of different imaging options. Rheum Dis Clin N Am 39:567–591
Halilaj E, Le Y, Hicks JL, Hastie TJ, Delp SL (2018) Modeling and predicting osteoarthritis progression: data from the osteoarthritis initiative. Osteoarthr Cartil 26:1643–1650
Harkey MS, Davis JE, Lu B, Price LL, Eaton CB, Lo GH, Barbe MF, Ward RJ, Zhang M, Liu S-H, Lapane KL, MacKay JW, McAlindon TE, Driban JB (2019) Diffuse tibiofemoral cartilage change prior to the development of accelerated knee osteoarthritis: data from the osteoarthritis initiative. Clin Anat 32:369–378
Harkey MS, Davis JE, Price LL, Ward RJ, MacKay JW, Eaton CB, Lo GH, Barbe MF, Zhang M, Pang J, Stout AC, Lu B, McAlindon TE, Driban JB (2020) Composite quantitative knee structure metrics predict the development of accelerated knee osteoarthritis: data from the osteoarthritis initiative. BMC Musculoskelet Disord 21:299
Janvier T, Jennane R, Valery A, Harrar K, Delplanque M, Lelong C, Loeuille D, Toumi H, Lespessailles E (2017) Subchondral tibial bone texture analysis predicts knee osteoarthritis progression: data from the osteoarthritis initiative: tibial bone texture & knee OA progression. Osteoarthr Cartil 25:259–266
Joseph GB, McCulloch CE, Nevitt MC, Neumann J, Gersing AS, Kretzschmar M, Schwaiger BJ, Lynch JA, Heilmeier U, Lane NE, Link TM (2018) Tool for osteoarthritis risk prediction (TOARP) over 8 years using baseline clinical data, X-ray, and MRI: Data from the osteoarthritis initiative. J Magn Reson Imaging 47:1517–1526
Kawahara T, Sasho T, Ohnishi T, Haneishi H (2019) Stage-specific meniscal features predict progression of osteoarthritis of the knee: a retrospective cohort study using data from the osteoarthritis initiative. BMC Musculoskelet Disord 20:33
Kemnitz J, Wirth W, Eckstein F, Ruhdorfer A, Culvenor AG (2017) Longitudinal change in thigh muscle strength prior to and concurrent with symptomatic and radiographic knee osteoarthritis progression: data from the osteoarthritis initiative. Osteoarthr Cartil 25:1633–1640
Kohn MD, Sassoon AA, Fernando ND (2016) Classifications in brief: Kellgren–Lawrence classification of osteoarthritis. Clin Orthop Relat Res 474:1886–1893
Kwee RM, Hafezi-Nejad N, Roemer FW, Zikria BA, Hunter DJ, Guermazi A, Demehri S (2018) Association of mucoid degeneration of the anterior cruciate ligament at MR imaging with medial tibiofemoral osteoarthritis progression at radiography: data from the osteoarthritis initiative. Radiology 287:912–921
LaValley MP, Lo GH, Price LL, Driban JB, Eaton CB, McAlindon TE (2017) Development of a clinical prediction algorithm for knee osteoarthritis structural progression in a cohort study: value of adding measurement of subchondral bone density. Arthritis Res Ther 19:95
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:1–34
Lo GH, Musa SM, Driban JB, Kriska AM, McAlindon TE, Souza RB, Petersen NJ, Storti KL, Eaton CB, Hochberg MC, Jackson RD, Kwoh CK, Nevitt MC, Suarez-Almazor ME (2018) Running does not increase symptoms or structural progression in people with knee osteoarthritis: data from the osteoarthritis initiative. Clin Rheumatol 37:2497–2504
Lo GH, Schneider E, Driban JB, Price LL, Hunter DJ, Eaton CB, Hochberg MC, Jackson RD, Kwoh CK, Nevitt MC, Lynch JA, McAlindon TE, OAI Investigators Group (2018) Periarticular bone predicts knee osteoarthritis progression: data from the osteoarthritis initiative. Semin Arthritis Rheum 48:155–161
Lo GH, Song J, McAlindon TE, Hawker GA, Driban JB, Price LL, Eaton CB, Hochberg MC, Jackson RD, Kwoh CK, Nevitt MC, Dunlop DD (2019) Validation of a new symptom outcome for knee osteoarthritis: the Ambulation Adjusted Score for knee pain. Clin Rheumatol 38:851–858
Lu B, Driban JB, Xu C, Lapane KL, McAlindon TE, Eaton CB (2017) Dietary fat intake and radiographic progression of knee osteoarthritis: data from the osteoarthritis initiative. Arthritis Care Res (Hoboken) 69:368–375
MacKay JW, Kapoor G, Driban JB, Lo GH, McAlindon TE, Toms AP, McCaskie AW, Gilbert FJ (2018) Association of subchondral bone texture on magnetic resonance imaging with radiographic knee osteoarthritis progression: data from the osteoarthritis initiative bone ancillary study. Eur Radiol 28:4687–4695
Mononen ME, Liukkonen MK, Korhonen RK (2019) Utilizing atlas-based modeling to predict knee joint cartilage degeneration: data from the osteoarthritis initiative. Ann Biomed Eng 47:813–825
Nevitt MC, Felson DT, Lester G (2006) The osteoarthritis initiative: protocol for the cohort study 1:74. https://www.oarsijournal.com/cms/10.1016/j.joca.2016.09.013/attachment/17129285-04bf-4f1c-a2f4-6c3f498da638/mmc2.pdf
Palmer JS, Jones LD, Monk AP, Nevitt M, Lynch J, Beard DJ, Javaid MK, Price AJ (2020) Varus alignment of the proximal tibia is associated with structural progression in early to moderate varus osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 28:3279–3286
Rathbun AM, Yau MS, Shardell M, Stuart EA, Hochberg MC (2017) Depressive symptoms and structural disease progression in knee osteoarthritis: data from the osteoarthritis initiative. Clin Rheumatol 36:155–163
Riddle DL, Stratford PW, Perera RA (2016) The incident tibiofemoral osteoarthritis with rapid progression phenotype: development and validation of a prognostic prediction rule. Osteoarthr Cartil 24:2100–2107
Roemer FW, Kwoh CK, Fujii T, Hannon MJ, Boudreau RM, Hunter DJ, Eckstein F, John MR, Guermazi A (2018) From early radiographic knee osteoarthritis to joint arthroplasty: determinants of structural progression and symptoms. Arthritis Care Res (Hoboken) 70:1778–1786
Runhaar J, Kloppenburg M, Boers M, Bijlsma JWJ, Bierma-Zeinstra SMA (2021) Towards developing diagnostic criteria for early knee osteoarthritis: data from the CHECK study. Rheumatology (Oxford) 60:2448–2455
Sharma L, Chang AH, Jackson RD, Nevitt M, Moisio KC, Hochberg M, Eaton C, Kwoh CK, Almagor O, Cauley J, Chmiel JS (2017) Varus thrust and incident and progressive knee osteoarthritis. Arthritis Rheumatol 69:2136–2143
Sloan M, Premkumar A, Sheth NP (2018) Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Jt Surg Am 100:1455–1460
Tiulpin A, Klein S, Bierma-Zeinstra SMA, Thevenot J, Rahtu E, van Meurs J, Oei EHG, Saarakkala S (2019) Multimodal machine learning-based knee osteoarthritis progression prediction from plain radiographs and clinical data. Sci Rep 9:20038
Waarsing JH, Bierma-Zeinstra SMA, Weinans H (2015) Distinct subtypes of knee osteoarthritis: data from the Osteoarthritis Initiative. Rheumatology (Oxford) 54:1650–1658
Wang Y, Teichtahl AJ, Abram F, Hussain SM, Pelletier J-P, Cicuttini FM, Martel-Pelletier J (2018) Knee pain as a predictor of structural progression over 4 years: data from the osteoarthritis initiative, a prospective cohort study. Arthritis Res Ther 20:250
Wang Y, Teichtahl AJ, Pelletier J-P, Abram F, Wluka AE, Hussain SM, Martel-Pelletier J, Cicuttini FM (2019) Knee effusion volume assessed by magnetic resonance imaging and progression of knee osteoarthritis: data from the osteoarthritis initiative. Rheumatology (Oxford) 58:246–253
Wirth W, Hunter DJ, Nevitt MC, Sharma L, Kwoh CK, Ladel C, Eckstein F (2017) Predictive and concurrent validity of cartilage thickness change as a marker of knee osteoarthritis progression: data from the osteoarthritis initiative. Osteoarthr Cartil 25:2063–2071
Xu C, Marchand NE, Driban JB, McAlindon T, Eaton CB, Lu B (2020) Dietary patterns and progression of knee osteoarthritis: data from the osteoarthritis initiative. Am J Clin Nutr 111:667–676
Zeng C, Lane NE, Hunter DJ, Wei J, Choi HK, McAlindon TE, Li H, Lu N, Lei G, Zhang Y (2019) Intra-articular corticosteroids and the risk of knee osteoarthritis progression: results from the osteoarthritis initiative. Osteoarthr Cartil 27:855–862
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2008) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed1000097
Funding
No funds, grants or other support were received for this investigation.
Author information
Authors and Affiliations
Contributions
PJ participated in data collection and analysis, design of the study and drafted the manuscript. AB and OM participated in the design of the study and helped to draft the manuscript. AC and KV conceived of the study and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no relevant conflicting interests.
Ethical approval
This systematic review was exempt from institutional ethical approval.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Joo, P.Y., Borjali, A., Chen, A.F. et al. Defining and predicting radiographic knee osteoarthritis progression: a systematic review of findings from the osteoarthritis initiative. Knee Surg Sports Traumatol Arthrosc 30, 4015–4028 (2022). https://doi.org/10.1007/s00167-021-06768-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-021-06768-5