Abstract
Purpose
To investigate the mid-term outcomes of an accelerated return to full weight bearing (WB) after matrix-induced autologous chondrocyte implantation (MACI).
Methods
This randomized study allocated 35 patients (37 knees) to a 6 week (n = 18) or 8 week (n = 19) return to full WB after MACI. Patients were evaluated pre-operatively and at 1, 2 and minimum 5 years (range 5.5–7 years), using the KOOS, SF-36, visual analogue pain scale, 6-min walk test and active knee range of motion (ROM). Peak isokinetic knee extensor and flexor strength was assessed, with limb symmetry indices (LSIs) calculated. Magnetic resonance imaging (MRI) was undertaken to evaluate the repair tissue, and an MRI composite score was calculated.
Results
While no group differences (n.s.) were observed, significant improvement was observed for all patient-reported outcome measures (p < 0.05), 6-min walk distance (p = 0.040), active knee flexion (p = 0.002) and extension (p < 0.0001) ROM, and the LSI for peak knee extensor strength (p < 0.0001). At final review, 87.5% (6 weeks) and 82.4% (8 weeks) of patients were satisfied overall. A non-significant decline (n.s.) was observed for the MRI composite score from 1-year post-surgery to final review, with no significant MRI-based differences (n.s.) between groups. At final review, two grafts (6-week n = 1, 8-week n = 1) demonstrated MRI-based graft failure, while an additional patient had progressed toward knee arthroplasty (8.1% failure rate at minimum 5 years).
Conclusions
The 6-week return to full WB after MACI provided comparable clinical and MRI-based outcomes beyond 5 years post-surgery, without jeopardizing the graft. This 6-week WB protocol is faster than those previously proposed and studied.
Level of Evidence
II.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Autologous chondrocyte implantation (ACI) is a regenerative surgical procedure for isolated chondral defects, that involves culturing and re-implanting a patient’s own chondrocytes. First and second generations of the surgical technique required suturing of a periosteal [4] or collagen [32] cover to retain the cell-based solution within the chondral defect. However, third-generation techniques (matrix-induced ACI) seed the cells onto a collagen membrane, which is then glued to the subchondral bone, with encouraging mid-term outcomes demonstrated overall [3, 5, 13, 14, 18, 19, 24, 37].
While a range of variables may contribute to the success of ACI, the importance of rehabilitation following ACI has been acknowledged and discussed [7, 16, 21, 23, 29,30,31]. This is important not only for the longer term restoration of strength and functional deficits, but also early post-surgery in the initial graft protective and progressive stimulation stages. Anecdotally, a persistent issue for orthopaedic specialists and patients is the lengthy period on crutches and time required to safely attain full weight bearing (WB) after surgery, together with the additional deconditioning and muscular atrophy this can create. Third-generation MACI has permitted an evolution of early WB pathways as patients transition back toward full WB gait [10], with several studies investigating a progressively accelerated WB regimen, at least compared to traditionally conservative programs [11, 14, 17, 25, 37].
The current study reports the mid-term (minimum 5 year) outcomes of a randomized controlled trial (RCT) that sought to investigate a 6-week (versus 8-week) return to full weight bearing after MACI [11]. It aims to demonstrate that the accelerated 6-week return to full WB, faster than those previously proposed and studied, provides a safe and clinically effective outcome sustained to a minimum 5-years post-surgery. Therefore, it was hypothesized that: (1) patients would demonstrate significantly improved outcomes over the post-operative timeframe to final follow-up, with no significant decline from 2 years to final review (minimum 5 years), and (2) there would be no significant clinical or radiological differences between the two WB pathways.
Materials and methods
Ethics approval for this study was obtained from the Hollywood Private Hospital (HPH145) Human Research Ethics Committee (HREC) and the trial was undertaken according to the Declaration of Helsinki. Between January 2010 and April 2014, this RCT allocated 35 patients (37 knees) to a 6-week (n = 18) or 8-week (n = 19) return to full WB after MACI to the medial or lateral femoral condyle (Table 1). Outcomes were assessed pre-operatively and at 1, 2 and minimum 5 years (range 5.5–7 years) post-surgery. Patients were originally randomized to one of the two WB regimens using a ‘random number generator’ via Microsoft Excel (two patients underwent MACI on both knees, and on the second knee were allocated to the alternative pathway to which they were randomized on the first knee). Only one investigator had access to the randomization list, and they were not involved in any of the surgical, rehabilitation or assessment components of the study (there was no patient contact). Figure 1 demonstrates the flow of patients throughout the post-operative timeline, while the original study protocol has been provided as a supplement.
The inclusion/exclusion criteria for this prospective RCT have been previously defined [13,23]. Briefly, patients were indicated for MACI if they were 15–65 years of age and had undergone MACI to address full thickness femoral condylar defects in the knee. Patients with ligamentous or meniscal deficiency were included providing it was addressed at the time of MACI. Patients with varus/valgus malalignment (> 5º anatomic tibiofemoral angle), or those that suffered from any ongoing, progressive inflammatory arthritis, osteoarthritis or rheumatoid arthritis, were excluded.
Surgery
The MACI surgical technique(s) has been previously described.13, 23 Briefly, an initial arthroscopic cartilage biopsy was undertaken followed by isolation, culturing and seeding of cells onto a type I/III collagen membrane (ACI-Maix Matricel GmbH, Germany). In a second surgery, undertaken via a mini-open (6-week n = 7, 8-week n = 8) or arthroscopic (6-week n = 11, 8-week n = 11) approach, the scaffold was glued to the cleaned and prepared subchondral bone.
Rehabilitation
In the early in-patient hospital period (1–3 nights), all patients, irrespective of group randomization, underwent continuous passive motion (CPM) within 12–24 h (set at 0–30°) for a minimum of 1 h daily, regular cryotherapy and elevation to control swelling, active ankle plantar- and dorsi-flexion to encourage circulation, and isometric contraction of the lower limb muscles to maintain muscle tone. A knee brace was worn for 24 h per day, and all patients underwent appropriate education on how to ambulate with two crutches permitting ≤ 20% of body weight through the operated limb (which was consistent across both WB rehabilitation groups for the first 2 weeks). All patients then participated in a progressive out-patient rehabilitation program, intensively over the first 12 post-operative weeks, and as required following that time up until 12 months. Apart from the gradient and time to attain full WB (Table 1), all other rehabilitation components were standardized for all patients and have been previously reported [11, 17]. As previously reported [11, 17], WB replication training for all patients was an important component of each rehabilitation session up until the time that full WB was achieved, and WB restrictions were learned via the electronic bathroom scale method [9, 22].
Clinical assessment
All clinical assessments were undertaken by a research assistant blinded to group allocation. Patient-reported outcome measures (PROMs) employed pre- and post-surgery (1, 2 and minimum 5 years) included: (1) a Visual Analogue Pain Scale (VAS) to assess the frequency (VAS-F) and severity (VAS-S) of knee pain on a scale of 0–10, (2) the 36-item Short Form Health Survey (SF-36) [2] to evaluate general health producing a mental (MCS) and physical (PCS) component score, with each subscale reported to the nearest 0.1 point, and (3) the Knee Injury and Osteoarthritis Outcome Score (KOOS) [33]. The KOOS was employed to assess knee pain, symptoms, activities of daily living (ADL), sport and recreation (Sport) and knee related quality of life (QOL), with each subscale reported to the nearest 0.1 point. A satisfaction questionnaire was employed at final follow-up to evaluate overall satisfaction, as well as satisfaction with the MACI surgery to relieve pain, improve the ability to perform daily and work activities, improve the ability to return to recreational activities and improve the ability to participate in sport. Descriptors of ‘Very Satisfied’, ‘Somewhat Satisfied’, ‘Somewhat Dissatisfied’ and ‘Very Dissatisfied’, were employed. The number of patients reporting each response for each domain was reported, as was the percentage of satisfied patients (reported to the nearest 0.1%). At all post-operative time-points, several objective evaluations were undertaken, including 6-min walk capacity (measured and reported to the nearest 1 m), maximal active knee flexion and extension range of motion (ROM) (measured and reported to the nearest 1 degree), and peak concentric knee extension and flexion isokinetic strength (peak torque, Nm) (measured and reported to the nearest 1 Nm). Strength was assessed using an isokinetic dynamometer (Isosport International, Gepps Cross, South Australia) at a single isokinetic angular velocity of 90°/s.
Magnetic resonance imaging (MRI) assessment
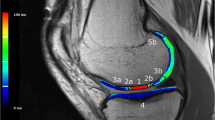
High-resolution MRI employing a Siemens Symphony 1.5 or 3 T scanner (Siemens, Erlangen, Germany; Philips, Best, the Netherlands; General Electric, Milwaukee, WI, USA) was undertaken to assess graft repair at all post-operative time-points. Standardized proton density and T2-weighted fat-saturated images were obtained in coronal and sagittal planes (slice thickness 3 mm, field of view 14–15 cm, 512 matrix in at least one axis for proton density images with a minimum 256 matrix in one axis for T2-weighted images). Axial proton density fat-saturated images were obtained (slice thickness 3–4 mm, field of view 14–15 cm, minimum 224 matrix in at least one axis). Pertinent parameters of graft repair (graft infill, signal intensity, border integration, surface contour, tissue structure, effusion, subchondral lamina and bone) were assessed [28], following the magnetic resonance observation of cartilage repair tissue (MOCART) scoring tool [27, 32, 35, 36]. Each variable was scored from 1 to 4 (1 = poor; 2 = fair; 3 = good; 4 = excellent) in comparison to the adjacent native cartilage, and an MRI composite score was calculated by weighting each variable [32], and summing the scores. MRI evaluation was performed by an independent, experienced radiologist, blinded to group allocation.
Data and statistical analyses
The mean (SD, range) of all measures were calculated pre-surgery (for PROMs), 1 and 2 years post-surgery, and final follow-up (minimum 5 years). Limb Symmetry Indices (LSIs) were calculated for strength measures (by dividing the peak values on the operated limb by that on the non-operated limb, reported to the nearest 0.1%). While analysis of variance (ANOVA) was used to investigate changes in clinical and MRI-based scores over the entire period, and between the two WB groups, t tests were specifically employed to evaluate change from 2 years to final review. To assess intra-observer reliability, 20 randomly selected MRI images were re-assessed a second time by the radiologist. For the morphological MRI scores, the kappa coefficient was employed, while the intra-class correlation coefficient was employed for the MRI composite score. Statistical analysis was performed using SPSS software (SPSS, Version 23.0, SPSS Inc., USA). Statistical significance was determined at p < 0.05. Prior to study onset, a priori power calculation was performed using G-Power (Dusseldorf, Germany) for the primary outcome variable (KOOS Pain), demonstrating that 28 knees (14 in each group) were required to reveal differences at the 5% significance level, with 90% power and employing a large effect size (1.1) as reported by previous research [38].
Results
No significant differences (n.s.) were observed between the two WB groups in pertinent patient demographics (age, body mass index), or prior injury or surgery history (duration of symptoms, number of prior procedures) (Table 2). All PROMs significantly improved (p < 0.05) over time (Table 3), though there were no group differences (n.s.) (Table 3). T tests indicated that there was no significant change (n.s.) in PROMs from 2 years post-surgery to final follow-up, apart from the KOOS Sport which significantly improved (p = 0.032) over that period. At final review, both groups demonstrated a similar percentage of patients that were satisfied with the ability of MACI to relieve their knee pain, though a greater percentage of patients that underwent the 6-week protocol appeared satisfied with their overall outcome (Table 4).
All objective measures (apart from the peak isokinetic flexor strength LSI) significantly improved (p < 0.05) over the post-operative timeline (Table 5), with no group differences (n.s.) (Table 5). While a significant improvement in the peak knee extensor strength LSI (p = 0.015) and significant increase in active knee extension ROM (p = 0.048) was observed from 2 years post-surgery to final follow-up (minimum 5 years), no other differences were observed.
No significant change (n.s.) was observed in the overall MRI composite score, or any other MRI-based variable, from 1 year to final follow-up (minimum 5 years) (Table 6). While there were no group differences (Table 6), when grouped together a non-significant decline was observed in tissue structure, subchondral lamina and bone, as well as the overall MRI composite score, from 2 years post-surgery to final review. Intra-observer reliability assessment indicated a significant correlation between MRI-based scores within each of the scoring variables (MRI composite score rho = 0.811; graft infill rho = 0.949; signal intensity rho = 1.00; border integration rho = 0.982; surface contour rho = 1.00; structure rho = 0.840; subchondral lamina rho = 1.00; subchondral bone rho = 0.920 and; effusion rho = 0.993).
At final review, two grafts (6-week n = 1, 8-week n = 1) demonstrated graft failure on MRI, defined by de-lamination or a graft bed devoid of repair tissue. Of these, the first patient (8-week WB protocol) displayed no graft tissue on 1-, 2- and minimum 5-year MRI, despite displaying early tissue infill on 3-month MRI (scored via the MOCART scoring tool as ‘fair’, < 50% infill). The second patient who underwent the 6-week WB protocol displayed viable tissue infill at 2-years post-surgery (scored via the MOCART scoring tool as ‘good’, > 50% infill), despite having failed on MRI at minimum 5-year follow-up. Both of these patients remained relatively asymptomatic within their ADLs. An additional patient in the 8-week WB group had demonstrated failure on MRI by 2 years post-surgery, though was not reviewed at final follow-up given he had already progressed toward total knee arthroplasty (TKA). However, this patient had also displayed no discernible tissue infill on MRI at either 3-month, 1- or 2-year MRI. Therefore, a graft failure rate of 8.1% was observed at final review (minimum 5 years).
Discussion
Previous studies have demonstrated safety and efficacy in a rehabilitation program that reduced the time required to attain full WB after MACI surgery, from 12 to 8 weeks [12, 14]. The most important finding from the current study was that further acceleration of this WB timeframe, namely 8–6 weeks, provided good patient outcomes without any adverse clinical or radiological outcomes up until and including 5 years post-surgery.
PROMs significantly improved, without any significant decline from 2 years to final review. Several other studies have demonstrated clinical improvement at mid-term follow-up after MACI [3, 5, 13, 14, 18, 19, 24]. Interestingly, the KOOS Sport was significantly better at final review when compared to 2-year outcomes, suggesting a longer period required to obtain the full benefit of MACI and of importance when counselling patients on expectations. This is also supported by the continued improvement in objective measures beyond 1-year, as well as the improvement from 2-years to final review in the LSI for peak knee extensor strength. This lengthy recovery period may be affected by the more conservative nature of rehabilitation overall, combined with other factors such as the long duration of pre-operative symptoms.
No significant change was observed from 1- and 2-years post-surgery through to final review (minimum 5 years) in any MRI-based scoring parameter, nor were there any group differences. Therefore, the 6-week return to full WB was well tolerated and did not affect graft status. Some mild (though non-significant) deterioration was observed from 2-years to final follow-up (tissue structure, subchondral lamina and bone), and ongoing review is required to see whether these changes may pre-dispose to subsequent graft failure. In particular, the subchondral bone, inclusive of both the subchondral bone plate and trabecular bone [20], is intimately associated with the cartilage [1]. The subchondral bone may be important for supplying nutrition to the cartilage [6], and the association between any ongoing adverse changes and overall graft status should be monitored closely. In the current study, three graft failures were observed, two of which were observed on MRI at (or before) 2-years post-surgery and both in the 8-week WB group. Of these, one was not reviewed at final follow-up given he had progressed toward TKA due to further knee deterioration. A third patient (6-week group) demonstrated repair tissue at 2-year review and, despite being relatively asymptomatic at final follow-up, demonstrated a graft bed devoid of repair tissue on MRI at 5-year review.
The important role of rehabilitation following ACI has been acknowledged [7, 16, 21, 23, 29,30,31]. Given the early requirement for graft protection, with respect to the third-generation technique (MACI) though more so its first- (periosteal-covered ACI) and second (collagen-covered ACI)-generation predecessors, rehabilitation and WB pathways have been conservative. Even upon initiation of the current study an 8-week return to full WB was considered ‘accelerated’ [14], though encouraging mid-term outcomes after a 6-week (versus 10-week) WB approach have since been published [37]. The current study supports these outcomes, suggesting the 6-week pathway is well tolerated and, while it did not demonstrate any further clinical benefit, it also did not jeopardize graft integrity. The earlier return to full WB may be of benefit anecdotally to the frustration often reported by patients seeking transition off their crutches, while improving early physical function and QOL [17].
The current study acknowledges some limitations. First, we acknowledge the relatively small patient cohort (n = 35) and the limited conclusions that may be drawn from the study, despite the positive outcomes across the majority of patients embarking on the 6-week WB protocol and the pre-study power calculation undertaken and being fulfilled. Second, it is acknowledged that only a 2-week period existed between groups with respect to their time to full WB. At the time of initiating the study it had been reported that an 8-week return to full WB (which was accelerated at that time) had demonstrated success in patients after MACI [14, 15], hence this was chosen as the control arm. While the study aimed to ascertain whether patients could be safely accelerated toward full WB, the research group was hesitant at that time to take patients off their crutches before 6 weeks, hence that time-point was chosen. Nonetheless, clinically, the 2-week delay remains burdensome and an anecdotally reported issue in considering surgery, together with the additional deconditioning and time off work that is often required when crutches are required.
Third, an issue that must be considered is the confidence in patients adhering to the WB protocols and being able to follow the WB restrictions. While the bathroom scale method was employed to teach WB restrictions and this still remains the most practical and widely used WB modality [9, 22], prior research has demonstrated improved accuracy with more practice [9]. This was an important part of every rehabilitation session. Furthermore, it should be acknowledged that the current study investigated the time to full WB in addition to the WB gradient, whereby full WB was ensured in each group at the designated time by cessation of crutch use. This guaranteed that the time to full WB was true across each group. Finally, the current study sought to investigate the morphological outcome of the graft as per the MRI-based scoring method employed, and acknowledge that other MRI-based imaging modalities can evaluate the biochemical characteristics of the repair tissue [8, 26, 34].
As the clinical relevance, this study demonstrated that an accelerated 6-week return to full WB after MACI results in encouraging and sustained post-operative clinical and radiological outcomes, without jeopardizing the integrity of the developing cartilage tissue. Anecdotally, this traditionally conservative early post-operative period is burdensome to the patient, promotes further physical deconditioning, often enforces a delayed period on crutches and away from work, and is a concern to patients considering MACI as a treatment pathway.
Conclusion
This study demonstrated sustained clinical outcomes beyond 5 years after MACI, with safety and efficacy demonstrated in the 6-week return to full WB. While the 6-week WB pathway provided no additional clinical benefit beyond the early post-operative stages [17], it was well tolerated by patients without adverse features and remains a protocol faster than those previously proposed and studied [12, 14].
References
Barr AJ, Campbell TM, Hopkinson D, Kingsbury SR, Bowes MA, Conaghan PG (2015) A systematic review of the relationship between subchondral bone features, pain and structural pathology in peripheral joint osteoarthritis. Arthritis Res Ther 17:228
Bartlett W, Gooding CR, Carrington RW, Briggs TW, Skinner JA, Bentley G (2005) The role of the Short Form 36 Health Survey in autologous chondrocyte implantation. Knee 12:281–285
Behrens P, Bitter T, Kurz B, Russlies M (2006) Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)—5-year follow-up. Knee 13:194–202
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331:889–895
Brittberg M, Recker D, Ilgenfritz J, Saris DBF, Group SES (2018) Matrix-applied characterized autologous cultured chondrocytes versus microfracture: five-year follow-up of a prospective randomized trial. Am J Sports Med 46:1343–1351
Castaneda S, Roman-Blas JA, Largo R, Herrero-Beaumont G (2012) Subchondral bone as a key target for osteoarthritis treatment. Biochem Pharmacol 83:315–323
Deszczynski J, Slynarski K (2006) Rehabilitation after cell transplantation for cartilage defects. Transplant Proc 38:314–315
Domayer SE, Welsch GH, Dorotka R, Mamisch TC, Marlovits S, Szomolanyi P et al (2008) MRI monitoring of cartilage repair in the knee: a review. SeminMusculoskeletRadiol 12:302–317
Ebert JR, Ackland TR, Lloyd DG, Wood DJ (2008) Accuracy of partial weight bearing after autologous chondrocyte implantation. Arch Phys Med Rehabil 89:1528–1534
Ebert JR, Edwards PK (2014) The evolution of progressive postoperative weight bearing after autologous chondrocyte implantation in the tibiofemoral joint. J Sport Rehabil 23:192–202
Ebert JR, Edwards PK, Fallon M, Ackland TR, Janes GC, Wood DJ (2017) Two-year outcomes of a randomized trial investigating a 6-week return to full weightbearing after matrix-induced autologous chondrocyte implantation. Am J Sports Med 45:838–848
Ebert JR, Fallon M, Ackland TR, Janes GC, Wood DJ (2020) Minimum 10-year clinical and radiological outcomes of a randomized controlled trial evaluating 2 different approaches to full weightbearing after matrix-induced autologous chondrocyte implantation. Am J Sports Med 48:133–142
Ebert JR, Fallon M, Wood DJ, Janes GC (2017) A prospective clinical and radiological evaluation at 5 years after arthroscopic matrix-induced autologous chondrocyte implantation. Am J Sports Med 45:59–69
Ebert JR, Fallon M, Zheng MH, Wood DJ, Ackland TR (2012) A randomized trial comparing accelerated and traditional approaches to postoperative weightbearing rehabilitation after matrix-induced autologous chondrocyte implantation: findings at 5 years. Am J Sports Med 40:1527–1537
Ebert JR, Robertson WB, Lloyd DG, Zheng MH, Wood DJ, Ackland T (2010) A prospective, randomized comparison of traditional and accelerated approaches to postoperative rehabilitation following autologous chondrocyte implantation: 2-year clinical outcomes. Cartilage 1:180–187
Edwards PK, Ackland T, Ebert JR (2014) Clinical rehabilitation guidelines for matrix-induced autologous chondrocyte implantation on the tibiofemoral joint. J Orthop Sports PhysTher 44:102–119
Edwards PK, Ackland TR, Ebert JR (2013) Accelerated weightbearing rehabilitation after matrix-induced autologous chondrocyte implantation in the tibiofemoral joint: early clinical and radiological outcomes. Am J Sports Med 41:2314–2324
Genovese E, Ronga M, Angeretti MG, Novario R, Leonardi A, Albrizio M et al (2011) Matrix-induced autologous chondrocyte implantation of the knee: mid-term and long-term follow-up by MR arthrography. Skeletal Radiol 40:47–56
Gobbi A, Kon E, Berruto M, Filardo G, Delcogliano M, Boldrini L et al (2009) Patellofemoral full-thickness chondral defects treated with second-generation autologous chondrocyte implantation: results at 5 years’ follow-up. Am J Sports Med 37:1083–1092
Goldring MB, Goldring SR (2010) Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci 1192:230–237
Hambly K, Bobic V, Wondrasch B, Van Assche D, Marlovits S (2006) Autologous chondrocyte implantation postoperative care and rehabilitation: science and practice. Am J Sports Med 34:1020–1038
Hambly K, Bobic V, Wondrasch B, Van Assche D, Marlovits S (2006) Autologous chondrocyte implantation postoperative care and rehabilitation: science and practice. Am J Sports Med 34:1–19
Hirschmuller A, Baur H, Braun S, Kreuz PC, Sudkamp NP, Niemeyer P (2011) Rehabilitation after autologous chondrocyte implantation for isolated cartilage defects of the knee. Am J Sports Med 39:2686–2696
Kon E, Di Martino A, Filardo G, Tetta C, Busacca M, Iacono F et al (2011) Second-generation autologous chondrocyte transplantation: MRI findings and clinical correlations at a minimum 5-year follow-up. Eur J Radiol 79:382–388
Kraeutler MJ, Belk JW, Carver TJ, McCarty EC (2018) Is delayed weightbearing after matrix-associated autologous chondrocyte implantation in the knee associated with better outcomes? A systematic review of randomized controlled trials. Orthop J Sports Med 6:2325967118770986
Kurkijarvi JE, Nissi MJ, Kiviranta I, Jurvelin JS, Nieminen MT (2004) Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) and T2 characteristics of human knee articular cartilage: topographical variation and relationships to mechanical properties. MagnReson Med 52:41–46
Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S (2006) Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol 57:16–23
Marlovits S, Striessnig G, Resinger CT, Aldrian SM, Vecsei V, Imhof H et al (2004) Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol 52:310–319
Mithoefer K, Hambly K, Logerstedt D, Ricci M, Silvers H, Della Villa S (2012) Current concepts for rehabilitation and return to sport after knee articular cartilage repair in the athlete. J Orthop Sports PhysTher 42:254–273
Reinold MM, Wilk KE, Macrina LC, Dugas JR, Cain EL (2006) Current concepts in the rehabilitation following articular cartilage repair procedures in the knee. J Orthop Sports PhysTher 36:774–794
Riegger-Krugh CL, McCarty EC, Robinson MS, Wegzyn DA (2008) Autologous chondrocyte implantation: current surgery and rehabilitation. Med Sci Sports Exerc 40:206–214
Robertson WB, Fick D, Wood DJ, Linklater JM, Zheng MH, Ackland TR (2007) MRI and clinical evaluation of collagen-covered autologous chondrocyte implantation (CACI) at 2 years. Knee 14:117–127
Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD (1998) Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports PhysTher 28:88–96
Tiderius CJ, Tjornstrand J, Akeson P, Sodersten K, Dahlberg L, Leander P (2004) Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC): intra- and interobserver variability in standardized drawing of regions of interest. Acta Radiol 45:628–634
Trattnig S, Pinker K, Krestan C, Plank C, Millington S, Marlovits S (2006) Matrix-based autologous chondrocyte implantation for cartilage repair with Hyalograft((R))C: 2-year follow-up by magnetic resonance imaging. Eur J Radiol 57:9–15
Welsch GH, Mamisch TC, Zak L, Blanke M, Olk A, Marlovits S et al (2010) Evaluation of cartilage repair tissue after matrix-associated autologous chondrocyte transplantation using a hyaluronic-based or a collagen-based scaffold with morphological MOCART scoring and biochemical T2 mapping: preliminary results. Am J Sports Med 38:934–942
Wondrasch B, Risberg MA, Zak L, Marlovits S, Aldrian S (2015) Effect of accelerated weightbearing after matrix-associated autologous chondrocyte implantation on the femoral condyle: a prospective, randomized controlled study presenting MRI-based and clinical outcomes after 5 years. Am J Sports Med 43:146–153
Wondrasch B, Zak L, Welsch GH, Marlovits S (2009) Effect of accelerated weightbearing after matrix-associated autologous chondrocyte implantation on the femoral condyle on radiographic and clinical outcome after 2 years: a prospective, randomized controlled pilot study. Am J Sports Med 37(Suppl 1):88S-96S
Funding
This research received some funding from the National Health and Medical Research Council (ID254622 and ID1003452) and the Hollywood Private Hospital Research Foundation (RF031 and RF050).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No benefits in any form have been received or will be received from a commercial party related to the subject of this article.
Ethical approval
This research was approved by the Hollywood Private Hospital (HPH145) Human Research Ethics Committee (HREC).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ebert, J.R., Fallon, M., Wood, D.J. et al. An accelerated 6-week return to full weight bearing after matrix-induced autologous chondrocyte implantation results in good clinical outcomes to 5 years post-surgery. Knee Surg Sports Traumatol Arthrosc 29, 3825–3833 (2021). https://doi.org/10.1007/s00167-020-06422-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-020-06422-6