Abstract
Purpose
The opioid epidemic has prompted an emphasis on investigating opioid-sparing alternatives for pain management following knee arthroscopy. This review evaluated the effects of perioperative nonopioid adjunct analgesia on postoperative opioid consumption and pain control in patients undergoing knee arthroscopy.
Methods
A systematic review and meta-analysis was performed using the following databases: PubMed, Embase, Web of Science, MEDLINE, and SCOPUS. Prospective comparative studies assessing the efficacy of various perioperative nonopioid analgesic strategies in patients undergoing knee arthroscopy were included. Twenty-five studies (n = 2408) were included.
Results
Pre-emptive nonopioid pain medications demonstrated a reduction in cumulative postoperative oral morphine equivalent (OME) consumption by 11.8 mg (95% CI − 18.3, − 5.4, p ≤ 0.0001) and VAS pain scores by 1.5 (95% CI − 2.3, − 0.7, p < 0.001) at 24 h compared to placebo. Postoperative nonopioid pain medications significantly reduced cumulative postoperative OME consumption by 9.7 mg (95% CI − 14.4, − 5.1, p < 0.001) and VAS pain scores by 1.0 (95% CI − 1.354, − 0.633, p < 0.001) at 24 h compared to placebo. Saphenous nerve blocks significantly reduced cumulative postoperative OME consumption by 6.5 mg (95% CI − 10.3, − 2.6, p = 0.01) and VAS pain scores by 0.8 (− 1.4, − 0.3, p = 0.03) at 24 h compared to placebo. Both preoperative patient education and postoperative cryotherapy reduced postoperative opioid consumption.
Conclusion
Perioperative nonopioid pharmacotherapy, saphenous nerve blocks, and cryotherapy for patients undergoing knee arthroscopy significantly reduce opioid consumption and pain scores when compared to placebo at 24 h postoperatively. These interventions should be considered in efforts to reduce opioid consumption in patients undergoing knee arthroscopy. More research is needed to determine which interventions can reduce pain outside of the immediate postoperative period and the potential synergistic effects of combining interventions.
Level of evidence
II.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Orthopaedic surgeons prescribe more opioids than any other surgical specialty [61]. A recent database study across various surgical specialties found that 94% of patients undergoing elective surgery received opioid prescriptions at discharge, and that all orthopaedic surgery patients were well in excess of the recommended opioid-prescription guidelines [57]. A considerable number of opioid-naïve orthopaedic surgery patients continue to use opioids well after the perioperative period and become chronic users [9]. The opioid epidemic has prompted a recent emphasis on investigating opioid-sparing alternatives for pain management. Given the general over-prescribing of opioids and the growing body of literature suggesting effective alternatives, orthopaedic surgeons must play a pivotal role in reducing postoperative opioid consumption [48, 50].
Knee arthroscopy represents one of the most commonly performed orthopaedic surgery procedures worldwide [17]. It has several purported advantages over open surgery, including improved intraoperative visualization, faster return to function, reduced postoperative pain, and improved patient satisfaction scores [32, 40]. However, there are no evidence-based clinical practice guidelines for postoperative pain management following arthroscopic surgery. Given the lack of guidelines, there is marked variability in prescribing patterns with the majority of surgeons prescribing an opioid postoperatively [15]. The literature suggests that surgeons are prescribing anywhere between 90 and 450 oral morphine equivalents (OMEs) after surgery [16]. There is evidence that following the above prescription practices results in an unnecessary and excessive amount of opioids prescribed for patients undergoing arthroscopic surgery [16].
It is well established that intraoperative, intra-articular injections are effective at reducing postoperative pain and opioid consumption postoperatively [62, 66]. However, more recently, there has been increased interest in the use of other perioperative adjuncts to reduce postoperative pain and opioid consumption for patients undergoing knee arthroscopy. Saphenous nerve blocks (SNB) can anesthetize sensory branches that innervate the skin and periosteum of the anteromedial knee, and have been shown to effectively control pain after knee arthroscopy without affecting motor function [20]. Various preoperative and postoperative nonopioid analgesia have been shown to manage postoperative pain and reducing opioid consumption [44, 47]. Similarly, less invasive strategies, including preoperative patient education and postoperative cryotherapy, have been shown to be useful adjuncts in pain management following knee arthroscopy [3, 27].
With the ongoing opioid epidemic, it is imperative to determine the optimal strategies to minimize opioid consumption while appropriately managing postoperative pain in patients undergoing outpatient knee arthroscopy. The purpose of this review was to perform a qualitative and quantitative synthesis of the current literature evaluating the effects of nonopioid adjunct analgesia on postoperative opioid use and pain control in patients undergoing outpatient knee arthroscopy.
Materials and methods
A systematic review and meta-analysis were performed on nonopioid perioperative analgesia adjuncts for patients undergoing outpatient knee arthroscopy. This review adhered to the recommendations outlined in the PRISMA and Cochrane Collaboration guidelines for the performance and reporting of systematic reviews [10, 34]. This review was prospectively registered in the PROSPERO database of systematic reviews.
Eligibility criteria
The inclusion and exclusion criteria were defined a priori. To be included, studies needed to be prospective comparative studies comparing any method of nonopioid analgesia in patients undergoing outpatient knee arthroscopy. Outcomes of interest were postoperative opioid consumption and postoperative pain scores. Exclusion criteria included: (1) patients undergoing anterior cruciate ligament (ACL) reconstruction, (2) studies comparing intraoperative analgesic methods including intra-articular injections, (3) studies with other joints involved that could not be stratified by joint, (4) case series with < 10 patients, and (5) articles that did not report the outcomes of interest.
Search strategy
A literature search on perioperative analgesia methods for knee arthroscopy was performed from inception to May 5th, 2020 across PubMed, Embase, Web of Science, MEDLINE, and SCOPUS. Search terms included terms surrounding the pathology and operation of interest: “knee arthroscopy”, “meniscectomy”, “chondroplasty”. Additionally, terms related to the treatment modalities including “nerve block”, “nonsteroidal anti-inflammatory”, and “cryotherapy” were included in the search. The search strategy can be located in the Supplementary Appendix 1. No language limits were placed on the search.
Study selection
Two independent reviewers (N.N. and J.D.) performed a blinded, duplicate screening of all articles that were identified from the systematic search. Title/abstract and full-text screening were performed using Rayyan software (Qatar Computing Research Institute, Doha, Qatar) [41]. Discrepancies at both the title/abstract and full-text stages were resolved by a senior author (A.G.). Inter-observer agreement for the assessments of study eligibility at title and abstract screening was calculated using Cohen’s kappa (κ) statistic and the κ values interpreted according to previously established cut-offs by McHugh et al. [33].
Data extraction
Data extraction was performed with an online collaborative data-extraction form (Google, California, USA) that was created a priori and tested prior to extraction [19]. Study design information, demographic data, details of interventions, and comparators as well as the length of follow-up were extracted. Opioid consumption at 24 h postoperatively (or cumulative total on postoperative day one) and at the last follow-up available were recorded. Opioids were converted to OMEs for consistency using a standardized conversion chart [38]. Our index instrument of choice was the Visual Analogue Scale (VAS), which utilizes a scale from 0 to 10, with higher scores representing increased pain [45]. All subjective pain scores were transformed to match our index instrument leading to mean pain scores between 0 and 10. Pain scores were recorded at 24 h postoperatively and at the last available follow-up recorded. Both pain scores and opioid consumption were reported as mean ± standard deviation (SD), when available. Adverse events were recorded by overall incidence and classified as major or minor if reported.
Statistical analysis
Mean differences (MDs) with 95% confidence intervals (CI) were used for continuous data. The MD from individual trials were then pooled to create an estimate of the effect for each outcome of interest. The MDs were weighted by sample size based on the inverse variance method. Quantitative synthesis was limited to studies with a control or placebo arm. Intervention groups were defined as pre-emptive analgesia (administered prior to anesthesia), postoperative analgesia, pre and postoperative analgesia, regional nerve blocks, and other adjuncts (with < 3 trials). The decision was made a priori to limit quantitative synthesis to interventions with three or more trials. The heterogeneity among studies made statistical comparisons among categories of adjunct analgesia inappropriate. The Ι2 test was categorized as follows: 0.0–24.9% was considered to indicate no heterogeneity; 25.0–49.9% was considered low heterogeneity; 50.0–74.9% was considered moderate heterogeneity; 75.0–100% was considered high heterogeneity [22]. The fixed effects model was used if there was no heterogeneity; otherwise, the random effects model was used. The synthesis of quantitative results and pooled analysis was performed using OpenMeta [Analyst] (Brown University, 2020).
Missing data
If values were presented in median and ranges, they were converted to mean and SD as per the Cochrane handbook and Hozo et al. [23]. If this was not possible, the SD was imputed using the SD of the other included studies as per Cochrane Handbook [10]. For data presented exclusively in graph format, we utilized a validated data-extraction software (WebPlotDigitizer, version 4.1; Ankit Rohatgi) to record outcomes.
Study appraisal
Two independent reviewers (N.N. and J.D.) assessed the overall quality of the evidence with the Methodological Index for Non-Randomized Studies (MINORS) for non-randomized studies [52]. A total of 12 domains are graded for comparative studies and combined for a global score out of 24. The Revised Cochrane Risk of Bias tool was utilized for randomized trials (Cochrane Risk of Bias 2.0) [54]. The respective domains for each tool were evaluated and assigned a label of low risk, some concerns, or high risk. The overall risk of bias of each study was then labeled low risk, some concerns, or high risk.
Results
Study selection
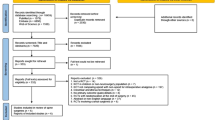
The results of the search strategy are outlined in Fig. 1. A total of 1996 related articles were identified after duplicates were removed. Of the 61 full-text articles reviewed, 25 studies (n = 2408) met the inclusion criteria for the qualitative review, 15 of which were included in the quantitative synthesis. Ten trials were evaluated qualitatively, because they lacked a control or placebo arm or the intervention evaluated was limited to < 3 trials. The agreement between the two reviewers was moderate (κ = 0.52) at the title/abstract stage and substantial (κ = 0.68) at the full-text stage.
Characteristics of the included studies are described in Table 1. Of the included studies, 22 were randomized controlled trials (RCTs) and three were prospective cohort studies. A total of 16 studies (n = 1905) examined perioperative nonopioid pain medications, six studies examined nerve blocks (n = 360), one study examined cryotherapy (n = 45), one examined patient education (n = 62), one study examined the impact of acupuncture, (n = 120) and one investigated postoperative pain wraps (n = 24).
The risk-of-bias summary for each included RCT is available in the Supplementary Appendix Fig. 1. Twelve studies were found to be of low risk, eight studies were found to be of moderate risk, and three studies were found to be of high risk of bias. The most common causes of bias were secondary to the randomization process and dealing with missing outcome data. Across the non-randomized studies, the mean MINORS score was 16/24. The most common reason studies were rated down was for retrospective sample size calculations. The complete MINORS assessment can be found in the Supplementary Appendix Fig. 2.
Nonopioid analgesics
Eight studies evaluated the efficacy of pre-emptive nonopioid pain medications (Table 2) [4, 13, 25, 28, 30, 35, 46, 47]. Seven studies examining preoperative analgesia had placebo groups and were included in the quantitative syntheses. Medications investigated included COX-2 inhibitors (4 studies), NSAIDs (2 studies), as well as gabapentin (1 study) and dextramorphan (1 study). At 24 h postoperatively, preoperative analgesics reduced cumulative OME consumption by 11.8 mg (95% CI − 18.3, − 5.4, p ≤ 0.001, I2 = 95.1%) compared to placebo (Fig. 2). Similarly, preoperative analgesic medications reduced the postoperative VAS pain score at 24 h by 1.5 (95% CI − 2.3, − 0.7, p < 0.001, I2 = 90.1%) (Fig. 2). Bali et al. [4] found that pre-emptive naproxen and codeine reduced postoperative pain scores and rescue opioid consumption in the immediate postoperative period when compared to naproxen alone. Table 2 demonstrates the outcomes of all studies included in the review.
Forest plots depicting the reduction in cumulative opioid consumption by 11.8 mg (95% CI − 18.3, − 5.4, p ≤ 0.001, I2 = 95.1%) (a) and VAS pain scores by 1.5 (95% CI − 2.3, − 0.7, p < 0.001, I2 = 90.1%) (b) at 24 h postoperatively in trials evaluating preoperative nonopioid analgesia compared to placebo. CI confidence interval
Five studies evaluated the efficacy of postoperative nonopioid analgesic medications in the management of postoperative pain, four of which were included in a quantitative synthesis [7, 37, 44, 47, 56]. Medications investigated included NSAIDs (2 studies), COX-2 inhibitors (2 studies), and zolpidem (1 study). At 24 h postoperatively, postoperative analgesics reduced the cumulative OME consumption by 9.7 mg (95% CI − 14.4, − 5.1, p < 0.001, I2 = 87.6%) when compared to placebo (Fig. 3). Postoperative analgesics reduced VAS pain scores at 24 h postop by 1.0 (95% CI − 1.354, − 0.633, p < 0.001, I2 = 0%) compared to placebo (Fig. 3). Tashjian et al. [56] found that postoperative administration of zolpidem, a sedative, reduced postoperative pain scores, fatigue, and opioid consumption in the week following knee arthroscopy when compared to a control group but not a placebo group.
Forest plots depicting the reduction in cumulative opioid consumption by 9.7 mg (95% CI − 14.4, − 5.1, p < 0.001, I2 = 87.6%) (a) and VAS pain scores by 1.0 (95% CI − 1.354, − 0.633, p < 0.001, I2 = 0%) (b) at 24 h postoperatively in trials evaluating postoperative nonopioid analgesia compared to placebo. CI confidence interval
Three studies compared the same nonopioid pain medications administered pre vs. postoperatively [39, 47, 69]. Given the heterogeneity in the timing, dosage, and type of medications used and variability in reporting, no quantitative analysis was undertaken. Reuben et al. and Zhou et al. both demonstrated improve pain control and reduced opioid consumption from pre-emptive NSAID (rofecoxib and meloxicam, respectively) administration when compared to postoperative administration [47, 69]. Conversely, Norris et al. [39] found no difference in pain scores or opioid consumption when comparing diclofenac administration pre and postoperatively.
Three studies examined the efficacy of different nonopioid medications administered pre- and postoperatively [12, 37, 59]. Ekman et al. [12] compared pre- and post-operative celecoxib to placebo and found significantly reduced opioid consumption and occurrence of opioid-related adverse events at 24 h. Nelson et al. [37] compared the administration of diclofenac pre- and postoperatively compared to only postoperatively and to placebo. They found both perioperative and postoperative alone administration of diclofenac reduced pain scores and opioid consumption compared to placebo without significant differences between the two groups. Finally, Uribe et al. [59] compared pre- and postoperative ibuprofen administration to postoperative ketorolac, and found no significant differences in pain scores or opioid consumption outside of immediate postoperatively.
Nerve blocks
Six studies evaluated the use of perioperative nerve blocks, all of which were included in the quantitative synthesis [1, 6, 14, 20, 24, 64]. All included studies utilized SNBs with one study evaluating an SNB + posterior obturator nerve block. At 24 h postoperatively, nerve blocks reduced the cumulative OME consumption by 6.5 mg (95% CI − 10.3, − 2.6, p = 0.01, I2 = 53.3%) when compared to placebo (Fig. 4). There was a significant reduction in VAS pain scores at 24 h by 0.8 (− 1.4, − 0.3, p = 0.03, I2 = 61.2%) when compared to placebo (Fig. 4).
Forest plots depicting the reduction in cumulative opioid consumption by 6.5 mg (95% CI − 10.3, − 2.6, p = 0.01, I2 = 53.3%) (a) and VAS pain scores by 0.8 (− 1.4, − 0.3, p = 0.03, I2 = 61.2%) (b) at 24 h postoperatively compared to placebo for studies evaluating perioperative saphenous nerve blocks. CI confidence intervals
Other adjuncts
Andelman et al. [3] performed a prospective cohort study on the role of preoperative patient education on proper opioid use after outpatient knee arthroscopy and found a significant reduction in total OME consumption at 4 week follow-up compared to the standard of care group [6 mg (7.8) vs. 23.8 mg (27.3), p = 0.001]. Lessard et al. [27] performed an RCT on the effects of cryotherapy following knee arthroscopy, and found a significant reduction in pain scores and opioid consumption. Usichenko et al. [60] performed an RCT on the use of auricular acupuncture in pain management following knee arthroscopy. They found no significant differences between opioid consumption or pain scores at all time points. Hayden et al. [21] compared a postoperative ‘pain wrap’ designed to inhibit nociceptive nerve endings to standard postoperative dressings. They found a decrease in average daily pain over 10 days but no reduction in opioid consumption.
Adverse events
The reporting of complications varied widely which prevented a quantitative synthesis or analysis. The total complication rates of each study arm can be found in Table 2. Sixteen studies recorded postoperative adverse events in details. There were no significant differences in postoperative adverse events in any study. There were three recorded major adverse events, two deep vein thromboses, and one pulmonary embolism. These major adverse events were not attributed to the analgesic intervention. The most common adverse events recorded across all groups including placebo were nausea and vomiting. One patient in the included studies examining nerve blocks had transient quadriceps weakness. Given the relatively short follow-up period of the included studies, the rates of chronic opioid use or long-term adverse effects were not reported.
Discussion
The primary finding of this study is that adjunct analgesia in the form of perioperative nonopioid pharmacotherapy, saphenous nerve blocks, and cryotherapy for patients undergoing simple knee arthroscopy all significantly reduce postoperative opioid consumption and pain scores when compared to placebo at 24 h postoperatively. Preoperative patient education was shown to reduce total postoperative opioid consumption when compared to the standard of care. Given the ongoing efforts to reduce opioid consumption, these interventions warrant serious consideration by surgeons.
Knee arthroscopy represents one of the most commonly performed orthopaedic surgeries worldwide, and may be an opportunity to reduce opioid prescription and consumption on a large scale. Daniels et al. [11] prospectively followed patients who did not receive a prescription for postoperative opioids, and found that the majority were satisfied with their pain levels and did not require rescue opioid medication. Despite this, insurance database research has shown that across 20,000 knee arthroscopies, 82% of opioid-naïve patients filled an opioid prescription postoperatively [65]. The interventions included in this review have the potential to reduce opioid consumption in this patient population.
Pre-emptive analgesia reduces both the central and peripheral sensitization phenomena that occurs with a surgical insult and has been shown to reduce postoperative pain across a number of surgical specialties [29]. Sensitization of central and peripheral nociceptors lowers the postoperative pain threshold and can even contribute to nerve fiber hypersensitivity which contributes to chronic neuropathic pain [29]. The majority of the included trials in this review focused on the efficacy of non-selective and selective (COX-2 inhibitors) NSAIDs. The efficacy of NSAIDs as a pre-emptive analgesic has been replicated across similar populations. Zhang et al. [67] demonstrated that a single preoperative dose of celecoxib (200 mg) in patients undergoing hip arthroscopy significantly reduced postoperative opioid consumption and pain scores at 24 h. The potential benefits of these medications must be weighed with the potential risks as non-selective NSAIDs carry the risk of gastrointestinal complications and selective NSAIDs carry the risk of cardiac events in certain populations. In this review, Montazeri et al. [35] demonstrated significant reductions in postoperative pain scores and opioid consumption with a single time pre-emptive dose of gabapentin. Pre-emptive gabapentin has antiallodynic and antihyperalgesic properties and has been shown to reduce pain and opioid consumption across a number of surgical interventions, but it also comes with associated risks [43]. There remains a paucity of data evaluating the benefits of pre-emptive analgesia outside the immediate postoperative period in patients undergoing knee arthroscopy.
Postoperative nonopioid pharmacotherapy appears to be an effective modality in controlling postoperative pain and reducing opioid consumption in patients undergoing knee arthroscopy. Nonsteroidal anti-inflammatory drugs reduce postoperative pain and inflammation by inhibiting prostaglandin formation and thus inhibiting the inflammatory response to the tissue insult induced by surgery. Although heterogeneity and lack of reporting prevented meta-analysis of outcomes past 24 h, there is evidence that postoperative NSAIDs reduce total opioid consumption after arthroscopic surgery. Nelson et al. [37] demonstrated that postoperative diclofenac reduced postoperative opioid requirements in the first 72 h after knee arthroscopy. More recent data from the shoulder arthroscopy population demonstrated that total opioid consumption at postoperative day 7 was lower in patients receiving ibuprofen in patients undergoing shoulder stabilization surgery [58].
It is important to highlight that the opioid-sparing agents included in this review carry their own risks and side-effect profile. Non-steroidal anti-inflammatories have a significant side-effect profile including increased risk of gastrointestinal bleeds, renal dysfunction, and cardiovascular events [68]. They are contraindicated in patients with renal disease, a history of peptic ulcers or gastric bleeds, and patients with a significant history of cardiovascular disease. Gabapentin, an antiepileptic, has also been commonly used for postoperative pain management, but can cause somnolence, dizziness, ataxia, and fatigue, and have the potential for misuse [36]. Given these side-effect profiles, these medications are not suitable for a considerable percentage of the population which may limit their generalizability and use in clinical practice.
Single-injection nerve blocks have consistently been shown to reduce short-term postoperative opioid consumption and pain scores for up to 24 h [1, 20, 64]. The studies included in this review evaluated adductor canal blocks (ACBs) which anesthetize the saphenous nerve, a purely sensory branch of the posterior femoral nerve which innervates the skin and tibial periosteum of the anteromedial aspect of the knee. The increased availability and popularity of ultrasound has allowed clinicians to perform SNBs with high rates of success. Unlike ACBs, sciatic and femoral nerve blocks block motor function to the quadriceps, which can lead to falls and delayed postoperative function [51]. Chisholm et al. [8] demonstrated that SNB provided similar pain control compared to traditional FNB in patients undergoing outpatient ACL reconstruction. In the current review, there were no falls or prolonged motor weakness and only one episode of transient quadriceps weakness was recorded.
Lessard et al. [27] first demonstrated the benefits of cryotherapy in simple knee arthroscopy, reporting reduction in opioid consumption and pain scores. Cryotherapy has been more extensively studied in arthroscopic ACL reconstruction where studies have demonstrated reductions in postoperative pain and opioid consumption when compared to placebo [5, 31, 63]. There is conflicting results as to whether the compressive cryotherapy outperforms non-compressive cryotherapy [53]. Regardless of technique, cryotherapy appears to be well tolerated and very few adverse events have been recorded [49].
Preoperative patient education has been well established as a method to improve postoperative surgical outcomes and reduce pain and opioid consumption. Andelman et al. [3] demonstrated that providing standardized education surrounding postoperative opioid use significantly reduced postoperative opioid consumption in patients undergoing knee arthroscopy. Syed et al. [55] demonstrated similar results in an RCT on arthroscopic rotator cuff repair, in which preoperative patient education significantly reduced opioid consumption postoperatively. They also demonstrated a reduction in postoperative pain scores, suggesting that preoperative education may allow patients to better cope and manage their pain without the use of opioids. Given the apparent benefits, preoperative patient education appears to be an effective low-cost, low-risk intervention that has the potential to reduce postoperative opioid consumption and pain scores.
The opioid epidemic remains a serious issue, with the United States seeing a 292% increase in premature deaths attributable to opioids between 2001 and 2016 [18]. Given that a large percentage of the population is first prescribed opioids as a postoperative analgesic, the orthopaedic community is in a position to reduce opioid prescribing and consumption [42]. A cohort study of patients undergoing minor surgery found that patients who received an opioid prescription were 44% more likely to become chronic opioid users compared to patients who did not receive an opioid prescription [2]. Considering that postoperative opioid consumption is a risk factor for chronic use, nonopioid analgesic strategies should be utilized to minimize or eliminate the use of opioids in the perioperative period [26].
This study represents a comprehensive systematic review and meta-analysis evaluating all methods of perioperative pain control in simple knee arthroscopy. It is strengthened by its inclusion of only prospective comparative studies, comprising the best available evidence on the topic. There are several limitations to a study of this nature. There was significant variability among the studies included regarding intervention type, timing, and dosage, as well as the patient-reported outcomes used. There was high heterogeneity within each meta-analysis which impacts the final estimate of the comparative effects between the treatment groups and placebo as well as the precision of that estimate. The high heterogeneity may have been secondary to differences in nonopioid analgesic used as well as differences timing and dosage between studies. The majority of studies failed to record postoperative opioid consumption past the 24 h mark. Given that the majority of patients require pain medications outside this time frame, the current literature fails to provide a complete understanding of the opioid-sparing effects of these interventions. Finally, given the heterogeneity among the different interventions, no network meta-analysis could be completed to compare multiple treatment modalities.
Conclusions
This study evaluates the currently available prospective comparative studies on perioperative analgesia adjuncts in patients undergoing simple knee arthroscopy. Several oral medications, predominately NSAIDs, reduce postoperative pain and opioid consumption whether given pre-emptively and/or postoperatively. Saphenous nerve blocks reduce postoperative pain and opioid consumption without interfering with quadriceps motor function. Cryotherapy and patient education both appear to be efficacious. More research is needed to determine which interventions can reduce pain outside of the immediate postoperative period and the potential synergistic effects of combining interventions.
Abbreviations
- OME:
-
Oral morphine equivalent
- VAS:
-
Visual analogue scale
- CI:
-
Confidence interval
- SD:
-
Standard deviation
- MD:
-
Mean difference
- RCT:
-
Randomized controlled trial
- SNB:
-
Saphenous nerve block
- NSAIDs:
-
Non-steroidal anti-inflammatories
- ACL:
-
Anterior cruciate ligament
- MINORS:
-
Methodological Index for Non-Randomized Studies
References
Akkaya T, Ersan O, Ozkan D, Sahiner Y, Akin M, Gümüş H, Ateş Y (2008) Saphenous nerve block is an effective regional technique for postmenisectomy pain. Knee Surg Sports Traumatol Arthrosc 16:855–858
Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM (2012) Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med Am Med Assoc 172:425–430
Andelman SM, Bu D, Debellis N, Nwachukwu C, Osman N, Gladstone JN, Colvin AC (2019) Preoperative patient education may decrease postoperative opioid use after meniscectomy. Arthrosc Sports Med Rehabil 2:e33–e38
Bali C, Ergenoglu P, Ozmete O, Akin S, Ozyilkan NB, Cok OY, Aribogan A (2016) Comparison of the postoperative analgesic effects of naproxen sodium and naproxen sodium-codeine phosphate for arthroscopic meniscus surgery. Rev Bras Anestesiol 66:151–156
Barber FA (2000) A comparison of crushed ice and continuous flow cold therapy. Am J Knee Surg 13:97–101
Bonet A, Koo M, Sabaté A, Otero I, Bocos J, Pi A (2015) Ultrasound-guided saphenous nerve block is an effective technique for perioperative analgesia in ambulatory arthroscopic surgery of the internal knee compartment. Rev Esp Anestesiol Reanim 62:428–435
Chelly JE, Nissen CW, Rodgers AJ, Smugar SS, Tershakovec AM (2007) The efficacy of rofecoxib 50 mg and hydrocodone/acetaminophen 7.5/750 mg in patients with post-arthroscopic pain. Curr Med Res Opin 23:195–206
Chisholm MF, Bang H, Maalouf DB, Marcello D, Lotano MA, Marx RG, Liguori GA, Zayas VM, Gordon MA, Jacobs J, YaDeau JT (2014) Postoperative analgesia with saphenous block appears equivalent to femoral nerve block in ACL reconstruction. HSS J 10:245–251
Cook DJ, Kaskovich SW, Pirkle SC, Mica MAC, Shi LL, Lee MJ (2019) Benchmarks of duration and magnitude of opioid consumption after total hip and knee arthroplasty: a database analysis of 69,368 patients. J Arthroplasty 34:638–644
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J (2019) Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev 10:ED000142
Daniels SD, Garvey KD, Collins JE, Matzkin EG (2019) Patient satisfaction with nonopioid pain management following arthroscopic partial meniscectomy and/or chondroplasty. Arthroscopy 35:1641–1647
Ekman EF, Wahba M, Ancona F (2006) Analgesic efficacy of perioperative celecoxib in ambulatory arthroscopic knee surgery: a double-blind, placebo-controlled study. Arthroscopy 22:635–642
Entezary SR, Farshadpour S, Alebouyeh MR, Imani F, Emami Meybodi MK, Yaribeygi H (2014) Effects of preoperative use of oral dextromethorphan on postoperative need for analgesics in patients with knee arthroscopy. Anesth Pain Med 4:e11187
Espelund M, Fomsgaard JS, Haraszuk J, Dahl JB, Mathiesen O (2014) The efficacy of adductor canal blockade after minor arthroscopic knee surgery—a randomised controlled trial. Acta Anaesthesiol Scand 58:273–280
Fujii MH, Hodges AC, Russell RL, Roensch K, Beynnon B, Ahern TP, Holoch P, Moore JS, Ames SE, MacLean CD (2018) Post-discharge opioid prescribing and use after common surgical procedure. J Am Coll Surg 226:1004–1012
Gardner V, Gazzaniga D, Shepard M, Grumet R, Rubin B, Dempewolf M, Bray C, Prietto C (2018) Monitoring postoperative opioid use following simple arthroscopic meniscectomy: a performance-improvement strategy for prescribing recommendations and community safety. J Bone Jt Surg Open Access 3:e0033
Garrett W, Swiontkowski M (2006) American Board of orthopaedic surgery practice of the orthopaedic surgeon: part-II, certification examination case mix. J Bone Jt Surg Am 88:660–667
Gomes T, Tadrous M, Mamdani MM, Paterson JM, Juurlink DN (2018) The burden of opioid-related mortality in the United States. JAMA Netw Open 1:e180217–e180217
Google Sheets (2020) Google LLC, Moutain View, California. https://www.google.ca/sheets. Accessed May 2020
Hanson NA, Derby RE, Auyong DB, Salinas FV, Delucca C, Nagy R, Yu Z, Slee AE (2013) Ultrasound-guided adductor canal block for arthroscopic medial meniscectomy: a randomized, double-blind trial. Can J Anaesth 60:874–880
Hayden JK, Cole BJ (2003) The effectiveness of a pain wrap compared to a standard dressing on the reduction of postoperative morbidity following routine knee arthroscopy: a prospective randomized single-blind study. Orthopedics 26:59–63
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13
Hsu LP, Oh S, Nuber GW, Doty R, Kendall MC, Gryzlo S, Nader A (2013) Nerve block of the infrapatellar branch of the saphenous nerve in knee arthroscopy: a prospective, double-blinded, randomized, placebo-controlled trial. J Bone Jt Surg Am 95:1465–1472
Ilan DI, Liporace FA, Rosen J, Cannavo D (2004) Efficacy of rofecoxib for pain control after knee arthroscopy: a prospective, randomized, double-blinded clinical trial. Arthroscopy 20:813–818
Johnson SP, Chung KC, Zhong L, Shauver MJ, Engelsbe MJ, Brummett C, Waljee JF (2016) Risk of prolonged opioid use among opioid-naïve patients following common hand surgery procedures. J Hand Surg Am 41:947–957.e3
Lessard LA, Scudds RA, Amendola A, Vaz MD (1997) The efficacy of cryotherapy following arthroscopic knee surgery. J Orthop Sports Phys Ther 26:14–22
Lierz P, Losch H, Felleiter P (2012) Evaluation of a single preoperative dose of etoricoxib for postoperative pain relief in therapeutic knee arthroscopy: a randomized trial. Acta Orthop 83:642–647
Lopez Valencia J, Koch Leopo A (2017) Preemptive analgesia in orthopedic surgery: a literature review. Clin Trials Orthop Disord 2:144
Mardani-Kivi M, Karimi Mobarakeh M, Haghighi M, Naderi-Nabi B, Sedighi-Nejad A, Hashemi-Motlagh K, Saheb-Ekhtiari K (2013) Celecoxib as a pre-emptive analgesia after arthroscopic knee surgery; a triple-blinded randomized controlled trial. Arch Orthop Trauma Surg 133:1561–1566
Martimbianco ALC, Gomes da Silva BN, de Carvalho APV, Silva V, Torloni MR, Peccin MS (2014) Effectiveness and safety of cryotherapy after arthroscopic anterior cruciate ligament reconstruction. A systematic review of the literature. Phys Ther Sport 15:261–268
Matsui N, Taneda Y, Ohta H, Itoh T, Tsuboguchi S (1989) Arthroscopic versus open synovectomy in the rheumatoid knee. Int Orthop 13:17–20
McHugh ML (2012) Interrater reliability: the kappa statistic. Biochemia Med 22:276–282
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Montazeri K, Kashefi P, Honarmand A (2007) Pre-emptive gabapentin significantly reduces postoperative pain and morphine demand following lower extremity orthopaedic surgery. Singap Med J 48:748–751
Morrison EE, Sandilands EA, Webb DJ (2017) Gabapentin and pregabalin: do the benefits outweigh the harms? J R Coll Physicians Edinb 47:310–313
Nelson WE, Henderson RC, Almekinders LC, DeMasi RA, Taft TN (1993) An evaluation of pre- and postoperative nonsteroidal antiinflammatory drugs in patients undergoing knee arthroscopy. A prospective, randomized, double-blinded study. Am J Sports Med 21:510–516
Nielsen S, Degenhardt L, Hoban B, Gisev N (2016) A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf 25:733–737
Norris A, Un V, Chung F, Thanamayooran S, Sandler A, Katz J (2001) When should diclofenac be given in ambulatory surgery: preoperatively or postoperatively? J Clin Anesth 13:11–15
Northmore-Ball MD, Dandy DJ, Jackson RW (1983) Arthroscopic, open partial, and total meniscectomy. A comparative study. J Bone Jt Surg Br 65:400–404
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan—a web and mobile app for systematic reviews. Syst Rev 5:210
Pasricha SV, Tadrous M, Khuu W, Juurlink DN, Mamdani MM, Paterson JM, Gomes T (2018) Clinical indications associated with opioid initiation for pain management in Ontario, Canada: a population-based cohort study. Pain 159:1562–1568
Penprase B, Brunetto E, Dahmani E, Forthoffer JJ, Kapoor S (2015) The efficacy of preemptive analgesia for postoperative pain control: a systematic review of the literature. AORN J 101:94–105.e8
Pham H, Pickell M, Yagnatovsky M, Kramarchuk M, Alaia MJ, Strauss EJ, Jazrawi LM, Campbell KA (2019) The utility of oral nonsteroidal anti-inflammatory drugs compared with standard opioids following arthroscopic meniscectomy: a prospective observational study. Arthroscopy 35:864–870.e1
Price DD, McGrath PA, Rafii A, Buckingham B (1983) The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 17:45–56
Rautoma P, Santanen U, Avela R, Luurila H, Perhoniemi V, Erkola O (2000) Diclofenac premedication but not intra-articular ropivacaine alleviates pain following day-case knee arthroscopy. Can J Anesth 47:220–224
Reuben SS, Bhopatkar S, Maciolek H, Joshi W, Sklar J (2002) The preemptive analgesic effect of rofecoxib after ambulatory arthroscopic knee surgery. Anesth Analg 94:55–59
Rossi MJ, Brand JC, Lubowitz JH (2019) Opioids after arthroscopy: we’re only halfway through the crisis. Arthroscopy 35:1633–1636
Secrist ES, Freedman KB, Ciccotti MG, Mazur DW, Hammoud S (2016) Pain management after outpatient anterior cruciate ligament reconstruction: a systematic review of randomized controlled trials. Am J Sports Med 44:2435–2447
Seymour R, Ring D, Higgins T, Hsu J (2017) Leading the way to solutions to the opioid epidemic. J Bone Jt Surg Am 99(21):e113
Sharma S, Iorio R, Specht LM, Davies-Lepie S, Healy WL (2010) Complications of femoral nerve block for total knee arthroplasty. Clin Orthop Relat Res 468:135–140
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J (2003) Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg 73:712–716
Song M, Sun X, Tian X, Zhang X, Shi T, Sun R, Dai W (2016) Compressive cryotherapy versus cryotherapy alone in patients undergoing knee surgery: a meta-analysis. SpringerPlus 5:1074
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898
Syed UAM, Aleem AW, Wowkanech C, Weekes D, Freedman M, Tjoumakaris F, Abboud JA, Austin LS (2018) Neer Award 2018: the effect of preoperative education on opioid consumption in patients undergoing arthroscopic rotator cuff repair: a prospective, randomized clinical trial. J Shoulder Elbow Surg 27:962–967
Tashjian RZ, Banerjee R, Bradley MP, Alford W, Fadale PD (2006) Zolpidem reduces postoperative pain, fatigue, and narcotic consumption following knee arthroscopy: a prospective randomized placebo-controlled double-blinded study. J Knee Surg 19:105–111
Thiels CA, Anderson SS, Ubl DS, Hanson KT, Bergquist WJ, Gray RJ, Gazelka HM, Cima RR, Habermann EB (2017) Wide variation and overprescription of opioids after elective surgery. Ann Surg 266:564–573
Thompson KA, Klein DS, Gonzalez-Lomas G, Alaia MJ, Strauss EJ, Jazrawi LM, Campbell KA (2019) Opioid use is reduced in patients treated with NSAIDS after arthroscopic shoulder instability repair: a randomized study. Orthop J Sports Med 7:2325967119S00256
Uribe AA, Arbona FL, Flanigan DC, Kaeding CC, Palettas M, Bergese SD (2018) Comparing the efficacy of IV ibuprofen and ketorolac in the management of postoperative pain following arthroscopic knee surgery. A randomized double-blind active comparator pilot study. Front Surg 5:59
Usichenko TI, Kuchling S, Witstruck T, Pavlovic D, Zach M, Hofer A, Merk H, Lehmann C, Wendt M (2007) Auricular acupuncture for pain relief after ambulatory knee surgery: a randomized trial. CMAJ 176:179–183
Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SRB (2011) Characteristics of opioid prescriptions in 2009. JAMA 305(13):1299–1301
Wang Y, Zeng C, Xie D, Yang Y, Wei J, Yang T, Li H, Lei G (2015) Single-dose intra-articular bupivacaine plus morphine after knee arthroscopic surgery: a meta-analysis of randomised placebo-controlled studies. BMJ Open 5:e006815
Waterman B, Walker JJ, Swaims C, Shortt M, Todd MS, Machen SM, Owens BD (2012) The efficacy of combined cryotherapy and compression compared with cryotherapy alone following anterior cruciate ligament reconstruction. J Knee Surg 25:155–160
Westergaard B, Jensen K, Lenz K, Bendtsen TF, Vazin M, Tanggaard K, Worm BS, Krogsgaard M, Børglum J (2014) A randomised controlled trial of ultrasound-guided blockade of the saphenous nerve and the posterior branch of the obturator nerve for postoperative analgesia after day-case knee arthroscopy. Anaesthesia 69:1337–1344
Wunsch H, Wijeysundera DN, Passarella MA, Neuman MD (2016) Opioids prescribed after low-risk surgical procedures in the United States, 2004–2012. JAMA 315:1654–1657
Zeng C, Gao S, Cheng L, Luo W, Li Y, Tu M, Tian J, Xu M, Zhang F, Jiang W, Wei L, Lei G (2013) Single-dose intra-articular morphine after arthroscopic knee surgery: a meta-analysis of randomized placebo-controlled studies. Arthroscopy 29:1450–1458.e2
Zhang Z, Zhu W, Zhu L, Du Y (2014) Efficacy of celecoxib for pain management after arthroscopic surgery of hip: a prospective randomized placebo-controlled study. Eur J Orthop Surg Traumatol 24:919–923
Zhao-Fleming H, Hand A, Zhang K, Polak R, Northcut A, Jacob D, Dissanaike S, Rumbaugh KP (2018) Effect of non-steroidal anti-inflammatory drugs on post-surgical complications against the backdrop of the opioid crisis. Burns Trauma 6:1
Zhou F, Du Y, Huang W, Shan J, Xu G (2017) The efficacy and safety of early initiation of preoperative analgesia with celecoxib in patients underwent arthroscopic knee surgery: a randomized, controlled study. Medicine (Baltimore) 96:e8234
Funding
No funding was received in the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to one or more of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, and (3) final approval of the version to be submitted. All authors have approved the submission of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Author (O.R.A.) is on the speaker’s bureau for CONMED. All other authors declare that they have no conflicts of interest.
Ethical approval
Not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gazendam, A., Ekhtiari, S., Horner, N.S. et al. Perioperative nonopioid analgesia reduces postoperative opioid consumption in knee arthroscopy: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc 29, 1887–1903 (2021). https://doi.org/10.1007/s00167-020-06256-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-020-06256-2