Abstract
Purpose
Altered quadriceps muscle activity can contribute to reduced ability of the muscle to quickly generate force and appropriately attenuate landing forces, exacerbating poor landing and movement strategies commonly seen after anterior cruciate ligament reconstruction (ACLR). The purpose was to evaluate if electromyographic (EMG) activity and knee biomechanics during a single-limb forward hop task are influenced by a history of ACLR.
Methods
Twenty-six individuals with a history of unilateral ACLR (age 20.2 ± 2.7 years, height 1.7 ± 0.1 m; weight 69.6 ± 12.4 kg; time from surgery, 2.9 ± 2.7 years; graft type, 21 bone-patellar-tendon bone, 5 hamstring) and 8 healthy controls (age 23.3 ± 1.8 years, height 1.7 ± 0.1 m; mass 66.3 ± 13.9 kg) volunteered. Sagittal plane knee kinetics and EMG of the vastus lateralis were synchronized and measured using a three-dimensional motion analysis system during a single-limb forward hop task. Mixed-effect models were used to assess the effect of group on kinetic and EMG variables.
Results
Kinetic outcomes (peak and rate of knee extension moment) and temporal muscle activity and activation patterns differed between the ACLR limb and healthy-control limb. Inter-limb asymmetries in the ACLR group were observed for all variables except EMG onset time; no limb differences were observed in the healthy cohort.
Conclusion
Years after ACLR, persistent quadriceps functional deficits are present, contributing to altered neuromuscular control strategies during functional tasks that may increase the risk of reinjury. To counteract these effects, emerging evidence indicates that clinicians could consider the use of motor learning strategies to improve neuromuscular control after ACLR.
Level of evidence
III.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A substantial portion of patients after anterior cruciate ligament reconstruction (ACLR) experience prolonged quadriceps strength deficits, contributing to the presence of long-term aberrant knee biomechanics, decreased force attenuation, and increased risk of reinjury [23, 35]. Although quadriceps strengthening is heavily emphasized after ACLR, and symmetry between limbs is used as a benchmark for progression back to functional activities, discrepancies in quadriceps neuromuscular control between the injured and uninjured limbs often persist [5, 7, 12, 13]. During both single- and double-limb landing tasks, individuals with a history of ACLR exhibit significant reductions in the ACL limb knee extension moment and vertical ground reaction force [16]. These findings highlight the potential unloading of the injured limb after ACLR, despite rehabilitation efforts to restore biomechanical function to pre-surgery levels [16].
Appropriate quadriceps strength is required for force absorption and joint loading during functional tasks, and thus research has continued to evaluate the underlying drivers of persistent deficits [17]. To this point, emerging evidence has indicated that the rate in which the quadriceps muscle can develop torque (rate of torque development) during both static and dynamic movements is altered in the ACL limb of patients [13]. Specifically, reductions in rate of torque development (during a static task) and rate of knee extension moment (during a running task) in the ACLR limb compared to the contralateral limb have been observed [13]. This work, however, did not evaluate the root of these quadriceps-dominant deficits, although they hypothesized that biomechanical alterations could be the consequence of decreased quadriceps neural drive [13]. To this point, global changes in neural activity may contribute to the reduction in the ability of the quadriceps to quickly generate force to appropriately attenuate landing forces, further exacerbating poor landing and movement strategies [29, 35]. To address this gap, factors related to quadriceps muscle activation strategies and their contribution to reductions in global knee function were evaluated.
Neuromuscular control, including the contribution of the timing of electromyographical (EMG) activity prior to landing has previously been studied using pre-activation patterns, where ACLR patients have been found to activate their quadriceps much sooner compared to healthy controls [7]. While evaluation of pre-activation patterns is important, analysis of neuromuscular control strategies during the load-absorbing phase is also needed. Understanding the control strategies used during landing can provide clinicians with relevant contextual information on modifiable components of neuromuscular control that can be targeted during rehabilitation after ACLR. Thus, the purpose of this study was to evaluate if quadriceps EMG activity and knee mechanics during a single-limb forward hop task are influenced by a history of ACLR. It was hypothesized that compared to healthy controls, ACLR participants would present with reductions in knee kinetic variables and altered neuromuscular control strategies.
Materials and methods
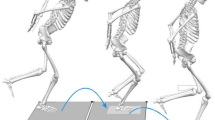
Twenty-six individuals with a history of unilateral ACLR and eight healthy controls participated in a single, cross-sectional controlled laboratory study. This study was approved by the University’s Institutional Review Board and informed consent was obtained prior to testing. The patients included in the study reported to the University of Connecticut’s Sport Optimization and Rehabilitation Laboratory for testing where subjects were required to perform a single-limb forward hop task. All individuals had previously been enrolled into a physician-directed rehabilitation program and were cleared by the treating physician to return to unrestricted sporting activities. Pre-injury and current activity level participation are included within Table 1. Sagittal plane knee kinematics and kinetics and EMG of the vastus lateralis (VL) were synchronized and measured using a three-dimensional motion analysis system during the forward hop task [22]. The patients were excluded if they: (1) had a previous history of knee surgery other than the current ACLR, (2) sustained a contralateral lower extremity injury within the past 6 months, (3) if they were pregnant or planning pregnancy, (4) if they had a demand-type cardiac pacemaker and (5) if they had an allergy to adhesives or any open skin lesions.
Biomechanical analyses
Sagittal plane knee kinematics and kinetics were collected during a single-limb forward hop task. A 12-camera motion capture system (Vicon, Oxford Metrics, London, England) sampling at 240.0 Hz, synchronized with two force plates sampling at 1200.0 Hz (Bertec Corp., Columbus, Ohio) and a wireless EMG system sampling at 1200.0 Hz (DTS System, Noraxon Inc, Scottsdale, AZ, USA) were utilized to quantify three-dimensional biomechanical and muscle activity data, respectively. Participants hopped forward the distance of their limb length, defined as the tip of the greater trochanter to the tip of the lateral malleolus [22]. The patients were allowed unlimited practice trials. Three successful trials were captured consecutively for each limb, where a trial was considered successful when the participants landed on the force platform and balanced on their injured limb for a least one second. The hop task was performed bilaterally and the order of limb testing was randomized. Trunk and lower limb joint rotations were defined based on 3D coordinates of 37 precisely positioned retro-reflective markers [trunk; (C7 spinous process, T10 spinous process, acromioclavicular joints, sternum), right and left limbs; (anterior and posterior superior iliac spines, iliac crest, greater trochanter, distal thigh, medial and lateral femoral epicondyles, tibial tuberosity, distal shank, lateral shank, medial and lateral malleoli, calcaneus, dorsal navicular, head of first and fifth metatarsal)]. A static trial [19] of each subject aligned with the laboratory coordinate system was recorded and a kinematic model including eight skeletal segments and 27 degrees of freedom was created using Visual3D software (C-Motion; Rockville, MD, USA). Ground reaction force data was sampled and synchronized with the kinematic data and filtered using a fourth-order, zero-lag, low-pass Butterworth filter at 12.0 Hz cut-off frequency [18]. Filtered kinematic and ground reaction force data were then submitted to a standard inverse dynamics approach within Visual3D. Kinetic outputs were normalized to body height and mass and represented as internal moments. Initial contact and toe-off were defined as the time when the vertical ground reaction force first exceeded 10.0 N [3, 18] and fell below 10.0 N, respectively. The rate of knee extension moment (RKEM) was calculated from heel strike to peak knee extension moment [13].
Electromyographic analyses
To quantify peak muscle activity of the VL, raw EMG signals were filtered using a high-pass Butterworth filter using a 12.0 Hz cut-off frequency. EMG data were then rectified and processed using a root mean square algorithm with a 50-millisecond moving window. EMG signals that were recorded from heel strike (defined as 10.0 N) to when peak knee extension moment (PKEM) was reached were used for statistical analysis. The average peak muscle activity obtained from this period of interest across the three trials was used. Dynamic EMG data recorded during the single-limb forward hop were then normalized to the peak muscle activity recorded across all trials. Muscle activity onset times of the VL relative to PKEM (EMG onset = time of PKEM − time of EMG “on”) were established using the Teager–Kaiser Energy Operator [32, 33] (EMG onset defined as median plus three standard deviations). This operator was applied only to band-pass filtered data and not signals that had been rectified and processed using a root mean square algorithm, as this would have potentially masked true muscle onset times.
Statistical analyses
Independent t tests were performed to assess differences between ACLR and healthy controls for demographic variables. Mixed-effect models, with individual included as a random factor [10], were used to assess the effect of group (ACL, healthy control) on PKEM, RKEM, peak VL muscle activity, and VL muscle activity onset time. Differences in subject age were controlled for by inclusion of age as a random factor [31]. Additionally, paired t tests were performed to assess differences between limbs within each group. An a priori power analysis based on previous work evaluating rate of knee extension moment [13] determined that at least 24 ACLR subjects were needed to detect differences between limbs. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software version 24.0 (IBM Corp., Armonk, NY, USA) and α level was set a priori at P ≤ 0.05.
Results
Kinetic outcomes and temporal muscle activity and activation patterns of the ACLR limb and healthy matched limb during a single-limb forward hop task were determined for both ACLR and healthy controls. Demographic information is provided in Table 1. Details (means ± SD) of both groups and limbs for all kinetic and muscle activity properties are presented in Table 2.
Kinetic variables
There was a significant effect of group (ACLR, healthy) on PKEM. Peak knee extension moment was lower in ACLR participants (1.3 ± 0.4 nm/kg2) compared to healthy controls (1.6 ± 0.2 nm/kg2, Table 2). There was also a significant effect of group on the RKEM, where there were deficits in the RKEM in the ACLR group (16.9 ± 4.5 nm/kg m s) compared to the healthy group (19.9 ± 2.1 nm/kg m s, Table 2). When assessing limb differences (Table 2) within the ACLR group, the ACLR limb exhibited reductions in both PKEM and RKEM compared to the contralateral limb. No differences between limbs within the healthy control group were observed.
Muscle activation properties
Similar to kinetic properties during the forward hop task, there was a significant effect of group on quadriceps muscle activity and activation patterns. Specifically, VL peak muscle activation was reduced in the ACL group compared to the healthy control group during the hop task (Table 2). The ACLR group also exhibited significant delays in VL muscle onset times, turning on the quadriceps muscle in their ACL limb approximately 0.5 ms after reaching PKEM, where the healthy control group activated their quadriceps 7.3 ms before PKEM (Table 2). Between limbs differences (Table 2) within the ACLR group were also observed reinforcing the observations in declined ACLR limb peak muscle activity during the forward hop task. No differences between limbs within the healthy control group were observed.
Discussion
The most important finding of the present study was that years after ACLR, patients continue to demonstrate altered neuromuscular control and quadriceps activation strategies during a dynamic single-limb landing task compared with healthy controls. Specifically, the ACLR limb exhibited reductions in PKEM, RKEM, peak VL muscle activity, and delayed onset time compared with healthy controls. These between group differences are reinforced by between limb differences in the ACLR group (deficits in PKEM, RKEM, and peak muscle activity of the VL) that were not observed within the healthy cohort. These results suggest that quadriceps functional deficits are resistant to the standard of care after ACLR, and protracted deficits in muscle activation impair knee mechanics during dynamic tasks.
Reductions in PKEM have been widely reported following ACLR during hop tasks [4, 11, 36, 38], walking [15, 28, 30], and running [24]. PKEM deficits observed in our ACLR cohort are consistent with previous work, and highlights the reduced underloading of the involved limb during the load-absorbing phase [21, 38]. While knee moments are commonly evaluated and reported after ACLR, more thoroughly evaluating how quickly the quadriceps is able to generate force during dynamic tasks (i.e., RTD) has become a recent focus of attention. Several studies evaluating RTD during less functional tasks such as isokinetic strength measurements have reported deficits within the injured limb of an ACLR cohort [12, 13]. Extending this work, Kline et al. [13] and Pamukoff et al. [24] have reported diminished RTD of the knee extensor moment (RKEM) during running, which is in agreement with our work. Taken together, our work highlights that those with a history of ACLR utilize reduced PKEM and RKEM strategies during functional movement tasks, which is clinically significant as it negatively influences the ability of the knee to adequately attenuate force. This has implications for short- and long-term knee joint health [26, 34, 37].
The underlying driver of these knee mechanical deficits remains unclear, as data that have directly evaluated adaptations in muscle activity in concert with aberrant knee mechanics during functional tasks are sparse. To this end, we evaluated synchronized peak muscle activity and muscle activation patterns during the hop task. Interestingly, we observed that there were reductions in quadriceps peak muscle activity and a delay in muscle onset time during the load-absorbing phase between the ACLR and healthy controls. The timing of muscle activation relative to PKEM is especially important as it can provide us with context regarding quadriceps muscle activity during the movement when there is increased demand on the knee to stabilize the lower extremity. Our work is novel as it examines this metric of quadriceps health during the energy absorption phase of ground contact. In relation to previous work [1, 7, 27], it appears that those with a history of ACLR have adaptations in VL muscle activity prior to and immediately following ground contact. Specifically, it appears that pre-activation strategies are employed to enhance joint stability prior to landing, by turning on the quadriceps muscle earlier, perhaps in an attempt to assist in the stabilization of the knee during the high physical demands of a single-leg landing task [7]. Others have observed that ACLR individuals often adopt increased co-activation strategies (i.e., increased quadriceps-to-hamstring co-activation) in an effort to increase joint stability during dynamic tasks [6, 9]. Similar to our work, ACLR individuals that utilize this increased co-activation strategies also exhibited reduced knee extensor torque compared to healthy controls [25]. Uniquely, most research evaluating RTD after ACLR, including the current study, has been performed in a cohort heavily dominated by bone–patellar tendon–bone autografts (BPTB) [13, 25]. Although we were unable to evaluate differences in quadriceps function between graft types, it has been hypothesized that individuals who receive a BPTB autograft may exhibit different impairments in quadriceps function compared to those receiving hamstring autografts [13, 25]. However, the data in this area are limited. Future studies are needed to evaluate whether differences exist in co-contraction strategies as well as hamstring muscle activation timing during dynamic and functional tasks and whether the magnitude of RTD deficits exist in patients receiving hamstring autografts.
Previous work evaluating RKEM and EMG onset has only included comparisons to the contralateral limb; narrowing the interpretations of these values to “normal” physiological function. Namely, comparison strictly to the contralateral limb has the potential to be confounded as neural alterations after ACLR have been known to occur bilaterally [20, 29] and may influence overall differences. To mitigate this concern, a healthy cohort was included. The strength of this approach is that it provides further evidence that patients undergoing ACLR are exhibiting these maladaptive strategies and that they persist in the years following surgery, despite rehabilitation efforts.
There were several limitations of this study. The ACLR patients included in this study were on average 2 years removed from surgery, hence the extent of these deficits during important recovery time points (such as return to play) remains unclear. Additionally, this cohort was only evaluated at a single time point after ACLR, limiting applicability. Future longitudinal studies should aim to evaluate how these metrics of knee health may change over the continuum. There was also difference in age between the ACLR and healthy control groups; however, this was controlled for in the analysis by including age as a covariate. This limitation is further mitigated by the inclusion of an inter-limb comparison within groups. The findings support that regardless of age, the ACLR group displayed significantly different kinetic and EMG variables compared to their contralateral limb; a finding that is not replicated within the healthy control group. Although this study was appropriately powered to evaluate differences in rate of knee extension moment amongst ACLR patients, it was not able to account for the influence of concomitant injury or gender on these outcomes.
Alterations in the timing of muscle activation relative to PKEM, along with reductions in the RKEM highlight a reduced ability for patients after ACLR to appropriately stabilize and control the lower extremity during a dynamic landing task compared to healthy counterparts. This is clinically relevant as it helps to identify areas where clinicians can potentially intervene to promote more optimal landing strategies during rehabilitation. Global quadriceps strengthening alone, without consideration of the neural deficits, has been shown to be insufficient in eliminating the inter-limb biomechanical deficits after ACLR. Based on the findings of the present study and previous work, it is clear that clinicians should consider the addition of therapies that can enhance neuromuscular control. Motor learning strategies such as gait retraining [8] and external cueing (e.g., biofeedback [2, 14]) are the two potential therapies that could be added to clinical practice that show promise in enhancing neuromuscular control after ACLR. Utilizing these motor learning strategies can help target and improve movement skill acquisition and quadriceps activation patterns that may allow patients to more appropriately attenuate forces upon landing [8]. Clinicians can feasibly integrate these treatment modalities into a structured rehabilitation program, prior to a patient resuming functional activities. This may help clinicians mitigate some of the common biomechanical and neuromuscular alterations observed in the years after ACLR.
Conclusion
Individuals after ACLR display deficits in PKEM and RKEM, which may negatively influence the ability to adequately attenuate forces during functional movement tasks. Further, reductions in peak muscle activity and a delay in quadriceps muscle onset time during the load-absorbing phase indicate that ACLR patients continue to experience altered neuromuscular control compared to healthy controls. Taken together, this work highlights that protracted impairments in knee mechanics and quadriceps activation strategies are present during the loading phase of a landing task after ACLR.
References
Besier TF, Lloyd DG, Ackland TR (2003) Muscle activation strategies at the knee during running and cutting maneuvers. Med Sci Sports Exerc 35:119–127
Bien DP, Dubuque TJ (2015) Considerations for late stage ACL rehabilitation and return to sport to limit re-injury risk and maximize athletic performance. Int J Sports Phys Ther 10:256–271
Borotikar BS, Newcomer R, Koppes R, McLean SG (2008) Combined effects of fatigue and decision making on female lower limb landing postures: central and peripheral contributions to ACL injury risk. Clin Biomech (Bristol, Avon) 23:81–92
Chang EW, Johnson S, Pollard C, Hoffman M, Norcross M (2018) Landing biomechanics in anterior cruciate ligament reconstructed females who pass or fail a functional test battery. Knee 25:1074–1082
Di Stasi SL, Logerstedt D, Gardinier ES, Snyder-Mackler L (2013) Gait patterns differ between ACL-reconstructed athletes who pass return-to-sport criteria and those who fail. Am J Sports Med 41:1310–1318
Friemert B, Franke S, Gollhofer A, Claes L, Faist M (2010) Group I afferent pathway contributes to functional knee stability. J Neurophysiol 103:616–622
Gokeler A, Hof AL, Arnold MP, Dijkstra PU, Postema K, Otten E (2010) Abnormal landing strategies after ACL reconstruction. Scand J Med Sci Sports 20:e12–19
Gokeler A, Neuhaus D, Benjaminse A, Grooms DR, Baumeister J (2019) Correction to: principles of motor learning to support neuroplasticity after ACL injury: implications for optimizing performance and reducing risk of second ACL injury. Sports Med 49:979
Grabiner MD, Weiker GG (1993) Anterior cruciate ligament injury and hamstrings coactivation. Clin Biomech (Bristol, Avon) 8:215–219
Holt NC, Danos N, Roberts TJ, Azizi E (2016) Stuck in gear: age-related loss of variable gearing in skeletal muscle. J Exp Biol 219:998–1003
Ithurburn MP, Paterno MV, Ford KR, Hewett TE, Schmitt LC (2017) Young athletes after anterior cruciate ligament reconstruction with single-leg landing asymmetries at the time of return to sport demonstrate decreased knee function 2 years later. Am J Sports Med 45:2604–2613
Johnson AK, Palmieri-Smith RM, Lepley LK (2018) Contribution of neuromuscular factors to quadriceps asymmetry after anterior cruciate ligament reconstruction. J Athl Train 53:347–354
Kline PW, Morgan KD, Johnson DL, Ireland ML, Noehren B (2015) Impaired quadriceps rate of torque development and knee mechanics after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med 43:2553–2558
Lepley AS, Gribble PA, Pietrosimone BG (2012) Effects of electromyographic biofeedback on quadriceps strength: a systematic review. J Strength Cond Res 26:873–882
Lepley AS, Gribble PA, Thomas AC, Tevald MA, Sohn DH, Pietrosimone BG (2016) Longitudinal evaluation of stair walking biomechanics in patients with ACL injury. Med Sci Sports Exerc 48:7–15
Lepley AS, Kuenze CM (2018) Hip and knee kinematics and kinetics during landing tasks after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. J Athl Train. https://doi.org/10.4085/1062-6050-334-16
Lewek M, Rudolph K, Axe M, Snyder-Mackler L (2002) The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 17:56–63
McLean SG, Fellin RE, Suedekum N, Calabrese G, Passerallo A, Joy S (2007) Impact of fatigue on gender-based high-risk landing strategies. Med Sci Sports Exerc 39:502–514
McLean SG, Lipfert SW, van den Bogert AJ (2004) Effect of gender and defensive opponent on the biomechanics of sidestep cutting. Med Sci Sports Exerc 36:1008–1016
Needle AR, Lepley AS, Grooms DR (2017) Central nervous system adaptation after ligamentous injury: a summary of theories, evidence, and clinical interpretation. Sports Med 47:1271–1288
Oberlander KD, Bruggemann GP, Hoher J, Karamanidis K (2013) Altered landing mechanics in ACL-reconstructed patients. Med Sci Sports Exerc 45:506–513
Palmieri-Smith RM, Lepley LK (2015) Quadriceps strength asymmetry after anterior cruciate ligament reconstruction alters knee joint biomechanics and functional performance at time of return to activity. Am J Sports Med 43:1662–1669
Palmieri-Smith RM, Thomas AC (2009) A neuromuscular mechanism of posttraumatic osteoarthritis associated with ACL injury. Exerc Sport Sci Rev 37:147–153
Pamukoff DN, Montgomery MM, Choe KH, Moffit TJ, Garcia SA, Vakula MN (2018) Bilateral alterations in running mechanics and quadriceps function following unilateral anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther 48:960–967
Pamukoff DN, Pietrosimone BG, Ryan ED, Lee DR, Blackburn JT (2017) Quadriceps function and hamstrings co-activation after anterior cruciate ligament reconstruction. J Athl Train 52:422–428
Paterno MV, Huang B, Thomas S, Hewett TE, Schmitt LC (2017) Clinical factors that predict a second ACL injury after ACL reconstruction and return to sport: preliminary development of a clinical decision algorithm. Orthop J Sports Med 5:2325967117745279
Pfeifer K, Banzer W (1999) Motor performance in different dynamic tests in knee rehabilitation. Scand J Med Sci Sports 9:19–27
Pietrosimone B, Blackburn JT, Padua DA, Pfeiffer SJ, Davis HC, Luc-Harkey BA et al (2018) Walking gait asymmetries 6 months following anterior cruciate ligament reconstruction predict 12-month patient-reported outcomes. J Orthop Res. https://doi.org/10.1002/jor.24056
Pietrosimone BG, McLeod MM, Lepley AS (2012) A theoretical framework for understanding neuromuscular response to lower extremity joint injury. Sports Health 4:31–35
Slater LV, Hart JM, Kelly AR, Kuenze CM (2017) Progressive changes in walking kinematics and kinetics after anterior cruciate ligament injury and reconstruction: a review and meta-analysis. J Athl Train 52:847–860
Snijders TAB (2005) Fixed and random effects. In: Everitt BS, Howell DC (eds) Encyclopedia of statistics in behavrioal science, vol 2. Wiley, Chicester, pp 664–665
Solnik S, DeVita P, Rider P, Long B, Hortobagyi T (2008) Teager-Kaiser Operator improves the accuracy of EMG onset detection independent of signal-to-noise ratio. Acta Bioeng Biomech 10:65–68
Solnik S, Rider P, Steinweg K, DeVita P, Hortobagyi T (2010) Teager-Kaiser energy operator signal conditioning improves EMG onset detection. Eur J Appl Physiol 110:489–498
Thomas AC, Hubbard-Turner T, Wikstrom EA, Palmieri-Smith RM (2016) Epidemiology of posttraumatic osteoarthritis. J Athl Train 52:491–496
Thomas AC, Lepley LK, Wojtys EM, McLean SG, Palmieri-Smith RM (2015) Effects of neuromuscular fatigue on quadriceps strength and activation and knee biomechanics in individuals post-anterior cruciate ligament reconstruction and healthy adults. J Orthop Sports Phys Ther 45:1042–1050
Trigsted SM, Post EG, Bell DR (2017) Landing mechanics during single hop for distance in females following anterior cruciate ligament reconstruction compared to healthy controls. Knee Surg Sports Traumatol Arthrosc 25:1395–1402
Welling W, Benjaminse A, Seil R, Lemmink K, Gokeler A (2018) Altered movement during single leg hop test after ACL reconstruction: implications to incorporate 2-D video movement analysis for hop tests. Knee Surg Sports Traumatol Arthrosc 26:3012–3019
Wren TAL, Mueske NM, Brophy CH, Pace JL, Katzel MJ, Edison BR et al (2018) Hop distance symmetry does not indicate normal landing biomechanics in adolescent athletes with recent anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther 48:622–629
Acknowledgements
This study was in part supported by the International Society of Biomechanics Student Congress Travel Grant 2019.
Funding
No funding was provided for the completion of this work.
Author information
Authors and Affiliations
Contributions
JB, AL, and LL are responsible for the design of the study. JB and LF were responsible for all recruitment and data collection procedures. JB, AL, LF and LL participated in the data analysis and interpretation. JB and LL drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval was agreed by the ethical committee of the institution.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This study was approved by the University of Connecticut’s Institutional Review Board.
Rights and permissions
About this article
Cite this article
Burland, J.P., Lepley, A.S., Frechette, L. et al. Protracted alterations in muscle activation strategies and knee mechanics in patients after Anterior Cruciate Ligament Reconstruction. Knee Surg Sports Traumatol Arthrosc 28, 3766–3772 (2020). https://doi.org/10.1007/s00167-019-05833-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-019-05833-4