Abstract
Purpose
Progression of osteoarthritis over time is poorly understood. The aim of the current study was to establish a timeline of “cartilage survival rate” per subregion of the knee in relation to mechanical alignment of the lower extremity. The study hypothesized that there are differences in progression of osteoarthritis between varus, valgus and physiologic lower extremity alignment.
Methods
Based on hip-knee-ankle standing radiographs at baseline, 234 knees had physiologic (180° ± 3°, mean 179.7°), 158 knees had varus (< 177°; mean 174.5°) and 66 knees valgus (> 183°; mean 185.2°) alignment (consecutive knees of the OAI “Index Knee” group, n = 458; mean age 61.7; 264 females). The Osteoarthritis Initiative (OAI; a multi-center, longitudinal, prospective observational study of knee osteoarthritis [30] using MRIs) defines progressive OA as a mean decrease of cartilage thickness of 136 µm/year and a mean decrease of cartilage volume by 5% over 1 year (DESS sequences, MRI). A Kaplan–Meier curve was generated for osteoarthritis progression based on OAI criteria.
Results
Osteoarthritis progression based on volume decrease of 5% in varus knees occurred after 30.8 months (medial femoral condyle), after 37 months (medial tibia), after 42.9 months (lateral femoral condyle) and 43.4 months (lateral tibia), respectively. In a valgus alignment progression was detectable after 31.5 months (lateral tibia), after 36.2 months (lateral femoral condyle), after 40.4 months (medial femoral condyle) and 43.8 months (medial tibia), respectively. The physiological alignment shows a progression after 37.8 months (medial femoral condyle), after 41.6 months (lateral tibia), after 41.7 months (medial tibia) and after 43 months (lateral femoral condyle), respectively.
Conclusion
Based on data from the OAI, the rate and location (subregion) of osteoarthritis progression of the knee is strongly associated with lower extremity mechanical alignment.
Level of evidence
Level I (prognostic study).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Knee osteoarthritis (OA) has a global prevalence of 3.8% [25] and is a major source of socioeconomic costs [18]. It has an increasing burden (years lived with disorder) of 64% over the last 2 decades [25] and is the most common cause of knee arthroplasty [2, 3].
In the past, an indirect approximation of articular cartilage measuring the minimum joint space width on radiographs represents the standard to detect progression of knee OA [1, 12]. Measuring minimum joint space width on radiographs is an indirect approach and is subject to error secondary to reproducibility of position of the joint, beam alignment, and distance between the joint and film [23]. The current gold standard to detect progression of knee OA is magnetic resonance imaging (MRI) [12, 40, 43]. MRI provides excellent soft tissue imaging and is accurate in measuring cartilage volume, thickness, and surface area [11, 14, 41, 42].
The Osteoarthritis Initiative (OAI) is a public free of charge database used to conduct longitudinal studies using prospective MRI data to analyze cartilage alterations in knee OA. A number of studies [13, 14, 26, 28, 42, 43] reported on changes in cartilage volume and thickness in ten tibial and six femoral subregions. Maschek et al. [26] showed a minimum loss of cartilage thickness of 136 µm in the most affected subregion (OV1) in knees with Kellgren and Lawrence grade (KLG) 2–4. Wluka et al. [45] defined OA progression as annual change in cartilage volume of 5.3%/year.
A number of factors are associated with progression of OA (age, previous knee injury, obesity) [18, 37]. Inter alia (BMI, physical activity, meniscal pathologies, cartilage or bone marrow lesions) [4, 24], limb alignment represents a risk factor for progression of OA of the knee [17, 20, 34,35,36]. Sharma et al. [36] showed a fourfold higher risk of OA progression with varus alignment (> 5°) and a odds ratio of 4.89 for progression of OA in valgus alignment (> 5°).
Although the quantitative loss of cartilage thickness and volume has been described in previous studies, nobody has studied yet, to our knowledge, the survival rate (Kaplan–Meier survival rate) over the time and can show a detailed timeline of cartilage loss in subregions of the knee.
Based on the OAI dataset, the study wants to describe a detailed timeline of cartilage survival. Progression of OA was analyzed to answer the following research questions: (1) Does mechanical alignment of the lower limb affect progression of OA (timeline of cartilage survival) in the different subregions of the knee? (2) Are there differences in the change of cartilage thickness and cartilage volume between the subregions of the knee?
Materials and methods
Cohort
The study investigates the Osteoarthritis Initiative (OAI) database, a multi-center, longitudinal, prospective observational study of knee OA [30]. A total of 4791 subjects aged between 45 and 79 years at risk of developing knee OA were enrolled in the OAI. Annual radiographs, MRI, and clinical assessment of knees and disease activity were performed for all participants over a period of 8 years.
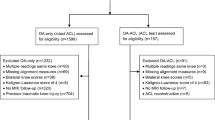
The “Core Image Assessment sample” at OAI was designed to provide longitudinal structural outcomes. 600 knees, part of the “Core Image Assessment sample”, were defined as “Index knees”: symptomatic knees with Kellgren and Lawrence (K&L) grades 2 or 3 radiographic evidence of OA at baseline [30]. Eckstein et al. [13, 42] performed quantitative cartilage measurements on MRI (sagittal DESS sequence) of these knees at baseline, 12, 24 and 48 months (kMRI quant Cart sagDESS, Project 9A) [31]. Of the 600 index knees, the hip-knee-ankle standing radiographs were assessed to determine limb alignment (available at a single measurement at 12 months by Cooke et al. [30] (“flXR Knee Alignment Cooke”), ICC 0.99). 142 knees were excluded because no alignment measurements were available leaving 458 knees to be included in the current study. Subjects were grouped based on lower limb alignment into varus alignment (n = 158, hip-knee-ankle angle < 177°), physiologic alignment (n = 234, hip-knee-ankle angle 180° ± 3°) and valgus alignment (n = 66, hip-knee-ankle angle > 183°). 458 knees were available for analysis at baseline, 451 knees at 12 months, 458 at 24 months and 56 at 48 months.
Image analyses
The cartilage volume and cartilage thickness were analyzed on sagittal double-echo steady-state (DESS) sequences. Reporting details and nomenclature for MRI evaluation are reported on the OAI website [30, 31] and by Eckstein et al. [10]. The medial and lateral femorotibial cartilage was segmented manually, using proprietary software (Chondrometrics GmbH), with two readers blinded to disease severity, alignment, and the order of image acquisition [44]. One expert reader, with more than 5 years of MRI-based cartilage segmentation experience, performed quality control readings of all segmentations.
The following cartilage volume parameters were analyzed (Fig. 1): central medial femur (total, OAI variable “BMFVCL”, in mm3 and normalized, “BMFVCN”, in mm), central lateral femur (total, “BLFVCL”, in mm3 and normalized, “BLFVCN”, in mm), central medial tibia (total, “WMTVCL”, in mm3 and normalized, “WMTVCN”, in mm) and central lateral tibia (total, “WLTVCL”, in mm3 and normalized, “WLTVCN”, in mm). The following thickness parameters (in mm) as femoral subregions were analyzed: central area of the weight-bearing lateral femur (“BLFMTH”), central area of the weight-bearing medial femur (“CBMFMTH”), internal part of the weight-bearing medial femur (“IBMFMTH”), external part of the weight-bearing medial femur (“EBMFMTH”). Following thickness parameters (in mm) as tibial subregions were analyzed: internal part of the weight-bearing medial (“IMTMTH”) and lateral (“ILTMTH”) tibia, central area of the medial (“CMTMTH”) and lateral (“CLTMTH”) tibia, external area of the medial (“EMTMTH”) and lateral (“ELTMTH”) tibia, anterior part of the medial (“AMTMTH”) and lateral (“ALTMTH”) tibia as well as posterior area of the medial (“PMTMTH”) and lateral (“PLTMTH”) tibia.
The analyzed subregions at the tibia (left) and the femur (right). Central medial tibia (cMT) includes variables “WMTVCL”, “WMTVCN” and “CMTMTH”, external medial tibia (eMT) includes “EMTMTH”, internal medial tibia (iMT) includes “IMTMTH”, anterior medial tibia (aMT) includes “AMTMTH”, posterior medial tibia (pMT) includes “PMTMTH”. At the lateral tibia following subregions with variables are described: central lateral tibia (cLT) with variables “WLTVCL”, “WLTVCN” and “CLTMTH”; external lateral tibia (eLT) with “ELTMTH”; internal lateral tibia (iLT) with “ILTMTH”; anterior lateral tibia (aLT) with “ALTMTH” and posterior lateral tibia (pLT) with “PLTMTH”. At the femur subregion central medial femur (ccMF) includes variables “BMFVCL”, “BMFVCN” and “CBMFMTH”; external central medial femur (ecMF) includes “EBMFMTH”, internal central medial femur (icMF) includes “IBMFMTH”; on the lateral femur subregion central lateral femur (ccLF) with “BLFVCL”, “BLFVCN” and “BLFMTH” is analyzed
Limb alignment (varus, valgus, physiologic) was identified on hip-knee-ankle radiographs (parameter: flXR_KneeAlign_Cooke01). Frontal alignment was defined as the angle between the mechanical axes of the femur and tibia [6, 7].
In the current study, progression of knee OA per year was defined by a minimum thickness loss of 136 µm [26] or/with volume loss of minimum 5% [45].
The study received IRB approval by the institutional review board at the authors’ institution (Hospital for Special Surgery, NY, USA, IRB #15013).
Statistical analyses
Demographics are shown in Table 1. Possible confounders (age, BMI and previous surgery) are analyzed using Student’s t test (BMI, age) and Pearson’s Chi square test (previous surgery) (Table 2).
Kaplan–Meier survival curves were generated for cartilage survival. A loss of more than 136 µm or 5% of volume was counted as progression of OA (“event” in Kaplan–Meier survival analysis). Cases without an event were censored. For every subregion (volume: 2 femoral, 2 tibial; thickness: 4 femoral, 10 tibial), three survival curves (alignment dependent: varus, valgus and physiological) were prepared. Significant differences in survival time between each subregion and/or alignment type were determined by log-rank tests. Effect size was calculated according to Hedge’s g. A p value of less than 0.05 was considered to be statistically significant. All statistical analyses were performed using IBM SPSS Statistics software version 23 (Armonk, NY: IBM Corp.).
Results
Comparison between alignments showed statistical significant differences (Tables 3, 4), effect sizes showed a large effect in every comparison (Hedge’s g 2.13–9.03), except at the lateral femoral condyle (varus vs physiologic alignment Hedge’s g 0.15).
Cartilage thickness survival rates and subregion differences in varus knees are demonstrated in Table 5. Table 6 summarizes the cartilage thickness survival rates and each difference between subregions in knees with valgus alignment. Physiologic aligned osteoarthritic knees showed differences in cartilage thickness survival rates as well as in their subregion analyses (Table 7).
Varus knees showed shortest cartilage volume survival rates at the medial femur (30.8 months, CI 95% 28.2–33.4), followed by the medial tibia (36.9 months, CI 95% 34.5–39.4) (Table 3). Valgus knees showed shortest cartilage volume survival at the lateral tibia (31.5 months, CI 95% 27.6–35.4) and at the lateral femur (36.2 months, CI 95% 32.4–40) (Table 7). Physiologic aligned osteoarthritic knees showed shortest volume survival rates at the medial femur (37.8 months, CI 95% 36–39.7) (Table 8).
Discussion
The main finding of the current study, as one of the first, is the detection of a cartilage survival rate over time (Kaplan–Meier) and shows a strong relation to malalignment. Varus and valgus alignment accelerate the OA progression rate compared to osteoarthritic knees with physiologic alignment. Differences of cartilage survival in subregions contribute to more precisely understanding of an “OA-progression-timeline” and create a “time-map” of cartilage survival.
The study reveals that the cartilage survival is strongly influenced by limb alignment similar to prior reports in the literature [27, 28, 34, 36]. The current study suggests that in knees with physiologic alignment, a more homogeneous progression of OA is observed compared to the varus or valgus group. In contrast, varus knees show a broader time range of the cartilage loss in the anterior and posterior subregion of the medial compartment suggesting that little posterior translation of the femur occurs. In valgus knees, however, posterior translation characterizes the progression of OA. These findings might be explained by differences in normal kinematics of the knee with a relatively constant medial condyle and posterior translation of the lateral condyle contact point during a knee bending [8, 9, 38].
In relation to cartilage thickness in varus and valgus alignment, a statistical significant difference of survival rates can be shown in the anterior and posterior subregion of the lateral compartment (lateral tibia anterior: p = 0.014; lateral tibia posterior: p = 0.002). This suggests that in valgus knees an anterior to posterior translation characterizes the progression of OA, whereas in varus knees direction of progression is in medial direction (medial tibia central: p = 0.001 medial external tibia: p = 0.001).
A closer look into differences between subregions in each alignment type shows in varus knees in the central medial tibia and central medial femur the shortest survival rates. Not surprisingly, external subregions on the medial tibia and femur are affected early too and the shortest survival rate of the contralateral compartment was determined in the internal lateral tibia. This might be related to beginning medial subluxation [21, 22].
The last affected regions were anterior and posterior. These findings lead to following assumptions: (1) prior studies reported that insufficiency of the ACL is a main factor for posterior medial wear in varus OA knees [19, 29]. Therefore, degeneration of the ACL may not be present in the majority of subjects in the OAI cohort. (2) Anteromedial wear pattern in varus OA knees are reported in anatomical studies analyzing bone wafers collected from total knee arthroplasties [19, 32, 39]. However, these findings cannot be confirmed with OAI–MRI measurements. Rajgopal et al. [32] described an anteromedial pattern of varus OA knees, although 87% showed an intact ACL. In 99.5%, anteromedial wear was present. White et al. [39] described ACL sufficient knees with anteromedial wear pattern in 40% (zone B) and in 43% with central medial wear pattern (zone BC).
These differences to the current study might be an expression of different definitions of medial tibial subregions. Rajgopal et al. [32] described three medial tibial subregions, White et al. [39] defined four medial tibial subregions, from anterior to posterior, respectively. Exact cutoff values were not reported. On the other hand, Raju et al. [33] showed in a cadaver study a more central medial cartilage wear. Biswal et al. [5] showed in a longitudinal MRI study that cartilage lesions located in the central region of the medial compartment progress faster than cartilage lesions in the anterior and posterior portions of the medial compartment. Everhart et al. [16] described a more rapid progress of cartilage defects in the medial compartment than in the lateral compartment.
In valgus OA knees, lateral central and internal tibial cartilage thickness shows the shortest survival rates. Lateral femoral cartilage thickness shows a significant cartilage loss relatively late. The shortest survival time at the tibial subregions, followed by femoral subregions is an important differentiation to varus aligned knees. Eckstein et al. [15] also showed the highest amount of cartilage loss at the internal and central lateral tibia followed by lateral femoral subregions in valgus knees. In valgus knees, the contralateral medial compartment shows a mean thickness cartilage survival rate of 43.7 months (medial tibial subregions), whereas in varus knee the contralateral compartment shows a mean thickness cartilage survival rate of 41.4 months (lateral tibial subregions). These findings suggest that the valgus osteoarthritic remains longer a unicompartmental disease than knees with varus OA.
Physiologic aligned osteoarthritic knees show a very homogeneous pattern of cartilage thickness loss. Central femoral cartilage thickness shows shortest cartilage survival rate followed by the central lateral tibial. The longest survival rate was detected in the posterior medial tibial subregion (46.2 months). A comparable pattern to varus aligned knees (central medial femur, central medial tibia and external medial tibia) or valgus aligned knees (central lateral tibia, internal lateral tibia and external lateral tibia) could not been shown.
The current study has several limitations. First, the cutoff values (cartilage loss of 136 µm thickness or 5% volume over 1 year) are described in recent studies, but are selected randomly in the current study. Second, these cutoff values are signs of cartilage-loss’ only, they do not predict progression of clinical symptoms as increased pain, decreased range of motion or decreased quality of life. The cutoff values are no disease value itself.
Conclusion
Data of the current study suggest that in varus OA, the main cartilage loss occurs on the medial femur (central and external), medial tibia (central and external) and internal lateral tibia. In valgus knees, the lateral tibia (central subregion first, followed by internal and external subregion) with a posterior component are affected first. This study is the first to determine the rate of OA progression depending on lower limb alignment. Therefore, we can now inform our patients in detail about when a significant loss of cartilage is to be expected and in which subregion this will occur. The therapy regimen may need to be adapted after reaching the cutoff values. OA progression occured after an average of 37.6 months in physiologic aligned knees, 31.2 months in varus knees and 30 months in valgus knees. This confirms a considerable influence of limb alignment on cartilage survival.
References
Altman RD, Brandt K, Hochberg M, Moskowitz R (1996) Design and conduct of clinical trials in patients with osteoarthritis: recommendations from a task force of the Osteoarthritis Research Society. Osteoarthr Cartil 4:217–243
Annual Report 2014. The Swedish Knee Arthroplasty Register. http://www.myknee.se/en/. Accessed 6 Jan 2019
Annual Report 2014. The Australian Orthopaedic Association National Joint Replacement Registry. https://aoanjrr.sahmri.com/annual-reports-2014. Accessed 6 Jan 2019
Beckwee D, Vaes P, Shahabpour M, Muyldermans R, Rommers N, Bautmans I (2015) The influence of joint loading on bone marrow lesions in the knee: a systematic review with meta-analysis. Am J Sports Med 43(12):3093–3107
Biswal S, Hastie T, Andriacchi TP, Bergman GA, Dillingham MF, Lang P (2002) Risk factors for progressive cartilage loss in the knee: a longitudinal magnetic resonance imaging study in forty-three patients. Arthritis Rheum 46(11):2884–2892
Cooke TD, Harrison L, Khan B, Scudamore A, Chaudhary MA (2002) Analysis of limb alignment in the pathogenesis of osteoarthritis: a comparison of Saudi Arabian and Canadian cases. Rheumatol Int 22(4):160–164
Cooke TD, Sled EA, Scudamore RA (2007) Frontal plane knee alignment: a call for standardized measurement. J Rheumatol 34(9):1796–1801
Dennis DA, Komistek RD, Mahfouz MR, Haas BD, Stiehl JB (2003) Multicenter determination of in vivo kinematics after total knee arthroplasty. Clin Orthop Relat Res (416):37–57
Dennis DA, Mahfouz MR, Komistek RD, Hoff W (2005) In vivo determination of normal and anterior cruciate ligament-deficient knee kinematics. J Biomech 38(2):241–253
Eckstein F, Ateshian G, Burgkart R, Burstein D, Cicuttini F, Dardzinski B, Gray M, Link TM, Majumdar S, Mosher T, Peterfy C, Totterman S, Waterton J, Winalski CS, Felson D (2006) Proposal for a nomenclature for magnetic resonance imaging based measures of articular cartilage in osteoarthritis. Osteoarthr Cartil 14(10):974–983
Eckstein F, Kwoh CK, Link TM, investigators OAI (2014) Imaging research results from the Osteoarthritis Initiative (OAI): a review and lessons learned 10 years after start of enrolment. Ann Rheum Dis 73(7):1289–1300
Eckstein F, Le Graverand MH (2015) Plain radiography or magnetic resonance imaging (MRI): which is better in assessing outcome in clinical trials of disease-modifying osteoarthritis drugs? Summary of a debate held at the World Congress of Osteoarthritis 2014. Semin Arthritis Rheum 45(3):251–256
Eckstein F, Maschek S, Wirt W et al (2009) One year change of knee cartilage morphology in the first release of participants from the Osteoarthritis Initiative progression subcohort—association with sex, body mass index, symptoms, and radiographic OA status. Ann Rheum Dis 68(5):674–679
Eckstein F, Nevitt M, Gimona A, Picha K, Lee JH, Davies RY, Dreher D, Benichou O, Le Graverand MP, Hudelmaier M, Maschek S, Wirth W, Osteoarthritis Initiative Investigators G (2011) Rates of change and sensitivity to change in cartilage morphology in healthy knees and in knees with mild, moderate, and end-stage radiographic osteoarthritis: results from 831 participants from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 63(3):311–319
Eckstein F, Wirth W, Hudelmaier M, Stein V, Lengfelder V, Cahue S, Marshall M, Prasad P, Sharma L (2008) Patterns of femorotibial cartilage loss in knees with neutral, varus, and valgus alignment. Arthritis Rheum 59(11):1563–1570
Everhart JS, Abouljoud MM, Poland SG, Flanigan DC (2018) Medial compartment defects progress at a more rapid rate than lateral cartilage defects in older adults with minimal to moderate knee osteoarthritis (OA): data from the OA initiative. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-018-5202-1
Felson DT, Niu J, Gross KD, Englund M, Sharma L, Cooke TD, Guermazi A, Roemer FW, Segal N, Goggins JM, Lewis CE, Eaton C, Nevitt MC (2013) Valgus malalignment is a risk factor for lateral knee osteoarthritis incidence and progression: findings from the Multicenter Osteoarthritis Study and the Osteoarthritis Initiative. Arthritis Rheum 65(2):355–362
Glyn-Jones S, Palmer AJR, Agricola R, Price AJ, Vincent TL, Weinans H, Carr AJ (2015) Osteoarthritis. Lancet 386(9991):376–387
Harman MK, Markovich GD, Banks SA, Hodge WA (1998) Wear patterns on tibial plateaus from varus and valgus osteoarthritic knees. Clin Orthop Relat Res 352:149–158
Hunter DJ, Zhang Y, Niu J, Goggins J, Amin S, LaValley MP, Guermazi A, Genant H, Gale D, Felson DT (2006) Increase in bone marrow lesions associated with cartilage loss: a longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum 54(5):1529–1535
Khamaisy S, Nam D, Thein R, Rivkin G, Liebergall M, Pearle A (2014) Limb alignment, subluxation, and bone density relationship in the osteoarthritic varus knee. J Knee Surg 28(3):207–212
Khamaisy S, Zuiderbaan HA, Thein R, Nawabi DH, Joskowicz L, Pearle AD (2014) Coronal tibiofemoral subluxation: a new measurement method. Knee 21(6):1069–1071
LaValley MP, McAlindon TE, Chaisson CE, Levy D, Felson DT (2001) The validity of different definitions of radiographic worsening for longitudinal studies of knee osteoarthritis. J Clin Epidemiol 54(1):30–39
Lim YZ, Wang Y, Wluka AE, Davies-Tuck ML, Teichtahl A, Urquhart DM, Cicuttini FM (2013) Are biomechanical factors, meniscal pathology, and physical activity risk factors for bone marrow lesions at the knee? A systematic review. Semin Arthritis Rheum 43(2):187–194
March L, Smith EU, Hoy DG, Cross MJ, Sanchez-Riera L, Blyth F, Buchbinder R, Vos T, Woolf AD (2014) Burden of disability due to musculoskeletal (MSK) disorders. Best Pract Res Clin Rheumatol 28(3):353–366
Maschek S, Wirth W, Ladel C, Hellio Le Graverand MP, Eckstein F (2014) Rates and sensitivity of knee cartilage thickness loss in specific central reading radiographic strata from the Osteoarthritis Initiative. Osteoarthr Cartil 22(10):1550–1553
Moyer R, Wirth W, Duryea J, Eckstein F (2016) Anatomical alignment, but not goniometry, predicts femorotibial cartilage loss as well as mechanical alignment: data from the Osteoarthritis Initiative. Osteoarthr Cartil 24(2):254–261
Moyer R, Wirth W, Eckstein F (2017) Longitudinal changes in magnetic resonance imaging-based measures of femorotibial cartilage thickness as a function of alignment and obesity: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 69(7):959–965
Mullaji AB, Marawar SV, Luthra M (2008) Tibial articular cartilage wear in varus osteoarthritic knees: correlation with anterior cruciate ligament integrity and severity of deformity. J Arthroplasty 23(1):128–135
Osteoarthritis-Initiative (2015) http://oai.epi-ucsf.org/datarelease/StudyOverview.asp. Accessed 12 Dec 2015
Osteoarthritis-Initiative (2015) https://oai.epi-ucsf.org/datarelease/ImageAssessments.asp. Accessed 12 Dec 2015
Rajgopal A, Noble PC, Vasdev A, Ismaily SK, Sawant A, Dahiya V (2015) Wear patterns in knee articular surfaces in varus deformity. J Arthroplasty 30(11):2012–2016
Raju PK, Kini SG, Verma A (2012) Wear patterns of tibiofemoral articulation in osteoarthritic knees: analysis and review of literature. Arch Orthop Trauma Surg 132(9):1267–1271
Sharma L, Chmiel JS, Almagor O, Felson D, Guermazi A, Roemer F, Lewis CE, Segal N, Torner J, Cooke TD, Hietpas J, Lynch J, Nevitt M (2013) The role of varus and valgus alignment in the initial development of knee cartilage damage by MRI: the MOST study. Ann Rheum Dis 72(2):235–240
Sharma L, Eckstein F, Song J, Guermazi A, Prasad P, Kapoor D, Cahue S, Marshall M, Hudelmaier M, Dunlop D (2008) Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum 58(6):1716–1726
Sharma L, Song J, Felson D, Cahue S, Shamiyeh E, Dunlop D (2001) The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA 286:188–195
Silverwood V, Blagojevic-Bucknall M, Jinks C, Jordan JL, Protheroe J, Jordan KP (2015) Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthr Cartil 23(4):507–515
Weidow J, Pak J, Kärrholm J (2002) Different patterns of cartilage wear in medial and lateral gonarthrosis. Acta Orthop Scand 73:326–329
White SH, Ludkowski PF, Goodfellow JW (1991) Anteromedial osteoarthritis of the knee. J Bone Jt Surg Br 73:582–586
Wirth W, Duryea J, Hellio Le Graverand MP, John MR, Nevitt M, Buck RJ, Eckstein F, Group OAII (2013) Direct comparison of fixed flexion, radiography and MRI in knee osteoarthritis: responsiveness data from the Osteoarthritis Initiative. Osteoarthr Cartil 21(1):117–125
Wirth W, Eckstein F (2008) A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging 27(6):737–744
Wirth W, Hellio Le Graverand MP, Wyman BT, Maschek S, Hudelmaier M, Hitzl W, Nevitt M, Eckstein F, Group OAII (2009) Regional analysis of femorotibial cartilage loss in a subsample from the Osteoarthritis Initiative progression subcohort. Osteoarthr Cartil 17(3):291–297
Wirth W, Larroque S, Davies RY, Nevitt M, Gimona A, Baribaud F, Lee JH, Benichou O, Wyman BT, Hudelmaier M, Maschek S, Eckstein F, Group OAII (2011) Comparison of 1-year vs 2-year change in regional cartilage thickness in osteoarthritis results from 346 participants from the Osteoarthritis Initiative. Osteoarthr Cartil 19(1):74–83
Wirth W, Nevitt M, Hellio Le Graverand MP, Benichou O, Dreher D, Davies RY, Lee J, Picha K, Gimona A, Maschek S, Hudelmaier M, Eckstein F, Investigators OAI (2010) Sensitivity to change of cartilage morphometry using coronal FLASH, sagittal DESS, and coronal MPR DESS protocols—comparative data from the Osteoarthritis Initiative (OAI). Osteoarthr Cartil 18(4):547–554
Wluka AE, Stuckey S, Snaddon J, Cicuttini FM (2002) The determinants of change in tibial cartilage volume in osteoarthritic knees. Arthritis Rheum 46(8):2065–2072
Funding
No funding was used to conducting this study. Outside the submitted work Dr. Boettner reports personal fees from Smith & Nephew, personal fees from Ortho Development Corporation, personal fees from Depuy and Dr. Faschingbauer reports personal fees from Deutsche Forschungsgemeinschaft (Research Fellowship, FA 1271/1-1, www.dfg.de).
Author information
Authors and Affiliations
Contributions
FM: planning/conception of the study, collection of data, analysis and interpretation, statistical analysis, writing and revising article; (orthopedic surgeon). KM: collection of data, analysis and interpretation, statistical analysis; (resident). SP, WW: collection of data, critical revision of the article; (resident, orthopedic surgeon). RH, BF: critical revision of the article, final approval of the article, overall responsibility; (surgeon in chief University of Ulm; orthopedic surgeon, attending at HSS).
Corresponding author
Ethics declarations
Conflict of interest
We certify that we have not signed any agreement with commercial interest related to this study, which would in any way limit publication of any and all data generated for the study or to delay publication for any reason. Dr. Boettner reports personal fees from Smith & Nephew, personal fees from Ortho Development Corporation, personal fees from Depuy, outside the submitted work. Dr. Faschingbauer reports personal fees from Deutsche Forschungsgemeinschaft (Research Fellowship, FA 1271/1-1, http://www.dfg.de), during the conduct of the study.
Ethical statement
The authors’ institutional review board approved this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Faschingbauer, M., Kasparek, M., Waldstein, W. et al. Cartilage survival of the knee strongly depends on malalignment: a survival analysis from the Osteoarthritis Initiative (OAI). Knee Surg Sports Traumatol Arthrosc 28, 1346–1355 (2020). https://doi.org/10.1007/s00167-019-05434-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-019-05434-1