Abstract
Purpose
Neurovascular structures around the ankle are at risk of injury during arthroscopic all-inside lateral collateral ligament repair for the treatment of chronic ankle instability. This study aimed to evaluate the risk of damage to anatomical structures and reproducibility of the technique amongst surgeons with different levels of expertise in the arthroscopic all-inside ligament repair.
Methods
Twelve fresh-frozen ankle specimens were used for the study. Two foot and ankle surgeons with different level of experience in the technique performed the procedure on 6 specimens each. The repair was performed following a standardized procedure as originally described. Then, an experienced anatomist dissected all the specimens to evaluate the outcome of the ligament repair, any injuries to anatomical structures and the distance between arthroscopic portals and the superficial peroneal nerve (SPN) and sural nerve.
Results
Dissections revealed no injury to the nerves assessed. Mean distance from the anterolateral portal and the SPN was of 4.8 (range 0.0–10.4) mm. The mean distance from the accessory anterolateral portal to the SPN and sural nerve was of 14.2 (range 7.1–32.9) mm and 28.1 (range 2.8–39.6) mm, respectively. The difference between the 2 surgeons’ groups was non-statistically significant for any measurement (mm). In all specimens both fascicles of the anterior talofibular ligament were reattached onto its original fibular footprint. The calcaneofibular ligament was not penetrated in any specimen.
Conclusions
The all-inside arthroscopic lateral collateral ligament repair is a safe and reproducible technique. The clinical relevance of this study is that this technique provides a safe and anatomic reattachment of the anterior talofibular ligament, with minimal risk of injury to surrounding anatomical structures regardless of the level of experience with the technique.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of arthroscopy in treating ankle instability has rapidly evolved in the last decade alongside arthroscopic stabilizing techniques. These techniques can be broadly divided into three groups: (1) an arthroscopically assisted percutaneous technique [1, 2], (2) an arthroscopic all-inside ligament repair [3], and (3) an arthroscopic ligamentoplasty [4, 5]. The clinical results of the arthroscopic techniques have proven similar or superior to open procedures [6]. The first technique to use arthroscopy for lateral ligament repair demonstrated good results but also a potential for complications due to nerve entrapment by the suture during the percutaneous gesture [1, 2, 7].
In 2013 Vega et al. were the first to propose an anatomic and arthroscopic all-inside lateral collateral ligament repair with a knotless anchor. Excellent clinical results were observed in the 16 patients of the study, and no major or neurological complications were reported [3]. The absence of nerve-related complications may be explained by the fully intra-articular technique [6].
Although a previous anatomic study validated the safety of the arthroscopic-assisted percutaneous procedure for the treatment of chronic ankle instability [8], to our knowledge this is the first anatomic study validating the arthroscopic all-inside lateral ligament repair.
The aim of this study was to evaluate the risk of damage and entrapment of neurological structures during the arthroscopic all-inside lateral collateral ligament repair. In addition, reproducibility between surgeons with a different level of expertise in this technique has been assessed to evaluate the learning curve.
It was hypothesized that the arthroscopic all-inside lateral ligament repair is a safe and reproducible technique, even in the hands of a less experienced surgeon.
Materials and methods
Twelve unpaired fresh frozen ankle specimens were used for the study (7 right and 5 left). Specimens were amputated below the knee and included 7 males and 5 females; mean age was 72 years (range 60–89 years).
Specimens with evident ankle stiffness or instability, deformities or signs of previous surgery were excluded from the study. Arthroscopic observation of an absent lateral collateral ligament was also an exclusion criterium. A partially or completely torn ligament was not considered a reason for exclusion.
Two foot and ankle surgeons experienced in ankle arthroscopy, but with different level of experience in the arthroscopic all-inside lateral ligament repair, performed the procedure in a protocolized manner as previously described [3, 9]. While the senior surgeon had an experience of over 300 cases treated, the junior had treated less than 10 patients. Each surgeon performed 6 cases independently with the same assistant in each procedure.
The specimens were held by the tibia with a specific device that allowed free ankle movement. Ankle dorsiflexion and no-distraction arthroscopic technique was performed.
Instruments used included an arthroscopic pump, a 4 mm 30° arthroscope, and a 3.5 mm motorized shaver. Specific instruments to perform the arthroscopic all-inside ligament repair branded Arthrex, Naples, FL included an automatic suture passer—MiniScorpion—and a non-automatic suture passer—Microsuture lasso curved 70°—two high resistance and non-absorbable sutures—#0 Fiberwire—and a knotless anchor—Pushlock 2.9 mm × 15 mm. Finally, one cannula—Passport cannula 6 mm × 20 mm—was used and introduced through the anterolateral portal to protect the superficial peroneal nerve during the procedure.
Arthroscopic procedure
Cutaneous landmarks were highlighted, and the superficial peroneal nerve was identified when possible with forced inversion of the ankle and 4th toe plantarflexion [10]. Standard anteromedial and anterolateral portals were created. An accessory anterolateral portal was placed 1–1.5 cm proximal to the fibular tip and just anterior to it. The arthroscope was introduced through the anteromedial portal, and the ATFL was identified occupying the floor of the lateral gutter. The ATFL and CFL were completely detached off the fibular origin with the help of an osteotome introduced through the anterolateral portal. Any remnant tissue left on the ATFL and CFL fibular footprints was debrided with a shaver to simulate a chronic ligament tear. Attention was paid not to open the joint capsule or excise any ligamentous tissue. A complete detachment of the ATFL and CFL was considered satisfactory when the fibular footprint areas were observed which are located over the distal and lateral aspects of the fibula.
At this point the procedure was carried out as reported in the original technique [3, 9]. With the ATFL and CFL detached from their insertion, the ligamentous tissue was grasped with the suture passer. First, a non-automatic suture passer was used. It was introduced through the anterolateral portal, and under direct arthroscopic visualization, the maximum possible amount of ligamentous tissue was penetrated from lateral to medial. Then, the suture passer was exchanged by the suture using a nitinol loop. Second, the automatic suture passer was used. The suture was charged into the grasping claw and the claw was introduced through the anterolateral portal, opened inside the joint, and grasped the capsule aiming to catch the maximum possible tissue. To protect surrounding structures, the tissue needs to be penetrated from lateral to medial inserting the needle inside the joint. The end result after removing the suture passer was the tissue being grasped by the suture.
Finally, the ligamentous tissue was reattached using the suture with one knotless anchor into the fibula through a previously drilled bone tunnel. The placement of the anchor in the fibula was determined by the ATFL footprint, which is located just distal to the fibular insertion of the anterior tibio-fibular ligament. Both suture ends were introduced inside the eyelet of the anchor and tensioned before the anchor was introduced. Then, the sutures were cut with arthroscopic scissors.
Anatomic study
After the arthroscopic procedure, specimens were dissected by an experienced anatomist as previously suggested [11]. An anterolateral skin window was created in the ankle. The perimeter of the window extended from the tibialis anterior to the calcaneal tendon and from 3 cm proximal to 3 cm distal of the tip of the fibula. Superficial dissection was performed first, preserving the anatomical plane of the crural fascia and the neurological structures lying on it. This allowed an initial evaluation of any entrapment of neurological structures and their relationship with the arthroscopic portals. A digital caliper (Ratio® 6369 H 15 150 mm -Ehlis S.A., 08740, Sant Andreu de la Barca, Barcelona, Spain, precision 0.01 mm) was used to measure the following: distance between the anterolateral portal and the superficial peroneal nerve, and distance between the accessory anterolateral portal and the superficial peroneal nerve as well as the sural nerve. Measures were obtained with the ankle in the neutral position. The shortest measured distance was noted in a table.
Finally, the crural fascia and the ankle joint capsule were removed to visualize the osteo-articular layer including the ankle lateral collateral ligament complex and the inferior extensor retinaculum. Careful evaluation was made of the ligament caught by each suture and its reinsertion onto the original fibular footprint. Any other anatomical structures entrapped by sutures were also noted.
The study was approved by the University of Barcelona with IRB number: 00003099.
Statistical analysis
Measurements obtained were assessed for normal distribution using the Kolmogorov–Smirnov test. The data in all groups was found to be non-normally distributed.
To compare continuous measurements the Wilcoxon–Mann–Whitney test was used for these non-normally distributed measurements. All tests were 2-tailed, with a p value of < 0.05 considered as significant.
No sample size calculation was performed given the lack of references in the literature for this technique.
Results
The anatomical study revealed that the lateral collateral ligament was anatomically reinserted onto its original fibular footprint in all specimens. No differences were noted between the two surgeons in terms of ligament grasping and/or reattachment. Sutures grasped both ATFL fascicles; the non-automatic suture passer grasped the superior ATFL fascicle, while the automatic suture passer grasped both ATFL fascicles. None of the sutures penetrated the CFL.
No cases of nerve injury either by portals creation or by suture entrapment were observed. In all cases the surrounding anatomic structures were intact, including the superficial peroneal nerve, the sural nerve or its communicating branch, and the peroneal or extensor tendons. Results can be found in Tables 1, 2 and 3.
The median distance between the anterolateral portal and the superficial peroneal nerve was 4.4 mm (mean 4.8 ± 2.7, range 0–10.4). The anterolateral portal was located medial to the superficial peroneal nerve in 9 cases, and lateral in 3 cases. For the experienced surgeon, the median distance was 4.7 mm (mean 5.2 ± 1.5, range 3.9–7.5), and for the non-experienced surgeon it was 3.9 mm (mean 4.5 ± 3.6, range 0–10.4).
The accessory anterolateral portal was located at a median distance of 11.9 mm (mean 14.2 ± 6.7, range 7.1–32.9) from the superficial peroneal nerve. The experienced surgeon located the accessory anterolateral portal at a median distance from the superficial peroneal nerve of 11.5 mm (mean 14.6 ± 9.2, range 7.1–32.9), and the non-experienced surgeon of 13.9 mm (mean 13.9 ± 3.6, range 10.0–18.7).
Regarding the sural nerve, it was observed at a median distance from the accessory anterolateral portal of 28.7 mm (mean 29.6 ± 6.8, range 20.8–39.6). For the experienced surgeon it was 28.7 mm (mean 29.8 ± 6.2, range 21.2–38.8), and 28.4 mm (mean 29.5 ± 8.0, range 20.8–39.6) for the non-experienced surgeon.
Differences between the 2 surgeons for any measurement (mm) were found to be non-statistically significant.
Discussion
The most important contribution of the study is the confirmation that the arthroscopic all-inside lateral collateral ligament repair is a safe and reproducible technique regardless of the surgeon’s level of experience.
To date, the open Broström procedure is considered the gold standard technique to treat chronic ankle instability [12, 13]. During the last decade, the role of arthroscopy has gained widespread popularity in the surgical management of the condition, partly due to the intra-articular pathology that is associated with ankle instability. Intra-articular pathology that can contribute to pain and dysfunction has been observed in 66–93% of unstable ankles [14,15,16,17,18]. Arthroscopic treatment of associated intra-articular pathology before an open Broström procedure has routinely been an adjunct to the procedure [15, 19]. More recently, arthroscopic techniques to treat ankle instability have been proposed instead of a “hybrid” procedure that entails arthroscopy plus a subsequent open Broström repair [3, 20]. Although arthroscopic techniques are varied, similar clinical results have been reported when comparing open versus arthroscopic procedures for the treatment of ankle instability [6]. Complications caused by nerve entrapment double their rate in cases of arthroscopic assisted technique with a percutaneous step compared to open techniques. This could be explained by the percutaneous passage of the sutures in the anterolateral aspect of the ankle. Although a safe zone for the percutaneous passage has been recently described [8], both clinical and cadaveric studies reported high risk of iatrogenic damage to both the superficial peroneal and sural nerves [1, 21]. The arthroscopic all-inside lateral collateral ligament repair is a fully arthroscopic technique that avoids percutaneous suture passage and, therefore, is inherently safer than its percutaneous counterparts. In the present study, there were no cases of suture entrapment of any anatomical surrounding structures. The ligament is sutured within the ankle joint and under arthroscopic visualization ensuring that no other anatomic structures or nerves are encountered. This study explains and complements the reported clinical outcomes of the technique that equally showed no neurological complications [3, 22, 23].
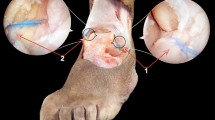
Neither the superficial peroneal nor the sural nerves were injured during the study despite the potential risk reported during the arthroscopic procedure. The standard anterolateral portal puts the superficial peroneal nerve at risk, and its injury is one of the most common complications during anterior ankle arthroscopy [24, 25]. To create a safe anterolateral approach, it is advised that the portal is placed just lateral to the peroneus tertius tendon, and medial to the superficial peroneal nerve when visible transcutaneously [3, 26] (Fig. 1). However, despite careful anterolateral portal creation [24, 26], the presence of anatomical variations of the nerve [27] demands caution during portal placement (Fig. 2). In the present study, the portal was located lateral to the nerve in 3 cases, and the median distance between the anterolateral portal and the superficial peroneal nerve was 4.4 mm (mean 4.8 ± 2.7, range 0.0–10.4). In one case, the nerve was observed in direct contact with the arthroscopic approach. Because of the superficial peroneal nerve variation and the risk of nerve entrapment during the procedure due to portal proximity, it is highly recommended to use a cannula in addition to a protocolized portal creation (Figs. 1, 2). Regarding the risk of nerve injury with the accessory anterolateral portal, the median distance between the portal and the superficial peroneal nerve was of 11.9 mm (mean 14.2 ± 6.7, range 7.1–32.9). For the sural nerve, the median distance between the accessory anterolateral portal and the nerve was of 28.7 mm (mean 29.6 ± 6.8, range 20.8–39.6). According to these figures, the accessory anterolateral portal must be considered a safe arthroscopic approach.
Dissection of the specimen after surgery was performed. An anterolateral skin window was created and superficial dissection was performed to identify the neurological structures and assess any lesions. Nerve distribution pattern is the most commonly found at the anterior are of the ankle. Canula was left at the anterolateral portal. Arthroscopic image shows intra-articular extent of the canula. (1) Medial dorsal cutaneous nerve (branch of superficial peroneal nerve). (2) Intermediate dorsal cutaneous nerve (branch of superficial peroneal nerve). (3) Sural nerve (lateral dorsal cutaneous nerve)
Dissection of the specimen after surgery was performed. An anterolateral skin window was created and superficial dissection was performed to identify the neurological structures and assess any lesions. Nerve distribution pattern is an anatomical variant where two communicating branches were found. Canula was left at the anterolateral portal. (1) Medial dorsal cutaneous nerve (branch of superficial peroneal nerve). (2) Communicating branch between medial dorsal cutaneous branch and intermediate dorsal cutaneous nerve. (3) Intermediate dorsal cutaneous nerve (branch of superficial peroneal nerve). (4) Communicating branch between the intermediate dorsal cutaneous nerve and the sural nerve. (5) Sural nerve (lateral dorsal cutaneous nerve)
As observed in the study, the ligament was reattached onto its original fibular footprint in all cases (Fig. 3). To evaluate the best technique, two different suture passers have been used. The use of a small suture passer -automatic or non-automatic- allowed for grasping of purely the ATFL while avoiding entrapment of surrounding structures. The non-automatic suture passer allowed for grasping of the superior fascicle of the ATFL (Fig. 4), while the automatic suture passer used did so for the superior and inferior ATFL fascicles (Fig. 5). The CFL was not grasped in any case. A new suture passer design and/or a technique modification is required to grasp the CFL. In any case, the need of repairing the CFL, when both the ATFL and CFL are injured, has been questioned in the literature with clinical as well as biomechanical studies that report excellent results after repairing of only the ATFL when both the ATFL and CFL are injured [28,29,30]. These good clinical results can be anatomically explained by the presence of arciform fibers connecting the inferior ATFL with the CFL [31]. With this in mind, the isolated ATFL repair would effectively address the CFL as well.
Dissection was advanced until a clear view of the lateral ankle ligaments was obtained. (1) Extensor digitorum longus tendons. (2) Inferior extensor retinaculum (3) Peroneus tertius tendon. (4) Peroneal tendons. (5) Anterior talofibular ligament (note that the suture has grasped both the superior and inferior ATFL’s fascicles)
Dissection showing the lateral ankle ligaments and position of the non-automatic suture passer (a) and arthroscopic view (b). (1) Inferior extensor retinaculum. (2) Peroneus tertius tendon. (3) Extensor digitorum longus tendons. (4) Superior extensor retinaculum. (5) ATFL’s superior fascicle. (6) ATFL’s inferior fascicle. (7) Arciform fibers of the lateral fibulotalocalcaneal ligament complex (connecting ATFL’s inferior fascicle and calcaneofibular ligament)
Dissection showing the lateral ankle ligaments and position of the automatic suture passer (a) and arthroscopic view (b). (1) Inferior extensor retinaculum. (2) Peroneus tertius tendon. (3) Extensor digitorum longus tendons. (4) Superior extensor retinaculum. (5) ATFL’s superior fascicle. (6) ATFL’s inferior fascicle. (7) Arciform fibers of the lateral fibulotalocalcaneal ligament complex (connecting ATFL’s inferior fascicle and calcaneofibular ligament)
The arthroscopic all-inside ligament repair is a moderately demanding technique and is not ideally suited for the novice ankle arthroscopist. However, as observed in this study, the technique can be efficiently reproduced by the surgeon versed in ankle arthroscopy who is willing to perform arthroscopic stabilization of the lateral ankle. Despite a rather flat learning curve, a thorough command of ankle arthroscopic skills and arthroscopic anatomy is mandatory.
Limitations of the study include the use of ankle specimens without lateral collateral ligament injury. Although the ligament injury was artificially reproduced, it was probably different to that experienced in-vivo after an ankle sprain or in patients with chronic ankle instability. In addition, the ligament tissue quality of cadaveric specimens could be different to that of the real patient, which could modify the grasping strength achieved with the suture. Another limitation is the absence of a biomechanical study to evaluate the repair. A biomechanical test assessing the repair strength would certainly increase the validity of the study.
The clinical relevance of the study is that the arthroscopic all-inside ligament repair is a safe technique, and allows an anatomic ATFL repair. The technique is a reproducible procedure with a flat learning curve for surgeons versed in ankle arthroscopy.
Conclusions
The arthroscopic all-inside lateral collateral ligament repair is a safe and reproducible technique even in the early stages of the learning curve. In addition, the technique can anatomically repair both fascicles of the ATFL with minimal risk of damage to surrounding anatomical structures.
References
Acevedo JI, Mangone PG (2015) Arthroscopic brostrom technique. Foot Ankle Int 36:465–473
Corte-real NM, Moreira RM (2015) Arthroscopic repair of chronic lateral ankle instability. Foot Ankle Int 5:213–217
Vega J, Golanó P, Pellegrino A, Rabat E, Peña F (2013) All-inside arthroscopic lateral collateral ligament repair for ankle instability with a knotless suture anchor technique. Foot Ankle Int 34:1701–1709
Guillo S, Cordier G, Sonnery-Cottet B, Bauer T (2014) Anatomical reconstruction of the anterior talofibular and calcaneofibular ligaments with an all-arthroscopic surgical technique. Orthop Traumatol Surg Res 100:S413–S417
Takao M, Oae K, Uchio Y, Ochi M, Yamamoto H (2005) Anatomical reconstruction of the lateral ligaments of the ankle with a gracilis autograft: a new technique using an interference fit anchoring system. Am J Sports Med 33:814–823
Guelfi M, Zamperetti M, Pantalone A, Usuelli FG, Salini V, Oliva XM (2018) Open and arthroscopic lateral ligament repair for treatment of chronic ankle instability: a systematic review. Foot Ankle Surg 24:11–18
Kim ES, Lee KT, Park JS, Lee YK (2011) Arthroscopic anterior talofibular ligament repair for chronic ankle instability with a suture anchor technique. Orthopedics 34:1–9
Acevedo JI, Ortiz C, Golanó P, Nery C (2015) ArthroBrostrom lateral ankle stabilization technique: an anatomic study. Am J Sports Med 43:2564–2571
Vega J, Guelfi M, Malagelada F, Peña F, Dalmau-Pastor M (2018) Arthroscopic all-inside anterior talofibular ligament repair through a three-portal and no-ankle-distraction technique. JBJS Essent Surg Tech 8:1–11
Stephens MM, Kelly PM (2000) Fourth toe flexion sign: a new clinical sign for identification of the superficial peroneal nerve. Foot Ankle Int 21:860–863
Dalmau-Pastor M, Vega J (2017) Letter regarding: cadaveric analysis of the distal tibiofibular syndesmosis. Foot Ankle Int 38:343–345
Bell SJ, Mologne TS, Sitler DF, Cox JS (2006) Twenty-six-year results after Broström procedure for chronic lateral ankle instability. Am J Sports Med 34:975–978
Guillo S, Bauer T, Lee JW, Takao M, Kong SW, Stone JW, Mangone PG, Molloy A, Perera A, Pearce CJ, Michels F, Tourné Y, Ghorbani A, Calder JD (2013) Consensus in chronic ankle instability: aetiology, assessment, surgical indications and place for arthroscopy. Orthop Traumatol Surg Res 99:S411–S419
Choi WJ, Lee JW, Han SH, Kim BS, Lee SK (2008) Chronic lateral ankle instability: the effect of intra-articular lesions on clinical outcome. Am J Sports Med 36:2167–2172
Hintermann B, Boss AP, Schäfer D (2002) Arthroscopic findings in patients with chronic ankle instability. Am J Sports Med 30:402–409
Komenda GA, Ferkel RD (1999) Arthroscopic findings associated with the unstable ankle. Foot Ankle Int 20:708–713
Lee J, Hamilton G, Ford L (2011) Associated Intra-articular ankle pathologies in patients with chronic lateral ankle instability: arthroscopic findings at the time of lateral ankle reconstruction. Foot Ankle Spec 4:284–289
Taga I, Shino K, Inoue M, Nakata K, Maeda A, Henry JH (1993) Articular cartilage lesions in ankles with lateral ligament injury. an arthroscopic study. Am J Sports Med 21:120–126
Hua Y, Chen S, Li Y, Chen J, Li H (2010) Combination of modified broström procedure with ankle arthroscopy for chronic ankle instability accompanied by intra-articular symptoms. Arthroscopy 26:524–528
Matsui K, Takao M, Miyamoto W, Innami K, Matsushita T (2014) Arthroscopic Broström repair with Gould augmentation via an accessory anterolateral port for lateral instability of the ankle. Arch Orthop Trauma Surg 134:1461–1467
Drakos MC, Behrens SB, Mulcahey MK, Paller D, Hoffman E, DiGiovanni CW (2013) Proximity of arthroscopic ankle stabilization procedures to surrounding structures: an anatomic study. Arthroscopy 29:1089–1094
Vega J, Allmendinger J, Malagelada F, Guelfi M, Dalmau-Pastor M (2017) Combined arthroscopic all-inside repair of lateral and medial ankle ligaments is an effective treatment for rotational ankle instability. Knee Surg Sport Traumatol Arthrosc. https://doi.org/10.1007/s00167-017-4736-y
Vega J, Montesinos E, Malagelada F, Baduell A, Guelfi M, Dalmau-Pastor M (2018) Arthroscopic all-inside anterior talo-fibular ligament repair with suture augmentation gives excellent results in case of poor ligament tissue remnant quality. Knee Surg Sport Traumatol Arthrosc. https://doi.org/10.1007/s00167-018-5117-x
de Leeuw PAJ, Golanó P, Sierevelt IN, van Dijk CN (2010) The course of the superficial peroneal nerve in relation to the ankle position: Anatomical study with ankle arthroscopic implications. Knee Surg Sport Traumatol Arthrosc 18:612–617
Zengerink M, van Dijk CN (2012) Complications in ankle arthroscopy. Knee Surg Sport Traumatol Arthrosc 20:1420–1431
Vega J, Dalmau-Pastor M, Malagelada F, Fargues-Polo B, Peña F (2017) Ankle arthroscopy: an update. J Bone Joint Surg Am 99:1395–1407
Ucerler H, Ikiz’ZA, Aktan (2005) The variations of the sensory branches of the superficial peroneal nerve course and its clinical importance. Foot Ankle Int 26:942–946
Ko KR, Lee W-Y, Lee H, Park HS, Sung K-S (2018) Repair of only anterior talofibular ligament resulted in similar outcomes to those of repair of both anterior talofibular and calcaneofibular ligaments. Knee Surg Sport Traumatol Arthrosc. https://doi.org/10.1007/s00167-018-5091-3
Lee KT, Lee J II, Sung KS, Kim JY, Kim ES, Lee SH, Wang JH (2008) Biomechanical evaluation against calcaneofibular ligament repair in the Brostrom procedure: a cadaveric study. Knee Surg Sport Traumatol Arthrosc 16:781–786
Lee KT, Park YU, Kim JS, Kim JB, Kim KC, Kang SK (2011) Long-term results after modified brostrom procedure without calcaneo-fibular ligament reconstruction. Foot Ankle Int 32:153–157
Vega J, Malagelada F, Manzanares Céspedes M-C, Dalmau-Pastor M (2018) The lateral fibulotalocalcaneal ligament complex: an ankle stabilizing isometric structure. Knee Surg Sport Traumatol Arthrosc. https://doi.org/10.1007/s00167-018-5188-8
Acknowledgements
This study was realized at ArthroLab™ in Munich (Germany). The authors would like to thank Arthrex GmbH for providing cadaveric specimens and all the surgical instruments needed for the study.
Funding
Arthrex GmbH provided the laboratory, surgical instruments and cadaveric specimens for the study. No other external funding was used.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the University of Barcelona with IRB number: 00003099.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guelfi, M., Vega, J., Malagelada, F. et al. The arthroscopic all-inside ankle lateral collateral ligament repair is a safe and reproducible technique. Knee Surg Sports Traumatol Arthrosc 28, 63–69 (2020). https://doi.org/10.1007/s00167-019-05427-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-019-05427-0