Abstract
Purpose
Age-related modifications of tendons, such as reduced tenocyte proliferation and modified extracellular matrix (ECM) turnover, have been previously described, but results are often incomplete and discordant. The aim of this study was to investigate, using morphological and molecular methods, the effect of ageing on human tendons and tenocytes, especially focusing on the collagen turnover pathways, in order to understand how the ageing process could influence tendon biology and structure.
Methods
Morphological analysis was performed on fragments from human semitendinosus and gracilis tendons harvested from 10 adult (mean age 41.8 ± 13.3 years) and 6 aged healthy patients (mean age 72.7 ± 7.0 years) by haematoxylin and eosin, Sirius red and Alcian blue staining. The expression of genes and proteins involved in collagen turnover and focal adhesions was assessed by real-time PCR, slot blot and zymography in cultured tenocytes. Cytoskeleton arrangement was studied by immunofluorescence and cell migration by wound healing assay.
Results
The structure and composition of ECM in ageing tendons are preserved as well as the expression of genes and proteins involved in collagen turnover pathways. Although morphological analysis revealed that ageing tenocytes tended to an impaired migration potential and that actin filaments are occasionally shorter and randomly distributed, the expression of proteins involved in focal adhesion formation is preserved.
Conclusion
Results of this study suggest that the structure of ageing tendons is preserved and that ageing tenocytes maintain their ability for ECM remodelling, supporting the hypothesis that ageing tendons maintain their biomechanical properties. The biological reliability of aged tendons has a clinical relevance, supporting the use of tendon autografts also in the elderly patients. Since the common and successful orthopaedic procedure of anterior cruciate ligament reconstruction using either autografts or allografts is becoming more common in older age groups, these findings suggest that the donor age would not significantly influence the clinical outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tendon mechanical properties are determined by the underlying composition and structure. Tendon dense regular connective tissue is characterized by a large amount of extracellular matrix (ECM) mainly containing interstitial collagen, proteoglycans (PGs), water and a relatively sparse population of specialized fibroblasts, the tenocytes. Tenocytes are responsible for tendon tissue homoeostasis, repair and adaptation to mechanical loading. Collagen accounts for the 60–85% of the dry mass and is predominantly represented by type I collagen (COL-I) fibrils and fibres, providing the major resistance to tensile mechanical forces. Type III collagen (COL-III) represents approximately 3% of the total and in normal tendons is mostly restricted to the endotenon and epitenon [19, 20]. PGs are protein/glycosaminoglycan (GAG) complexes representing the noncollagenous components in the ECM. These molecules, interacting with collagen, play a pivotal role in affecting the viscoelastic properties of the tissue and allowing for the reciprocal gliding of collagen fibres [42].
A marked loss in skeletal muscle mass and strength has been evidenced in ageing, with a decrease in the mechanical properties of tendons. In the last few years, an increased incidence of tendon injuries and pathology has been described [8, 16, 32, 35], suggesting that age-related changes in tendon may modify its structure and its mechanical properties. Some of these modifications are reduced tenocyte proliferation [23, 38, 43], imbalance between anabolic and catabolic pathways in ECM, decreased collagen cross-linking [1, 38] and variations in the GAG content [28, 31]. Since current data on this topic are incomplete and conflicting, in this study we were interested in characterizing the overall effect of ageing on tendon structure and tenocyte metabolism.
Due to low morbidity in the site of explant and to tendon properties, hamstring autografts are often used in tendon and ligament reconstructive procedures [10]. Proponents of allografts have encouraged the use of allografts harvested from younger donors, but recent studies on the biomechanical properties of donor tissue used for reconstruction have reported that the structural properties of allograft are generally independent of age [5, 13, 14, 35] and donor age had no effect on post-operative improvement [15]. However, the use of allografts in surgical practice is currently discussed, with controversial results evidenced in the literature [9, 12, 21, 29].
The aim of this study was to investigate the effect of ageing on human tendons and tenocytes, with particular attention to collagen turnover pathways, in order to understand how the ageing process could influence tendon biology and structure. For the first time, to our knowledge, the overall analysis of tendon structure and tenocyte collagen turnover pathways is provided. Results of this study will be helpful to highlight the safety of autograft tendon in some surgical procedures becoming more common in ageing population.
Materials and methods
Fragments from human semitendinosus and gracilis tendons (fragment size 0.5–1 cm) were harvested from 10 adult patients (mean age 41.8 ± 13.3 years) during ACL reconstruction and from 6 aged healthy patients (mean age 72.7 ± 7.03 years) operated for total knee arthroplasty. For each sample, we analysed the mid-substance of the collected tendons, representing the region with the typical structure of the dense regular connective tissue.
Tendon fragments were immediately fixed in the operating theatre in 10% formalin in 0.1 M phosphate-buffered saline (PBS), pH 7.4, routinely dehydrated, paraffin-embedded and serially cut (thickness 5 µm). Sections were stained with freshly made haematoxylin–eosin to evaluate the cell and tissue morphology. To obtain specific stain for fibrillary collagen, slides were deparaffinized and immersed for 30 min in saturated aqueous picric acid containing 0.1% Sirius red F3BA (Sigma-Aldrich, Milan, Italy) [18]. Sections were stained with Alcian blue in sodium acetate buffer, pH 5.8 containing different MgCl2 concentration in order to selectively stain different mucopolysaccharides, such as GAGs and PGs. More in detail, in buffer containing 0.025 M MgCl2 all acid mucopolysaccharydes stain blue. Using 0.3 and 0.65 M MgCl2, respectively, sulphated acid mucopolysaccharides and highly sulphated acid mucopolysaccharides can be observed. The slides were photographed by a digital camera connected to a Nikon Eclipse 80i microscope.
Sirius red- and Alcian blue-stained sections were analysed in blind by three different operators using a semiquantitative five point scoring system to assess the degree of staining: 0 corresponds to very faint staining, 0.5 faint staining, 1 moderate staining, 2 strong staining and 3 very strong staining.
Tenocytes were obtained from all tendon fragments of both experimental groups. Tendon fragments were rinsed with sterile PBS, plated in T25 flasks, incubated in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% heat-inactivated foetal bovine serum (FBS), antibiotics (100 U/mL penicillin, 0.1 mg/mL streptomycin) and ascorbic acid (200 µM) at 37 °C in a humidified atmosphere containing 5% CO2. When tenocytes grew out from the explant, they were trypsinized (0.025% trypsin-0.02% EDTA) for secondary cultures and plated in T75 flasks. Viability was assessed by the Trypan blue exclusion method. For evaluations, confluent human tenocytes were used between the fourth and fifth passage in T25 flasks. For protein analysis, cells were cultured in serum-free DMEM. Tenocytes and cell supernatants were prepared in duplicate and were analysed after 24, 48 and 72 h.
Cell growth was assessed by growth curves. Tenocytes were plated in triplicate samples in 6-well multi-well plates at the same cell density. Cell number was determined after 24, 48 and 72 h in tenocytes in the proliferative phase.
For fluorescence microscopy, tenocytes were cultured on 12-mm-diameter round coverslips put into 24-well culture plates for 48 h, as previously described [25]. Briefly, for actin cytoskeleton analysis, cells were incubated with 50 µM rhodamine–phalloidin (Sigma-Aldrich), and for vimentin, tubulin and vinculin detection, cells were incubated for 1 h at room temperature, respectively, with the monoclonal primary antibodies anti-vimentin (1:100 in PBS, Novocastra), anti-tubulin (1:2000 in PBS, Sigma-Aldrich) or anti-vinculin (1:600 in PBS, Sigma-Aldrich). The cells were photographed by a digital camera connected to a Nikon Eclipse 80i microscope.
Gene expression was analysed by real-time RT-PCR as previously reported in samples run in triplicate [25]. GAPDH was used as endogenous control to normalize the differences in the amount of total RNA in each sample. The primers sequences were the following: GAPDH: sense CCCTTCATTGACCTCAACTACATG, antisense TGGGATTTCCATTGATGACAAGC; LH2b: sense CCGGAAACATTCCAAATGCTCAG, antisense GCCAGAGGTCATTGTTATAATGGG; TIMP-1: sense GGCTTCTGGCATCCTGTTGTTG, antisense AAGGTGGTCTGGTTGACTTCTGG; vinculin: sense GGAGGTGATTAACCAGCCAAT, antisense AATGATGTCATTGCCCTTGC; FAK: sense GTCTGCCTTCGCTTCACG, antisense GAATTTGTAACTGGAAGATGCAAG. Each sample was analysed in triplicate in a Bioer LineGene 9600 thermal cycler (Bioer, China) after 40 cycles. The cycle threshold (Ct) was determined, and gene expression levels relative to that of GAPDH were calculated.
Collagen type I and III (COL-I, COL-III), matrix metalloproteinase (MMP)-1 protein levels secreted by tenocytes were assessed in duplicate samples by slot blot in serum-free cell culture medium, as previously detailed [25]. Membranes were incubated for 1 h at room temperature in monoclonal antibody to COL-I (1:1000 in TBST) (Sigma-Aldrich, Italy), COL-III (1:2000 in TBST) (Sigma-Aldrich, Italy), MMP-1 (1 µg/mL in TBST) (Millipore, Italy). Immunoreactive bands, revealed by the Amplified Opti-4CN substrate (Amplified Opti-4CN, Bio-Rad, Italy), were scanned densitometrically (UVBand, Eppendorf, Italy).
SDS-zymography was used to analyse MMP-2 activity, as previously described [25]. MMP gelatinolytic activity, detected after staining the gels with Coomassie brilliant blue R250 as clear bands on a blue background, was quantified by densitometric scanning (UVBand, Eppendorf, Italy).
Cell migration in adult and ageing tenocytes was analysed by wound healing assay in 6-well multi-well plates [24]. The “scratch” was created in confluent tenocytes using a p200 pipet tip. Cell debris were removed by DMEM washing and multi-well plates were incubated at 37 °C and observed under an inverted microscope at different time points. Digital images were captured by a digital camera after 4, 8, 12 and 16 h, and the size of the “scratch” was measured to obtain the migration potential.
The protocol study was approved by the local ethics committee (San Raffaele Hospital Ethical Committee, Milan) of the coordinating institution (IRCCS Policlinico San Donato) (protocol Tendon Ageing—June 13, 2013), and patients signed an informed consent. Inclusion and exclusion criteria are listed in Table 1.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 6.0 software (GraphPad Software Inc.). Data were typical results from two replicate experiments for each of the patients-derived cell lines cultured in duplicate and were expressed as mean ± standard error (SEM). Comparison of groups was made using independent samples two-tailed t test. Differences associated with P values lower than 5%, at a confidence level of 95%, were considered to be significant.
Results
Tendon structure
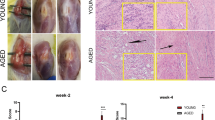
Light microscopy analysis revealed that tendon structure was maintained and collagen fibrils were parallel and tightly aligned, with a slightly wavy appearance in both experimental groups, with tenocytes in between (Fig. 1). Sirius red (Fig. 2a, b)- and Alcian blue (Fig. 3a, b)-stained specimens revealed that collagen, GAG and PG content were not modified by the ageing process.
Haematoxylin–eosin-stained longitudinal sections of the mid-region of adult and ageing tendons. Collagen fibres are arranged in parallel bundles having an undulating distribution and containing flattened tenocytes. Tenocytes were thin and arranged in rows between collagen fibres. Some rounded nuclei were evident, suggesting the presence of tenoblasts. Structure alterations related to tendon pathology, such as loss of collagen fibre alignment, lipoid degeneration or increased vascularization, were not observed. Original magnification ×20
a Microphotographs showing adult and ageing tendons stained using Alcian blue containing different molarities of MgCl2 and allowing the detection of differently sulphated GAGs. Original magnification ×20. b Bar graphs showing the assessment by semiquantitative score of the intensity of Alcian blue staining of adult and ageing tendons
Cell growth
Cell proliferation evaluated by growth curves showed that ageing tenocytes exhibited a slower proliferation rate compared to adult tenocytes, but the difference was not statistically significant (Table 2).
Expression of genes and proteins related to collagen turnover
Slot blot analysis revealed that COL-I and COL-III protein levels were similar in cell culture supernatants of adult and ageing tenocytes, suggesting a comparable synthesis rate (Fig. 4a, b). The expression of LH2b, involved in collagen cross-linking, was not significantly affected by ageing (Fig. 4c).
Bar graphs displaying COL-I (a) and COL-III (b) protein levels analysed by slot blot in culture medium of adult and ageing tenocytes. Data are expressed as mean ± SEM. c Bar graphs showing mRNA levels for LH2b in adult and ageing tenocytes assessed by real-time PCR. Data were normalized on GAPDH gene expression and are expressed as mean ± SEM for at least two independent experiments for samples run in duplicate
The analysis of collagen degradation pathways revealed that MMP-1 levels (Fig. 5a, b), and MMP-2 (Fig. 5c, d) and TIMP-1 gene expression (Fig. 5e) resulted similarly expressed in adult and ageing tenocytes.
Representative slot blot for MMP-1 levels in tenocyte serum-free cell culture medium (a) and bar graphs showing MMP-1 protein levels after densitometric analysis of immunoreactive. Data are expressed as densitometric units ± SEM. b Representative SDS-zymography showing MMP-2 activity in serum-free cell supernatants of adult and ageing tenocytes and bar graphs showing MMP-2 levels after densitometric analysis of lytic bands (c). Data are expressed as densitometric units ± SEM. d Bar graphs showing TIMP-1 gene expression after normalization on GAPDH mRNA levels. Data are expressed as mean ± SEM
Cytoskeleton arrangement
Microtubule network and vimentin intermediate filaments were similarly expressed and distributed in adult and ageing tenocytes (Fig. 6a). Fluorescent microscopy analysis for F-actin (Fig. 6a, b) revealed that, interestingly, in ageing tenocytes actin filaments were occasionally shorter and randomly oriented in the cytoplasm (see arrows in Fig. 6a, b). Furthermore, in some ageing tenocytes actin filaments were not detectable in some parts of the cytoplasm, likely according to actin depolymerization (see asterisks in Fig. 6a, b).
a Fluorescence microscopy analysis of the actin, tubulin and vimentin cytoskeleton in adult and ageing tenocytes. Microtubule network displayed a normal arrangement and organization, originating from a brightly stained organizing centre located in the perinuclear area. Vimentin intermediate filaments were similarly expressed and distributed in adult and ageing tenocytes: they were dispersed in the cytoplasm, forming a typical network around the nucleus, from which they irradiated out into the cell periphery in fine lace-like threads. Original magnification ×40. b Photomicrographs of microfilament distribution in ageing tenocytes evidenced by rhodamine–phalloidin labelling and showing actin filaments arrangement. Original magnification ×60. Arrows point to shorter and randomly oriented actin filaments and asterisks indicate parts of the cytoplasm where actin filaments were not detectable
Wound healing assay
In order to understand whether ageing tenocytes maintain their motility potential, we analysed cell migration in adult and ageing tenocytes by wound healing assay by measuring the “scratch” size at different intervals. The comparison of the scratch size revealed that the migration potential is not modified in ageing tenocytes (Fig. 7).
Expression of focal adhesion proteins
In order to investigate whether ageing tenocytes retain their ability to form focal adhesions needed for cell migration, the expression of some key proteins forming the adhesion plaque was analysed. The focal adhesion kinase (FAK) and vinculin gene expression was unchanged in ageing compared to adult tenocytes (Fig. 8a–c).
Discussion
The main findings of this study, aimed at characterizing the effect of the ageing process on tendon and tenocytes, reveal that ageing tendons maintain their morphological and biological properties.
The process of ageing is commonly associated with increased prevalence of tendinopathy and tendon injuries [4, 7, 11], with a prevalence of 30% in a population older than 70 years [26, 35]. The mechanisms underlying tendon degeneration and rupture during ageing are unknown but may arise because of age-associated changes in tendon structure and mechanical properties, influenced by ECM components arranged to withstand tensile and compressive mechanical forces.
Some age-related modifications of tendons were previously described, such as decreased tenocyte proliferation [23, 32, 38, 42], imbalance in collagen turnover pathways, decreased collagen cross-linking [38] and variations in the GAG content [30, 34], suggesting that increased incidence of tendon injuries and pathology in the elderly could be a consequence of changes in tendon structure [8, 16, 35, 36].
Morphological analysis revealed that tendon structure is maintained in adult and ageing healthy tendons, and cellularity was similar, as previously reported [37, 41], as well as collagen and GAG/PG content, suggesting that tendon structure is maintained in ageing healthy tendons.
Tenocytes are specialized fibroblasts able to synthesize and degrade tendon ECM, playing a key role in the maintenance of tendon ECM homoeostasis and, therefore, determining the tendon ability to resist mechanical forces and repair in response to injury [20]. An imbalance in the synthesis and degradation of ECM components was suggested responsible for structure alterations and degeneration of the tendon [17]. Therefore, we were interested in characterizing the effect of ageing on the overall expression of genes and proteins involved in collagen turnover and ECM remodelling in human cultured tenocytes.
Collagen content is the result of a finely regulated dynamic balance between its synthesis and degradation. Collagen breakdown is driven by MMPs, a large family of proteases playing a major role in ECM turnover during adaptation of tendon to mechanical loading and repair, thus determining tendon strength. An inverse correlation between MMP-1 gene/protein expression and the amplitude of tensile mechanical load was demonstrated, suggesting that low levels of MMP-1 are related to a more stable tendon structure and therefore less susceptible to damage [3].
The first step in collagen degradation is driven by MMP-1 exerting a collagenolytic activity and able to cleave the intact collagen triple helix, followed by other proteases [33, 40]. MMP activation and activity are regulated at the post-translational level by TIMPs [6, 27] and able to inhibit all MMP members to varying degrees, although functional differences have been identified, and TIMP-1 is the main inhibitor of MMP-1.
In this study, COL-I and COL-III levels secreted by adult and ageing tenocytes are similar, as well as the expression of MMP-1, MMP-2 and TIMP-1. These results suggest that collagen turnover-related mechanisms are maintained in ageing tenocytes.
Since there is no evident overall decline in tenocyte synthetic or degradative pathways during ageing, different mechanisms could be responsible for age-related modifications of tendons, possibly related to an impaired collagen maturation by LH2b, one of the isoforms of LH2 generally overexpressed in fibrotic processes, responsible for collagen cross-linking of the newly synthesized collagen [39]. Collagen cross-linking is an important requirement for collagen maturation in relation to the development of tendon elastic properties, providing collagen fibril stabilization and strength [34]. Age-induced down-regulation of LH2b might be responsible for a less stable tendon, more susceptible to collagen degradation and, finally, more susceptible to damage. Our results suggest that LH2b mRNA levels are similar in adult and ageing tenocytes, suggesting a comparable collagen maturation, although a tendency to decrease was observed in ageing cells.
Mechanical loads acting on a connective tissue are perceived by resident cells as stimuli that are transmitted through constituents of the ECM, ECM receptors and intracellular structures. Tenocyte activity in ECM remodelling is regulated by cell–ECM interactions mediated by a mechanotransduction mechanisms based on integrins that, together with actin cytoskeleton and tendon cell primary cilium [3, 22], provide a bridge through which forces can be transmitted between inside and outside of the cells. It was observed that in some ageing tenocytes actin filaments were shorter, randomly oriented and occasionally undetectable, thus suggesting they underwent some depolymerization. Since tenocytes are able to sense and respond to changes in their mechanical environment using a mechanotransduction system based on the actin cytoskeleton [3], we can hypothesize that ageing tenocytes could experience a decreased mechanoresponsiveness and, therefore, could be less efficient in maintaining ECM homoeostasis in response to different mechanical load. Furthermore, the organization of the actin cytoskeleton is a good indicator of the motile capacity. Cell migration, playing a key role during tissue repair and regeneration, is allowed by cell protrusions driven by actin polymerization and stabilized by ECM adhesion. Cells that migrate poorly exhibit a more disorganized actin cytoskeleton and have shorter actin filaments as compared to robust migrators [30]. A wound healing assay was used to understand whether tenocyte motility is affected by ageing, and no significant differences were found. Interestingly, it was suggested that a less organized actin cytoskeleton is related to a reduced ability to form focal adhesions [2]. Since focal adhesions are needed for a cell to generate the traction necessary to migrate [30], and tenocyte motility is required for tendon repair, we analysed the expression of some key proteins of the adhesion plaque in order to understand whether ageing tenocytes could be less efficient in tissue healing and, therefore, responsible for a slow recovery rate of aged individuals after tendon injury and for long-term alterations in tendon ECM. FAK and vinculin resulted similarly expressed in adult and ageing tenocytes, supporting the hypothesis of a similar ability to form focal adhesions and to migrate.
Results of this study suggest that the structure of ageing tendons is preserved and that ageing tenocytes maintain their ability for ECM remodelling, supporting the hypothesis that ageing tendons maintain their biomechanical properties. These findings allow to formulate new clinical hypothesis stimulating further research in a larger number of subjects and provide new information useful to plan surgery requiring the use of tendon autograft, suggesting that tendon autografts can be used in the elderly patients.
Conclusion
Results of this study, suggesting that ageing process does not modify tendon structure tenocyte biological activity, support the use of tendon autografts also in older patients.
References
Ackerman JE, Bah I, Jonason JH, Buckley MR, Loiselle AE (2017) Aging does not alter tendon mechanical properties during homeostasis, but does impair flexor tendon healing. J Orthop Res. doi:10.1002/jor.23580
Arnesen SM, Lawson MA (2006) Age-related changes in focal adhesions lead to altered cell behavior in tendon fibroblasts. Mech Ageing Dev 127:726–732
Arnoczky SP, Tian T, Lavagnino M, Gardner K (2004) Ex vivo static tensile loading inhibits MMP-1 expression in rat tail tendon cells through a cytoskeletally based mechanotransduction mechanism. J Orthop Res 22:328–333
Astrom M, Rausing A (1995) Chronic Achilles tendinopathy. A survey of surgical and histopathologic findings. Clin Orthop Relat Res 316:151–164
Blevins FT, Hecker AT, Bigler GT, Boland AL, Hayes WC (1994) The effects of donor age and strain rate on the biomechanical properties of bone-patellar tendon-bone allografts. Am J Sports Med 22:328–333
Brew K, Dinakarpandian D, Nagase H (2001) Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta 1477:267–283
Chard MD, Cawston TE, Riley GP, Gresham GA, Hazleman BL (1994) Rotator cuff degeneration and lateral epicondylitis: a comparative histological study. Ann Rheum Dis 53:30–34
Clayton RA, Court-Brown CM (2008) The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury 39:1338–1344
Corry IS, Webb JM, Clingeleffer AJ, Pinczewski LA (1999) Arthroscopic reconstruction of the anterior cruciate ligament: a comparison of patellar tendon autograft and four-strand hamstring tendon autograft. Am J Sports Med 27:444–454
Denti M, Vetere DL, Bandi M, Volpi P (2006) Comparative evaluation of knee stability following reconstruction of the anterior cruciate ligament with the bone-patellar tendon-bone and the double semitendinosus-gracilis methods: 1- and 2-year prospective study. Knee Surg Sports Traumatol Arthrosc 14:637–640
Dressler MR, Butler DL, Wenstrup R, Awad HA, Smith F, Boivin GP (2002) A potential mechanism for age-related declines in patellar tendon biomechanics. J Orthop Res 20:1315–1322
Edgar CM, Zimmer S, Kakar S, Jones H, Schepsis AA (2008) Prospective comparison of auto and allograft hamstring tendon constructs for ACL reconstruction. Clin Orthop Relat Res 466:2238–2246
Flahiff CM, Brooks AT, Hollis J, Vander Schilden JL, Nicholas RW (1995) Biomechanical analysis of patellar tendon allografts as a function of donor age. Am J Sports Med 23:354–358
Greaves LL, Hecker AT, Brown CH (2008) The effect of donor age and low-dose gamma irradiation on the initial biomechanical properties of human tibialis tendon allografts. Am J Sports Med 36:1358–1366
Hampton D, Lamb J, Klimkiewicz JJ (2012) Effect of donor age on patellar tendon allograft ACL reconstruction. Orthopedics 8:e1173–e1176
Hess GW (2010) Achilles tendon rupture: a review of etiology, population, anatomy, risk factors, and injury prevention. Foot Ankle Spec 3:29–32
Ireland D, Harrall R, Curry V, Holloway G, Hackney R, Hazleman B, Riley G (2001) Multiple changes in gene expression in chronic human Achilles tendinopathy. Matrix Biol 20:159–169
Junqueira LC, Bignolas G, Brentani RR (1979) Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11:447–455
Kannus P (2000) Structure of the tendon connective tissue. Scand J Med Sci Sports 10:312–320
Kjaer M (2004) Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84:649–698
Kleipool AE, Zijl JA, Willems WJ (1998) Arthroscopic anterior cruciate ligament reconstruction with bone-patellar tendon-bone allograft or autograft: a prospective study with an average follow up of 4 years. Knee Surg Sports Traumatol Arthrosc 6:224–230
Lavagnino M, Arnoczky SP, Gardner K (2011) In situ deflection of tendon cell-cilia in response to tensile loading: an in vitro study. J Orthop Res 29:925–930
Lavagnino M, Gardner K, Arnoczky SP (2013) Age-related changes in the cellular, mechanical, and contractile properties of rat tail tendons. Connect Tissue Res 54:70–75
Liang CC, Park AY, Guan JL (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2:329–333
Menon A, Pettinari L, Martinelli C, Colombo G, Portinaro N, Dalle-Donne I, d’Agostino MC, Gagliano N (2013) New insights in extracellular matrix remodeling and collagen turnover related pathways in cultured human tenocytes after ciprofloxacin administration. Muscles Ligaments Tendons J. 11(3):122–131
Milgrom C, Schaffler M, Gilbert S, van Holsbeeck M (1995) Rotator-cuff changes in asymptomatic adults. J Bone Joint Surg Br 77:296–298
Murphy G, Willenbrock F, Crabbe T, O’Shea M, Ward R, Atkinson S, O’Connell J, Docherty A (1994) Regulation of matrix metalloproteinase activity. Ann N Y Acad Sci 732:31–41
Peffers MJ, Thorpe CT, Collins JA, Eong R, Wei TK, Screen HR, Clegg PD (2014) Proteomic analysis reveals age-related changes in tendon matrix composition, with age- and injury-specific matrix fragmentation. J Biol Chem 289:25867–25878
Peterson RK, Shelton WR, Bomboy AL (2001) Allograft versus autograft patellar tendon anterior cruciate ligament reconstruction: a 5-year follow-up. Arthroscopy 17:9–13
Reed MJ, Ferara NS, Vernon RB (2001) Impaired migration, integrin function, and actin cytoskeletal organization in dermal fibroblasts from a subset of aged human donors. Mech Ageing Dev 122:1203–1220
Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL (1994) Glycosaminoglycans of human rotator cuff tendons: changes with age and in chronic rotator cuff tendinitis. Ann Rheum Dis 53:367–376
Ruzzini L, Abbruzzese F, Rainer A, Longo UG, Trombetta M, Maffulli N, Denaro V (2014) Characterization of age-related changes of tendon stem cells from adult human tendons. Knee Surg Sports Traumatol Arthrosc 22:2856–2866
Sakai T, Gross J (1967) Some properties of the products of reaction of tadpole collagenase with collagen. Biochemistry 6:518–528
Silver FH, Christiansen D, Snowhill PB, Chen Y, Landis WJ (2000) The role of mineral in the storage of elastic energy in turkey tendons. Biomacromol 1:180–185
Takahashi H, Tajima G, Kikuchi S, Yan J, Kamei Y, Maruyama M, Sugawara A, Saigo T, Doita M (2017) Morphology of the fibular insertion of the posterolateral corner and biceps femoris tendon. Knee Surg Sports Traumatol Arthrosc 25:184–191
Tempelhof S, Rupp S, Seil R (1999) Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg 8:296–299
Thorpe CT, McDermott BT, Goodship AE, Clegg PD, Birch HL (2016) Ageing does not result in a decline in cell synthetic activity in an injury prone tendon. Scand J Med Sci Sports 26:684–693
Thorpe CT, Streeter I, Pinchbeck GL, Goodship AE, Clegg PD, Birch HL (2010) Aspartic acid racemization and collagen degradation markers reveal an accumulation of damage in tendon collagen that is enhanced with aging. J Biol Chem 285:15674–15681
Walker LC, Overstreet MA, Yeowell HN (2005) Tissue-specific expression and regulation of the alternatively-spliced forms of lysyl hydroxylase 2 (LH2) in human kidney cells and skin fibroblasts. Matrix Biol 23:515–523
Woessner FJ (1991) Matrix metalloproteinases and their inhibitors in connective tissue remodelling. FASEB J 5:2145–2154
Wood LK, Brooks SV (2016) Ten weeks of treadmill running decreases stiffness and increases collagen turnover in tendons of old mice. J Orthop Res 34:346–353
Yoon JH, Halper J (2005) Tendon proteoglycans: biochemistry and function. J Musculoskelet Neuronal Interact 5:22–34
Yu TY, Pang JH, Wu KP, Chen MJ, Chen CH, Tsai WC (2013) Aging is associated with increased activities of matrix metalloproteinase-2 and -9 in tenocytes. BMC Musculoskelet Disord 14:2
Authors’ contribution
NG was responsible for the concept and design of the study, data acquisition, analysis and interpretation, and for drafting the article. AM and RC were involved in data acquisition and analysis, and critically revised the manuscript. FC interpreted the data and critically revised the manuscript. PR was responsible for data analysis and interpretation, and for drafting and critically revising the article. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
The protocol study was approved by the Local Ethics Committee (San Raffaele Hospital Ethical Committee, Milan) of the coordinating Institution (IRCCS Policlinico San Donato) (protocol Tendon Aging - June 13, 2013).
Informed consent
Patients signed an informed consent.
Rights and permissions
About this article
Cite this article
Gagliano, N., Menon, A., Cabitza, F. et al. Morphological and molecular characterization of human hamstrings shows that tendon features are not influenced by donor age. Knee Surg Sports Traumatol Arthrosc 26, 343–352 (2018). https://doi.org/10.1007/s00167-017-4661-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-017-4661-0