Abstract
Purpose
Patellofemoral osteoarthritis (PFOA) occurs in approximately half of anterior cruciate ligament (ACL)-injured knees within 10–15 years of trauma. Risk factors for post-traumatic PFOA are poorly understood. Patellofemoral alignment and trochlear morphology may be associated with PFOA following ACL reconstruction (ACLR), and understanding these relationships, particularly early in the post-surgical time period, may guide effective early intervention strategies. In this study, patellofemoral alignment and trochlear morphology were investigated in relation to radiographic features of early PFOA 1-year post-ACLR.
Methods
Participants (aged 18–50 years) had undergone ACLR approximately 1 year prior to being assessed. Early PFOA was defined as presence of a definite patellofemoral osteophyte on lateral or skyline radiograph. Sagittal and axial plane alignment and trochlear morphology were estimated using MRI. Using logistic regression, the relationship between alignment or morphology and presence of osteophytes was evaluated.
Results
Of 111 participants [age 30 ± 8.5; 41 (37%) women], 19 (17%) had definite osteophytes, only two of whom had had patellofemoral chondral lesions noted intra-operatively. One measure of patellar alignment (bisect offset OR 1.1 [95% confidence interval 1.0, 1.2]) and two measures of trochlear morphology (sulcus angle OR 1.1 [1.0, 1.2], trochlear angle OR 1.2 [1.0, 1.5]) were associated with patellofemoral osteophytes.
Conclusions
Patellofemoral malalignment and/or altered trochlear morphology were associated with PFOA 1 year following ACLR compared to individuals post-ACLR without these features. Clarifying the role of alignment and morphology in post-traumatic PFOA may contribute to improving early intervention strategies aimed at secondary prevention.
Level of evidence
IV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patellofemoral osteoarthritis (PFOA) occurs in approximately half of all anterior cruciate ligament (ACL)-injured knees within 10–20 years of trauma, regardless of whether or not ACL reconstruction (ACLR) is performed [8, 14, 31]. The most common early manifestation of knee OA following ACLR is isolated to the patellofemoral joint (12 and 20% at one and 10-year post-ACLR, respectively) [7, 10]. Health-related quality of life, typically impaired after ACL injury [13], appears to be compromised even further in those who develop post-traumatic OA [10, 24, 25, 27]. Therefore, identifying risk factors for PFOA early after ACLR may offer new insights for preventing PFOA and associated impacts on quality of life.
Risk factors that contribute to high rates of post-traumatic PFOA are not well known [10, 16]. Altered knee biomechanics may accelerate development of PFOA [8, 10] since knee biomechanics, including patellofemoral maltracking, are altered following ACL injury and reconstruction [8, 10, 11, 15, 28, 37]. Patellofemoral alignment and trochlear morphology are associated with prevalence and severity of non-traumatic PFOA [22]. Importantly, it is unknown whether patellar alignment or morphology is risk factors for a post-traumatic PFOA phenotype.
If post-traumatic OA, or a subset thereof, constituted a ‘malalignment phenotype’, this population might benefit from distinct intervention strategies aimed at secondary prevention. This provides the rationale to evaluate the relationship between alignment/morphology and PFOA early after ACLR—particularly because patellofemoral alignment may be modifiable [3, 5, 40] and intervening early may offer the greatest potential for modifying disease outcome [29]. Thus, the aim of this study was to investigate measures of patellofemoral alignment and trochlear morphology in relation to radiographic features of PFOA 1-year post-ACLR. It was hypothesized that in PFOA, the patella would be positioned more proximal and be more laterally displaced and tilted; and the trochlea would be shallower, compared to ACLR knees without PFOA.
Materials and methods
A medical chart review identified consecutive patients who had undergone ACLR approximately 12 months previous and who were aged 18–50 years at the time of surgery. Exclusion criteria were: (1) previous injury/symptoms in the ACL-injured knee; (2) ≥5 years between ACL injury and reconstruction; (3) ≥15 months since ACLR at enrolment; (4) inability to read or speak English; (5) subsequent injury or follow-up surgery to ACLR knee; (6) presence of any other condition influencing daily function; and (7) contraindications for imaging.
ACLR
Arthroscopic single-bundle ACLR with hamstring tendon autograft procedures were performed between 2010 and 2011 by one of two orthopaedic surgeons in Melbourne, Australia (TSW, HGM). Surgical details were reported previously [7]. At the time of surgery, PF articular cartilage lesions were noted (defined as Outerbridge grade ≥2 [4]). Participants underwent ACLR at a mean 14 months (median 3 months) after injury. All patients received similar physiotherapy treatment following surgery.
Outcome measures
Basic demographic information was acquired (age, sex, BMI, date of injury, date of surgery) in addition to radiographs of both knees, and MRI of the ACLR knee.
Radiography—osteophytes
Posteroanterior, lateral and skyline radiographs were obtained for both knees in all participants. To assess the patellofemoral joint, lateral radiographs were acquired in full weight bearing with a SynaFlexer frame (Bioclinica/Synarc) holding knees flexed 30° and feet externally rotated 10°. Skyline radiographs were obtained in non-weight bearing with knees flexed 30°. For this study, early PFOA was defined as a definite osteophyte (equivalent to Kellgren Lawrence grade ≥2/osteophyte), a new paradigm to assess early OA proposed by Felson et al. [9, 12, 20]. Radiographs were used to define early OA because criteria for MRI-defined early PFOA are not well established, and because MRI features of early OA such as patellofemoral bone marrow lesions and minor cartilage defects are prevalent even in young asymptomatic knees [38].
Two trained observers (AGC, KMC) independently scored osteophytes for the medial and lateral patellofemoral compartments (femoral trochlea and patella were read together, with the median ridge included in the medial compartment). Interrater reliability (kappa coefficient) was 0.78 (95% CI 0.67, 0.88) [7]. Both raters were blinded to MRI findings (alignment, morphology), and consensus was used to resolve any discrepancies.
MRI—patellofemoral alignment and trochlear morphology
All MRI scans were acquired on a 3.0 Tesla scanner (Philips Achieva, The Netherlands) using a 16-channel knee coil (Invivo) with knees near full extension. To estimate alignment, a proton density-weighted 3D VISTA sequence was used (repetition time/echo time 1300 ms/27 ms; field of view 150 mm2; 0.35 mm isotropic; echo train length 64 ms; scan time 6 min 11 s).
Image slices were selected using InteleViewer software version 4-3-4-P59 (Intelerad, Canada). Three slices were selected: the median patellar slice in sagittal and axial planes, and the axial slice with the most prominent posterior condylar line [34]. Selected slices were exported into .jpg format.
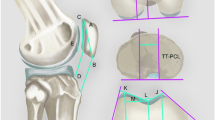
Motion Analysis software version 9.9.0.1 (eHAB, Australia) was used to estimate alignment and morphology, and all values were assessed to within one decimal point. In the sagittal plane, two measures were assessed, Insall–Salvati ratio [17] and patellotrochlear index [1] (Fig. 1). In the axial plane, two parameters of patellar displacement were assessed (bisect offset [35] and lateral displacement [5]), as were two measures of tilt (lateral patellar tilt angle [5] and patellar tilt angle [35]) (Fig. 2). Trochlear morphology estimates in the axial plane included sulcus angle, lateral trochlear inclination and trochlear angle [35] (Fig. 3). The MRI reader (EMM) was blinded to radiograph findings. All measures were calculated twice, and intra-rater reliability was established with intra-class correlation coefficients (ICC [1, 3]) ranging from 0.94 to 0.99.
a Insall–Salvati Ratio: ratio of (i) tendon length (TL) from patella tendon attachments (posteriorly) at tibia and patella to (ii) longest patella length (PL) from inferior to superior patella. Larger number indicates higher position of patella. b Patellotrochlear index: ratio of (i) length of trochlear cartilage (TC) overlap to (ii) patellar cartilage (PC) length. Smaller number indicates less cartilage overlap
a Bisect offset: percentage of the widest patella length (PL) that is lateral to the line through deepest part of trochlea running perpendicular to posterior condylar line (PCL). A higher percentage is more laterally displaced. b Lateral displacement: percentage of patella that lies lateral to a perpendicular line to the anterior condylar line (ACL) that runs through the highest point on the lateral femoral condyle. A higher percentage is more laterally displaced. c Lateral patellar tilt: angle (θ) between PCL and the interior bony margin of the patella lateral facet (PF). A higher angle is less lateral tilt. d Patellar tilt: angle (θ) between PCL and PL. A higher angle is more lateral tilt
a Sulcus angle: angle (θ) joining the lateral (LF) and medial (MF) bony facet margins and the lowest point of the sulcus. Higher number indicates shallower sulcus. b Lateral trochlear inclination: angle (θ) between posterior condylar line (PCL) and LF. A larger angle indicates a deeper sulcus laterally. c Trochlear angle: angle (θ) between PCL and anterior condylar line (AL). Higher angle indicates deeper sulcus laterally
Ethical approval for this study was granted by The University of Melbourne (ID: 1136167) and The University of Queensland (ID: 2012000567 and ID: 2013001448) Human Research Ethics Committees. Participants provided written informed consent prior to participation. All procedures conformed to the Declaration of Helsinki principles.
Statistical analysis
Logistic regression modelling was used to evaluate the relationship of alignment or morphology measures to prevalent early PFOA (medial, lateral and any compartment). Age, sex and BMI were considered for inclusion in the model. If any participants with PFOA at 1 year had had cartilage damage noted at the time of surgery, sensitivity analyses were performed with those participants removed to account for the possibility of pre-existing OA. Sample size calculations were not done a priori because this was an ancillary analysis of an existing cohort. Statistical significance was set at p < 0.05. All statistical analyses were completed using Stata Intercooled 12.0 (StataCorp, Texas, USA).
Results
Of 334 patients screened, 186 met eligibility criteria. Of those, eight were unreachable, 35 lived too far away to attend, and 31 declined invitation. One more participant was later excluded from analysis because their MRI revealed a full ACL graft tear. This resulted in complete MR images for 111 participants [mean ± SD age 30 ± 8.5, 41 (37%) women, BMI 25.9 ± 3.8] (see Table 1 for demographics by group). Median time between injury and surgery was 13 weeks (interquartile range 20; range 1, 1463). The mean time between surgery and study MRI acquisition was 14 ± 2 months (median 13 months, range 11, 19). Demographics for those who were eligible but did not participate in the study (n = 74) did not differ from the study sample (age, sex, pre-injury level of sports activity, time from injury to ACLR or rate of concomitant injuries).
Early PFOA was present in the ACLR knee of 19 participants (17%) compared to 8 (7%) in the contralateral knee (Table 1). Five of these participants had bilateral PFOA. Those with OA of the ACLR knee were older than those without; thus, age was included in all logistic regression models. Seven (37%) of the 19 with OA were aged over 40 years, one of whom had bilateral PFOA. Twelve participants had patellofemoral articular cartilage lesions assessed intra-operatively, two of whom had OA of the ACLR knee at the 1-year evaluation.
Alignment and morphology
Table 2 provides summary statistics for all alignment and morphology measures, including covariates. Due to the relatively low number of osteophytes in medial and lateral compartments separately, compartment-specific evaluations were not performed.
Alignment
In the sagittal plane, there were no significant associations between alignment and PFOA (Table 2).
In the axial plane, bisect offset was larger (i.e. more laterally displaced) in those with early PFOA, with an odds ratio (OR) of 1.1 (95% CI 1.0, 1.2). The remaining measures were not significantly different.
Morphology
Two trochlear morphology measures were associated with early PFOA. Participants with a shallower sulcus angle had higher odds of prevalent PFOA (OR 1.1 [1.0, 1.2]); participants with a more anteriorly protruding lateral facet (i.e. higher trochlear angle) had higher odds (OR 1.2 [1.0, 1.5]). Lateral trochlear inclination was not associated with PFOA.
Sensitivity analyses
Two participants with PFOA had arthroscopically assessed patellofemoral chondral lesions. In sensitivity analyses with those two cases removed, bisect offset and sulcus angle values did not change (same as reported in Table 2). Trochlear angle values did not change either, but the measure was no longer statistically significant.
Discussion
The most important finding of the present study was that each of (1) patellar lateral displacement, (2) sulcus angle or (3) trochlear angle were independently associated with radiographic indices of early PFOA 1-year post-ACLR. In this sample of 111 participants, 17% had early PFOA. While data for comparison to asymptomatic controls are not available, this rate of early PFOA is substantially higher than the prevalence of symptomatic knee OA among non-obese men and women aged 25–44 living in the USA, which ranges from 0.7 to 2.1% [21]. While longitudinal research is required to explore alignment and morphology as possible causative risk factors for PFOA, the findings of the present study may indicate that a malalignment phenotype of post-traumatic PFOA exists. Identifying and characterising this phenotype may offer new insights for early intervention and secondary prevention strategies.
Alignment and PFOA
In the sagittal plane, observed Insall–Salvati ratios were consistent with values previously reported 1 year after ACLR (1.1 ± 0.2) [23], and with other musculoskeletal knee conditions including suspected ACL injury and PFOA [1, 33, 36]. For patellotrochlear index, results for this study’s OA group were the same as values reported for people under 40 years old with knee injury and mild cartilage defects (0.6 ± 0.1), and slightly larger than those without cartilage defects (0.5 ± 0.1) [1].
There was no significant relationship between sagittal plane patellar alignment and early PFOA. Both cross-sectional and longitudinal studies (over 2.5 years) found that patella alta is positively associated with non-traumatic PFOA features [22]. This contrasts with findings in a study of post-traumatic OA, in which 7 years after ACLR, those with moderate to severe PFOA had lower positioned patellae on radiographs (Insall–Salvati ratio 0.86 ± 0.1) than those without PFOA (0.95 ± 0.1) [19]. Together, these data suggest that the relationship between sagittal plane patellar alignment and PFOA differs post-ACLR compared to non-traumatic OA, with patella alta and baja potentially altering patellofemoral joint stress in different ways [39]. Sagittal plane patellar alignment may also take longer than 1-year post-ACLR to cause detectable OA. At this time, the role of patella height in post-traumatic PFOA remains unclear.
To our knowledge, axial plane patellar alignment has not been reported in patients’ post-ACLR, with or without OA. Mean bisect offset for those with OA in this study (57.1 ± 8.0) was similar to knees with patellofemoral pain (59.6%) [30] but lower than other cohorts with patellofemoral pain (69 ± 13) [32] or PFOA (71.6 ± 13.1) [5]. Lateral patellar tilt angle was smaller (i.e. greater lateral patellar tilt, 10.7 ± 5.5) than knees with PFOA (16.1 ± 9.5) [5] yet similar to those with isolated PFOA (11.1 ± 7) [18]. Patellar tilt angle, in contrast, was slightly less laterally tilted in the present OA sample (8.8 ± 4.6) than in PF pain (12.5 ± 7.6) [32]. These contrasting findings could be explained by the different landmarks or methods used to determine alignment, and it is currently unknown which of these measures is best suited to an ACLR population.
Trochlear morphology and PFOA
While trochlear morphology post-ACLR has not been reported to our knowledge, previous studies have reported higher prevalence of trochlear dysplasia in individuals with ACL injuries [2, 26]. In the present study, the trochlea appears to be deeper on average than in various knee conditions with or without PFOA [1, 36]. It is unclear whether values measured in the present study are due to true differences from other study samples or due to methodological differences. Regardless, the results of the present study indicate that a shallower trochlear groove may predispose the patient to post-traumatic PFOA. This is consistent with a systematic review that provides strong evidence that shallower trochleae are cross sectionally associated with higher prevalence of non-traumatic PFOA [22].
There are several limitations to the current study. First, this is a cross-sectional study and associations do not infer causality. Second, pre-injury or pre-operative images of the reconstructed knee were not available; thus, it is possible that PFOA existed prior to, and independently of, ACL trauma. However, this is unlikely since there was a substantially higher proportion of ACLR knees with OA compared with the rate in contralateral knees. In addition, results did not change in sensitivity analyses to account for participants more likely to have had pre-existing PFOA (i.e. those with cartilage lesions assessed intra-operatively). Older age in those with PFOA was also accounted for by using age as a covariate. As this is one of the first studies to explore patellofemoral alignment and trochlear morphology in early post-traumatic PFOA, adjustments for multiple testing were not made. Due to a low number of osteophytes in medial and lateral compartments, it was not possible to evaluate compartment-specific relationships.
This study is clinically relevant in that clarifying the role of alignment and morphology in the onset of post-traumatic PFOA may inform intervention strategies in a subset of patients who may be at increased risk of developing post-traumatic PFOA. Tailored interventions designed to address malalignment (e.g. patellar taping, bracing, targeted neuromuscular control exercises) [3, 5, 6, 40] are examples of approaches that could be tested in futures studies to prevent or delay post-traumatic PFOA.
Conclusions
Patellar lateral displacement (bisect offset) and trochlear morphology (sulcus angle, trochlear angle) were associated with radiographic indices of early PFOA 1 year after ACLR. Further longitudinal studies are required to evaluate the role of alignment and morphology as risk factors for post-traumatic PFOA.
Abbreviations
- 3D VISTA:
-
Three-dimensional volume isotropic turbo spin echo acquisition
- ACL:
-
Anterior cruciate ligament
- ACLR:
-
Anterior cruciate ligament reconstruction
- ANOVA:
-
Analysis of variance
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- ICC:
-
Intra-class correlation coefficients
- MRI:
-
Magnetic resonance imaging
- OA:
-
Osteoarthritis
- OR:
-
Odds ratio
- PF:
-
Patellofemoral
- SEM:
-
Standard error of measure
References
Ali SA, Helmer R, Terk MR (2010) Analysis of the patellofemoral region on MRI: association of abnormal trochlear morphology with severe cartilage defects. AJR Am J Roentgenol 194(3):721–727
Botchu R, Obaid H, Rennie WJ (2013) Correlation between trochlear dysplasia and anterior cruciate ligament injury. J Orthop Surg 21(2):185–188
Callaghan M, Guney H, Reeves N, Bailey D, Doslikova K, Maganaris C, Hodgson R, Felson D (2016) A knee brace alters patella position in patellofemoral osteoarthritis: a study using weight bearing magnetic resonance imaging. Osteoarthr Cartil 24(12):2055–2060
Cameron ML, Briggs KK, Steadman JR (2003) Reproducibility and reliability of the outerbridge classification for grading chondral lesions of the knee arthroscopically. Am J Sports Med 31(1):83–86
Crossley K, Marino G, Macilquham M, Schache A, Hinman R (2009) Can patellar tape reduce the patellar malalignment and pain associated with patellofemoral osteoarthritis? Arthritis Rheumatol 61(12):1719–1725
Crossley KM, Vicenzino B, Lentzos J, Schache AG, Pandy MG, Ozturk H, Hinman RS (2015) Exercise, education, manual-therapy and taping compared to education for patellofemoral osteoarthritis: a blinded, randomised clinical trial. Osteoarthr Cartil 23(9):1457–1464
Culvenor AG, Collins NJ, Guermazi A, Cook JL, Vicenzino B, Khan KM, Beck N, van Leeuwen J, Crossley KM (2015) Early knee osteoarthritis is evident one year following anterior cruciate ligament reconstruction: a magnetic resonance imaging evaluation. Arthritis Rheumatol 67(4):946–955
Culvenor AG, Cook JL, Collins NJ, Crossley KM (2013) Is patellofemoral joint osteoarthritis an under-recognised outcome of anterior cruciate ligament reconstruction? A narrative literature review. Br J Sports Med 47(2):66–70
Culvenor AG, Engen CN, Øiestad BE, Engebretsen L, Risberg MA (2015) Defining the presence of radiographic knee osteoarthritis: a comparison between the Kellgren and Lawrence system and OARSI atlas criteria. Knee Surg Sports Traumatol Arthrosc 23(12):3532–3539
Culvenor AG, Lai CC, Gabbe BJ, Makdissi M, Collins NJ, Vicenzino B, Morris HG, Crossley KM (2014) Patellofemoral osteoarthritis is prevalent and associated with worse symptoms and function after hamstring tendon autograft ACL reconstruction. Br J Sports Med 48(6):435–439
Culvenor AG, Schache AG, Vicenzino B, Pandy MG, Collins NJ, Cook JL, Crossley KM (2014) Are knee biomechanics different in those with and without patellofemoral osteoarthritis after anterior cruciate ligament reconstruction? Arthritis Care Res Hoboken 66(10):1566–1570
Felson DT, Niu J, Guermazi A, Sack B, Aliabadi P (2011) Defining radiographic incidence and progression of knee osteoarthritis: suggested modifications of the Kellgren and Lawrence scale. Ann Rheum Dis 70(11):1884–1886
Filbay SR, Ackerman IN, Russell TG, Macri EM, Crossley KM (2014) Health-related quality of life after anterior cruciate ligament reconstruction: a systematic review. Am J Sports Med 42(5):1247–1255
Friel NA, Chu CR (2013) The role of ACL injury in the development of posttraumatic knee osteoarthritis. Clin Sports Med 32(1):1–12
Hart HF, Culvenor AG, Collins NJ, Ackland DC, Cowan SM, Machotka Z, Crossley KM (2016) Knee kinematics and joint moments during gait following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Br J Sports Med 50:597–612
Hoffelner T, Resch H, Moroder P, Atzwanger J, Wiplinger M, Hitzl W, Tauber M (2012) No increased occurrence of osteoarthritis after anterior cruciate ligament reconstruction after isolated anterior cruciate ligament injury in athletes. Arthroscopy 28(4):517–525
Insall J, Salvati E (1971) Patella position in the normal knee joint 1. Radiology 101(1):101–104
Iwano T, Kurosawa H, Tokuyama H, Hoshikawa Y (1990) Roentgenographic and clinical findings of patellofemoral osteoarthrosis. With special reference to its relationship to femorotibial osteoarthrosis and etiologic factors. Clin Orthop Relat Res 252:190–197
Jarvela T, Paakkala T, Kannus P, Jarvinen M (2001) The incidence of patellofemoral osteoarthritis and associated findings 7 years after anterior cruciate ligament reconstruction with a bone-patellar tendon-bone autograft. Am J Sports Med 29(1):18–24
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16(4):494–502
Losina E, Weinstein AM, Reichmann WM, Burbine SA, Solomon DH, Daigle ME, Rome BN, Chen SP, Hunter DJ, Suter LG (2013) Lifetime risk and age at diagnosis of symptomatic knee osteoarthritis in the US. Arthritis Care Res Hoboken 65(5):703–711
Macri E, Stefanik J, Khan K, Crossley K (2016) Is tibiofemoral or patellofemoral alignment or trochlear morphology associated with patellofemoral osteoarthritis? A systematic review. Arthritis Care Res Hoboken 68(10):1453–1470
Muellner T, Kaltenbrunner W, Nikolic A, Mittlboeck M, Schabus R, Vecsei V (1999) Anterior cruciate ligament reconstruction alters the patellar alignment. Arthroscopy 15(2):165–168
Neuman P, Englund M, Kostogiannis I, Fridén T, Roos H, Dahlberg LE (2008) Prevalence of tibiofemoral osteoarthritis 15 years after nonoperative treatment of anterior cruciate ligament injury a prospective cohort study. Am J Sport Med 36(9):1717–1725
Neuman P, Kostogiannis I, Friden T, Roos H, Dahlberg LE, Englund M (2009) Patellofemoral osteoarthritis 15 years after anterior cruciate ligament injury—a prospective cohort study. Osteoarthr Cartil 17(3):284–290
Ntagiopoulos PG, Bonin N, Sonnery-Cottet B, Badet R, Dejour D (2014) The incidence of trochlear dysplasia in anterior cruciate ligament tears. Int Orthop 38(6):1269–1275
Øiestad BE, Holm I, Engebretsen L, Risberg MA (2011) The association between radiographic knee osteoarthritis and knee symptoms, function and quality of life 10–15 years after anterior cruciate ligament reconstruction. Br J Sport Med 45(7):583–588
Papannagari R, Gill TJ, Defrate LE, Moses JM, Petruska AJ, Li G (2006) In vivo kinematics of the knee after anterior cruciate ligament reconstruction: a clinical and functional evaluation. Am J Sport Med 34(12):2006–2012
Pollard TC, Gwilym SE, Carr AJ (2008) The assessment of early osteoarthritis. J Bone Jt Surg Br 90(4):411–421
Powers CM, Shellock FG, Beering TV, Garrido DE, Goldbach RM, Molnar T (1999) Effect of bracing on patellar kinematics in patients with patellofemoral joint pain. Med Sci Sports Exerc 31(12):1714–1720
Risberg MA, Oiestad BE, Gunderson R, Aune AK, Engebretsen L, Culvenor A, Holm I (2016) Changes in knee osteoarthritis, symptoms, and function after anterior cruciate ligament reconstruction: a 20-year prospective follow-up study. Am J Sport Med 44(5):1215–1224
Salsich GB, Perman WH (2013) Tibiofemoral and patellofemoral mechanics are altered at small knee flexion angles in people with patellofemoral pain. J Sci Med Sport 16(1):13–17
Shabshin N, Schweitzer ME, Morrison WB, Parker L (2004) MRI criteria for patella alta and baja. Skeletal Radiol 33(8):445–450
Stefanik JJ, Roemer FW, Zumwalt AC, Zhu Y, Gross KD, Lynch JA, Frey-Law LA, Lewis CE, Guermazi A, Powers CM (2012) Association between measures of trochlear morphology and structural features of patellofemoral joint osteoarthritis on MRI: the MOST study. J Orthop Res 30(1):1–8
Stefanik JJ, Zumwalt AC, Segal NA, Lynch JA, Powers CM (2013) Association between measures of patella height, morphologic features of the trochlea, and patellofemoral joint alignment: the MOST study. Clin Orthop Relat Res 471(8):2641–2648
Tsavalas N, Katonis P, Karantanas AH (2012) Knee joint anterior malalignment and patellofemoral osteoarthritis: an MRI study. Eur Radiol 22(2):418–428
Van de Velde SK, Gill TJ, DeFrate LE, Papannagari R, Li G (2008) The effect of anterior cruciate ligament deficiency and reconstruction on the patellofemoral joint. Am J Sports Med 36(6):1150–1159
van der Heijden RA, de Kanter JL, Bierma-Zeinstra SM, Verhaar JA, van Veldhoven PL, Krestin GP, Oei EH, van Middelkoop M (2016) Structural abnormalities on magnetic resonance imaging in patients with patellofemoral pain a cross-sectional case-control study. Am J Sport Med: 44(9):2339-2346
Ward SR, Terk MR, Powers CM (2007) Patella alta: association with patellofemoral alignment and changes in contact area during weight-bearing. J Bone Jt Surg Am 89(8):1749–1755
Wong YM, Chan ST, Tang KW, Ng GY (2009) Two modes of weight training programs and patellar stabilization. J Athlet Train 44(3):264–271
Acknowledgements
We gratefully acknowledge support for this work, including funding from the Queensland Orthopaedic Physiotherapy Network, a University of Melbourne Research Collaboration grant and a University of British Columbia research grant. E. Macri received funding support from the Australian Endeavour Award Research Fellowship and Vanier Canada Graduate Scholarship (CIHR). A. Culvenor received funding from the European Union Seventh Framework Programme (Grant Agreement Number 607510).
Authors’ contributions
EM, KC, AG and KK formulated the research question with input from HM and TW. KC, AG, HM, TW developed the original study design, and EM, KC, AG, TR and KK developed the secondary study design. HM and TW completed all surgeries. AG completed data collection. TR developed software program for analysing alignment and morphology and trained EM in software use. KC and AG evaluated X-rays for OA findings. EM and KC developed protocol for analysing alignment and morphology. EM completed alignment and morphology measures. EM did statistical analysis with intellectual input from KC, AG, KK. EM led manuscript writing with intellectual input from all authors. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by the Queensland Orthopaedic Physiotherapy Network, a University of Melbourne Research Collaboration grant and a University of British Columbia research grant. E. Macri received funding support from the Australian Endeavour Award Research Fellowship and Vanier Canada Graduate Scholarship (CIHR). A. Culvenor received funding from the European Union Seventh Framework Programme (Grant Agreement Number 607510). Dr. Whitehead reports personal fees from a Smith and Nephew Clinical Fellowship and personal fees from Smith and Nephew speaking engagement, outside the submitted work. Dr. Morris reports personal fees from Oceania Orthopaedics Clinical Fellowship, outside the submitted work.
Ethical approval
This study was approved by the appropriate ethics committees (The University of Melbourne, ID 1136167, and The University of Queensland, IDs 2012000567 and 2013001448) and have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Informed consent
Participants provided written informed consent prior to participation.
Rights and permissions
About this article
Cite this article
Macri, E.M., Culvenor, A.G., Morris, H.G. et al. Lateral displacement, sulcus angle and trochlear angle are associated with early patellofemoral osteoarthritis following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 26, 2622–2629 (2018). https://doi.org/10.1007/s00167-017-4571-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-017-4571-1