Abstract
Purpose
The aim of this study is to report the effects of autologous PRP injections on time to return to play and recurrence rate after acute grade 2 muscle injuries in recreational and competitive athletes.
Methods
Seventy-five patients diagnosed with acute muscle injuries were randomly allocated to autologous PRP therapy combined with a rehabilitation programme or a rehabilitation programme only. The primary outcome of this study was time to return to play. In addition, changes in pain severity and recurrence rates were evaluated.
Results
Patients in the PRP group achieved full recovery significantly earlier than controls (P = 0.001). The mean time to return to play was 21.1 ± 3.1 days and 25 ± 2.8 days for the PRP and control groups, respectively (P = 0.001). Significantly lower pain severity scores were observed in the PRP group throughout the study. The difference in the recurrence rate after 2-year-follow-up was not statistically significant between groups.
Conclusions
A single PRP injection combined with a rehabilitation programme significantly shortened time to return to sports compared to a rehabilitation programme only. Recurrence rate was not significantly different between groups.
Level of evidence
I.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prevalence rates for muscle strains have been reported between 12 and 16 % [4, 19, 21], and reinjury rates can be as high as 34 % during the first year [19, 21]. Muscle injuries can lead to prolonged absence from sport and lengthy rehabilitation [2, 5, 29].
The use of platelet-rich plasma (PRP) for muscle injures is based on the effects that growth factors have on the stimulation and acceleration of tissue regeneration. PRP has been studied as a primary or adjunctive treatment for acute tendon rupture [3, 6], articular cartilage injury [10], ligament sprains [28], and osteoarthritis [26]. However, only a few studies with conflicting findings are available in the literature regarding its use in muscle injuries [12, 24, 25].
Moreover, these previous studies focused only on the short-term effects of PRP in muscle injuries, but they do not analyse the long-term effects of PRP therapy including the recurrence rate. Although numerous risk factors for muscle injury have been identified, the greatest risk factor for a recurrence remains a previous injury to that muscle. Therefore, the optimization of both preventative and management techniques is essential especially in competitive athletes [9, 18, 20, 21, 31].
The aim of this randomized controlled trial is to report the effects of autologous PRP injections on time to return to play and recurrence rate after acute grade 2 muscle injuries in recreational and competitive athletes. To our knowledge, this is the first study to describe the recurrence rate after the use of PRP for acute muscle injuries after a 2-year-follow-up.
Materials and methods
Patients above 18 years admitted to the hospital with a suspected muscle injury of the lower extremity were screened for eligibility. The eligibility criteria for this study were as follows: (1) age between 18 and 40 years, (2) acute muscle injury (7 days since injury onset) including hamstrings, gastrocnemius and quadriceps, (3) all muscle injuries involved in the study were classified by ultrasound examination as grade 2 [22]. All involved patients were competitive or recreational athletes according to the level of competition [30].
Exclusion criteria
Patients who (1) had received any form of injection therapy for the current injury, (2) had used nonsteroidal anti-inflammatory drugs within 1 week before randomization, (3) were unable to comply with the rehabilitation programme, and (4) had previous surgeries or pathologies of the involved muscle were excluded from the study.
From January 2012 to November 2012, 83 consecutive patients were admitted to our hospital with a diagnosis of muscle strains in the lower extremity (including hamstrings, gastrocnemius, and quadriceps) and were screened for eligibility. Eight patients did not meet the inclusion criteria: three were unable to comply with the study protocol, and five declined to participate. The remaining 75 patients were included after signing an informed consent and subsequently were randomized. A radiologist trained in interventional musculoskeletal injections performed all diagnostic ultrasonography assessments. For each patient, complete sociodemographic and injury characteristics were documented electronically in the medical record.
Design and randomization
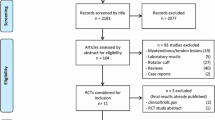
This study was a randomized, single-blind (evaluator), controlled trial. A schematic CONSORT flow diagram of the study conduct is shown in Fig. 1.
Randomization was performed by letting patients choose between two sealed and opaque envelopes, each of which held the allocation to one of the two treatment groups, so the chance to be allocated to one of the groups was the same for every patient.
Patients allocated to group 1 (intervention group) received a single intralesion injection of autologous PRP. Patients allocated to group 2 (control group) did not received any intervention and directly started with the rehabilitation programme. All patients, regardless of group allocation, were subjected to the same standardized rehabilitation protocol supervised by a physical therapist three times per week until the end of treatment. The rehabilitation protocol consisted of four phases (Table 1). Progression from one phase to another depended mainly on pain and range of motion improvement. The exercises performed in the therapist’s practice were complemented with a supervised home rehabilitation programme explained to each patient during the first visit.
All included athletes with a clinically diagnosed muscle injury followed the same standardized rehabilitation programme, and their progress was supervised by experienced physiotherapists. The rehabilitation programme consisted of progressive agility and trunk stabilization exercises. This programme has shown to be effective in promoting earlier return to play and preventing injury recurrence [29].
Intervention
In addition to rehabilitation exercises, a single intralesion injection of autologous PRP was administered to patients in the PRP group. The PRP injection was given immediately after randomization, with a mean of 2.3 days (range 1–4 days) after injury onset (Fig. 2).
Extraction of 40 ml of blood aseptically was performed using a multiple needle gauge 20 g for vacutainer holder system. It was placed in 8 tubes with 6 ml EDTA serum (ethylenediaminetetraacetic acid). Two of them were used to perform serology, haematology control, and immunohematological donor/patient, and the remaining 6 tubes with 6 ml EDTA were intended to obtain PRP. They were previously centrifuged for 3 min at 1400 rpm. The product obtained was separated into laminar flow cabinet and referred to a dry 10-ml tube, which was again centrifuged for 4 min at 3000 rpm to achieve greater product concentration to 2 cm. A process quality control was performed to the product through a haematology analyser ROCHE XT prior to infusion of PRP to the donor/patient.
There is no consensus on the volume of PRP that must be administered [8, 13]. In our study, we measured the volume of the muscle injury and administered the equal amount of PRP. For instance, if the volume of the muscle injury was 3 ml, we would inject 3 ml of PRP. The autologous PRP was delivered into the damaged area under ultrasound guidance. No activating agent was added to the PRP before the injection. Further, no local anaesthetic was administered to the overlying skin before PRP administration (Fig. 2).
Patients were asked to reduce their activities for the following 24 h, and they were allowed to start the rehabilitation programme 2 days after the injections.
A sports physical therapist with more than 10 years of experience conducted these sessions. At each visit, patients were asked to complete the visual analogue scale (VAS) for pain at rest and during active motion. A 10-cm scale with 0 as “no pain” and 10 as “the worst imaginable pain” was used for assessment.
A standard clinical examination to assess the patient’s readiness to return to play was then performed by a sports physical therapist blinded to the treatment allocation.
Outcome measures
We used time to return to play as the primary outcome measure of this study. Time to return to play was defined as the time (in days) from the date of injury onset until the patient fulfilled the criteria to return to play. The determination of fitness for return to play was based on recent clinical sports medicine recommendations. It establishes that athletes can be ready to return to unrestricted sporting activities once full range of motion, strength, and functional abilities (e.g., jumping, running, cutting) can be performed without complaints of pain or stiffness [14]. Patients who fulfilled the criteria to return to play were allowed to resume full activities and progressively increase their training load until reaching their preinjury levels. The secondary outcomes of interest were changes in pain severity between the 2 groups throughout the duration of the study. These were assessed using the VAS at rest and during resisted motion which participants completed after randomization (baseline) and at each follow-up visit.
Finally, the recurrence rate was registered in each group. Patients were called 2, 12, and 24 months after returning to sports to be inquired about injury recurrence. Subjects were considered to have a recurrence if they had a mechanism of injury likely leading to strain with clinical symptoms in a previous injured muscle as tenderness to palpation within the muscle–tendon unit, pain with resisted motion, or a limitation of daily/sport activity. All suspected recurrences were confirmed with ultrasound examination.
The research protocol was approved by the Ethics Committee of Research Protocols of the Hospital Italiano de Buenos Aires (IRB00003580), study protocol number 1772.
Statistical analysis
Sample size calculation
The primary outcome measure, determined before the start of the trial, was time to return to play in days. Assuming a power of 90 % (β = 0.10, α = 0.05) and a mean difference of 5 days with a standard deviation (SD) of 5 days between the treatment groups, our power analysis indicated that a minimum of 22 patients per group were needed to detect significant differences. In order to account for possible loss to follow-up of 20 %, a minimum of 27 patients per group needed to be enrolled in the study.
Continuous variables are expressed as mean (SD) or median [interquartile range], according to the data distribution, and categorical variables as percentage (%). VAS measured at baseline and in follow-up was compared using either t test for each time point. Categorical data comparisons were performed with Chi-square test. VAS change across time was evaluated between groups considering effect of time in VAS decline. Regression coefficients and P values were reported in this manuscript. A P value lower than 0.05 was considered statistically significant. Database management and analysis was performed with Stata statistical software: release 12. (College Station, TX: StataCorp LP).
Results
The baseline characteristics of the 35 patients in the intervention group and the 40 patients in the control group with complete follow-up data are shown in Table 1. Demographic characteristics and potential risk factors appeared to be well balanced at baseline so that no significant differences were found between the two groups regarding mean age, distribution of gender, level of competition, characteristics of injury, and involved muscles.
Primary outcome
The mean time to return to play was 21.1 ± 3.1 days and 25 ± 2.8 days for the PRP and control groups, respectively (P = 0.001). The mean time to return to play for each group of muscles was 23.5 ± 3.5 days for hamstrings, 22.2 ± 2.9 for gastrocnemius, and 23.4 ± 3.2 for quadriceps. This difference was not significant (n.s.).
Secondary outcomes
Changes in VAS score at rest
The mean baseline (pretreatment) VAS score was similar in the two treatment arms (intervention/control: 4.7 (0.9)/4.8 (1.2) points) (n.s.). There was substantial improvement in pain, regardless treatment group, at all-time points. However, patients in the PRP group had significantly lower pain severity scores than controls at all-time points (beta regression coefficient = −0.198 standard error 0.106, 95 % CI −0.406 to 0.010, P = 0.023).
Changes in VAS score with resisted motion
The mean baseline (pretreatment) VAS score with resisted motion was similar in the two treatment arms (intervention/control: 5.9 (1.1)/6.3 (1.1) points) (n.s.). There was substantial improvement in pain, regardless treatment group, at all-time points. However, patients in the PRP group had significantly lower pain severity scores than controls (beta regression coefficient = −0.272 standard error 0.115 95 % CI −0.500 to 0.045, P = 0.019).
Recurrence rate
Two of the 35 athletes (5.7 %) in the intervention group and 4 of the 40 athletes (10 %) in the control group suffered a recurrent muscle strain within the first year of returning to sports (n.s.). No patient suffered recurrent strains between 12 and 24 months after the initial injury.
Discussion
The most important finding of this randomized controlled trial was that a single injection of autologous PRP combined with a rehabilitation programme significantly shortened time to return to sports after an acute grade 2 muscle injury compared with a control group. Moreover, the rate of recurrence during the first 2 years after treatment was not statistically significant between groups.
Several growth factors within PRP have been evaluated in muscle healing [15]. Transforming growth factor-b1 and PGE2 may function synergistically to balance the level of fibrosis during skeletal muscle healing [17]. Acceleration of functional restoration was found in a human trial of elite athletes injected with ultrasound-guided PRP following muscle injury. These high-level athletes returned to sport at full strength in as early as half the expected recovery time without any evidence of excess fibrosis [27]. Rettig et al. [25], in a retrospective case-control study, investigated the effects of an autologous PRP injection on time to return to play after acute hamstring injuries in professional National Football League (NFL) players. Ten professional players diagnosed with an acute hamstring injury were retrospectively divided into PRP (n = 5) and control (n = 5) groups. Patients in the PRP group were injected once under ultrasound guidance, and both groups went through the same rehabilitation programme. The difference between the 2 groups regarding return to sports was not statistically significant. However, this study had two important limitations that were lack of randomization and the limited number of patients.
Hamid et al. [12], in a recent randomized controlled trial, allocated 28 patients to receive either PRP combined with a rehabilitation programme or a rehabilitation programme only. Patients in the PRP group achieved full recovery significantly earlier than controls. The mean time to return to play was 42.5 days in the control group and 26.7 days in the PRP group. In our series, the mean time to return to play in the treatment and control groups was 21.2 and 25 days, respectively. Although the recovery time was also significantly shorter in patients treated with PRP, the difference was only of 4 days. We believe that although this difference is significant from a statistical point of view probably, it is only clinically relevant to elite professional athletes involved in highly competitive leagues with several games per week. A striking point of Hamid’s study is the notable prolonged period of rehabilitation in the control group. Most previous studies showed an average recovery time for grade 2 hamstring injuries of between 3 and 4 weeks [1, 7, 16, 23, 25, 27, 32].
Regarding pain, we found that patients in the PRP group had significantly lower pain severity scores than controls at all-time points. This improvement is clinically relevant because pain reduction is not only an alleviation of symptoms but forms the basis for patients to move faster in the stages of rehabilitation and thus return to sports more rapidly.
Finally, an interesting finding of our study that has not been previously reported was that the difference in the recurrence rate was not statistically significant between groups. However, due to the small total number of recurrences we obtained in our study, future studies involving a larger number of patients are needed to accurately determine whether the PRP has some advantage regarding recurrences.
There are some limitations with this study. Firstly, the decision of time to return to play used in the current study was based mainly on clinical criteria. We did not asses strength with isokinetic tests because they are not easily accessible in our environment, and therefore, we considered it unhelpful to use a tool in the investigation that we would not be able to use easily in our future practice. Secondly, because ethical considerations prevented us from drawing blood from the controls and discarding it, the control patients were probably aware of their treatment allocation. Thirdly, we did not asses the individual muscles. However, in our practice, recovery times of the different muscles of the lower limb are similar and previous studies did not report significant differences in recovery time between the specific muscles studied [11].
Muscle tears are part of the most common sports injuries, leaving athletes off the field. It has become an important area of research. However, the indiscriminate use of platelet-rich plasma without solid foundation to support it is a common practice nowadays. We believe that our study provides scientific data to clarify the clinical use of platelet-rich plasma.
Conclusion
This randomized controlled trial showed that a single injection of autologous PRP combined with a rehabilitation programme significantly shortened time to return to sports after an acute grade 2 muscle injury compared with a control group. The rate of recurrence during the first 2 years after treatment was not statistically significant between groups.
References
Ahmad CS, Redler LH, Ciccotti MG, Maffulli N, Longo UG, Bradley J (2013) Evaluation and management of hamstring injuries. Am J Sports Med 41:2933–2947
Askling C, Saartok T, Thorstensson A (2006) Type of acute hamstring strain affects flexibility, strength, and time to return to pre-injury level. Br J Sports Med 40:40–44
Aspenberg P, Virchenko O (2004) Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand 75:93–99
Bennell KL, Crossley K (1996) Musculoskeletal injuries in track and field: incidence, distribution and risk factors. Aust J Sci Med Sport 28:69–75
Croisier J-L, Forthomme B, Namurois M-H, Vanderthommen M, Crielaard J-M (2002) Hamstring muscle strain recurrence and strength performance disorders. Am J Sports Med 30:199–203
De Mos M, van der Windt AE, Jahr H, van Schie HTM, Weinans H, Verhaar JAN, van Osch GJVM (2008) Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med 36:1171–1178
Ekstrand J, Healy JC, Waldén M, Lee JC, English B, Hägglund M (2012) Hamstring muscle injuries in professional football: the correlation of MRI findings with return to play. Br J Sports Med 46:112–117
Engebretsen AH, Myklebust G, Holme I, Engebretsen L, Bahr R (2010) Intrinsic risk factors for hamstring injuries among male soccer players: a prospective cohort study. Am J Sports Med 38:1147–1153
Engebretsen L, Steffen K, Alsousou J, Anitua E, Bachl N, Devilee R, Everts P, Hamilton B, Huard J, Jenoure P, Kelberine F, Kon E, Maffulli N, Matheson G, Mei-Dan O, Menetrey J, Philippon M, Randelli P, Schamasch P, Schwellnus M, Vernec A, Verrall G (2010) IOC consensus paper on the use of platelet-rich plasma in sports medicine. Br J Sports Med 44:1072–1081
Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA (2009) Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med 37:2259–2272
Hallén A, Ekstrand J (2014) Return to play following muscle injuries in professional footballers. J Sports Sci 32:1229–1236
Hamid MS, Mohamed Ali MR, Yusof A, George J, Lee LPC (2014) Platelet-rich plasma injections for the treatment of hamstring injuries: a randomized controlled trial. Am J Sports Med 42:2410–2418
Hamilton BH, Best TM (2011) Platelet-enriched plasma and muscle strain injuries: challenges imposed by the burden of proof. Clin J Sport Med 21:31–36
Heiderscheit BC, Sherry MA, Silder A, Chumanov ES, Thelen DG (2010) Hamstring strain injuries: recommendations for diagnosis, rehabilitation, and injury prevention. J Orthop Sports Phys Ther 40:67–81
Kasemkijwattana C, Menetrey J, Bosch P, Somogyi G, Moreland MS, Fu FH, Buranapanitkit B, Watkins SS, Huard J (2000) Use of growth factors to improve muscle healing after strain injury. Clin Orthop Relat Res 370:272–285
Malliaropoulos N, Isinkaye T, Tsitas K, Maffulli N (2011) Reinjury after acute posterior thigh muscle injuries in elite track and field athletes. Am J Sports Med 39:304–310
Menetrey J, Kasemkijwattana C, Day CS, Bosch P, Vogt M, Fu FH, Moreland MS, Huard J (2000) Growth factors improve muscle healing in vivo. J Bone Joint Surg Br 82:131–137
Orchard JW (2001) Intrinsic and extrinsic risk factors for muscle strains in Australian football. Am J Sports Med 29:300–303
Orchard J, Best TM, Verrall GM (2005) Return to play following muscle strains. Clin J Sport Med 15:436–441
Orchard J, Marsden J, Lord S, Garlick D (1997) Preseason hamstring muscle weakness associated with hamstring muscle injury in Australian footballers. Am J Sports Med 25:81–85
Orchard J, Seward H (2002) Epidemiology of injuries in the Australian Football League, seasons 1997–2000. Br J Sports Med 36:39–44
Peetrons P (2002) Ultrasound of muscles. Eur Radiol 12:35–43
Petersen J, Thorborg K, Nielsen MB, Budtz-Jørgensen E, Hölmich P (2011) Preventive effect of eccentric training on acute hamstring injuries in men’s soccer: a cluster-randomized controlled trial. Am J Sports Med 39:2296–2303
PRP Therapy Dallas (2013) Athletes using PRP therapy. http://prptherapydallas.com/athletes-using-prp-therapy/
Rettig AC, Meyer S, Bhadra AK (2013) Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players clinical effects and time to return to play. Orthop J Sports Med 1:2325967113494354
Sánchez M, Anitua E, Azofra J, Aguirre JJ, Andia I (2008) Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: a retrospective cohort study. Clin Exp Rheumatol 26:910–913
Sanchez M, Anitua E, Andia I (2005) Application of autologous growth factors on skeletal muscle healing. http://www.plateletrichplasma.com/pdf/OrthopedicPRP/Sports%20Medicine/66-SanchezRegMed2005.pdf
Sánchez M, Anitua E, Azofra J, Andía I, Padilla S, Mujika I (2007) Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med 35:245–251
Sallay PI, Friedman RL, Coogan PG, Garrett WE (1996) Hamstring muscle injuries among water skiers. Functional outcome and prevention. Am J Sports Med 24:130–136
Sherry MA, Best TM (2004) A comparison of 2 rehabilitation programs in the treatment of acute hamstring strains. J Orthop Sports Phys Ther 34:116–125
Stein T, Linke RD, Buckup J, Efe T, von Eisenhart-Rothe R, Hoffmann R, Jäger A, Welsch F (2011) Shoulder sport-specific impairments after arthroscopic Bankart repair: a prospective longitudinal assessment. Am J Sports Med 39:2404–2414
Wright-Carpenter T, Klein P, Schäferhoff P, Appell HJ, Mir LM, Wehling P (2004) Treatment of muscle injuries by local administration of autologous conditioned serum: a pilot study on sportsmen with muscle strains. Int J Sports Med 25:588–593
Acknowledgments
This study was approved by the Ethical committee of the Italian Hospital from Buenos Aires. IRB: 00003580, study protocol number 1772.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rossi, L.A., Molina Rómoli, A.R., Bertona Altieri, B.A. et al. Does platelet-rich plasma decrease time to return to sports in acute muscle tear? A randomized controlled trial. Knee Surg Sports Traumatol Arthrosc 25, 3319–3325 (2017). https://doi.org/10.1007/s00167-016-4129-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-016-4129-7