Abstract
Purpose
Bone marrow concentrate (BMC) and platelet-rich plasma (PRP) are used extensively in regenerative medicine. The aim of this study was to determine differences in the cellular composition and cytokine concentrations of BMC and PRP and to compare two commercial BMC systems in the same patient cohort.
Methods
Patients (29) undergoing orthopaedic surgery were enrolled. Bone marrow aspirate (BMA) was processed to generate BMC from two commercial systems (BMC-A and BMC-B). Blood was obtained to make PRP utilizing the same system as BMC-A. Bone marrow-derived samples were cultured to measure colony-forming units, and flow cytometry was performed to assess mesenchymal stem cell (MSC) markers. Cellular concentrations were assessed for all samples. Catabolic cytokines and growth factors important for cartilage repair were measured using multiplex ELISA.
Results
Colony-forming units were increased in both BMCs compared to BMA (p < 0.0001). Surface markers were consistent with MSCs. Platelet counts were not significantly different between BMC-A and PRP, but there were differences in leucocyte concentrations. TGF-β1 and PDGF were not different between BMC-A and PRP. IL-1ra concentrations were greater (p = 0.0018) in BMC-A samples (13,432 pg/mL) than in PRP (588 pg/mL). The IL-1ra/IL-1β ratio in all BMC samples was above the value reported to inhibit IL-1β.
Conclusions
The bioactive factors examined in this study have differing clinical effects on musculoskeletal tissue. Differences in the cellular and cytokine composition between PRP and BMC and between BMC systems should be taken into consideration by the clinician when choosing a biologic for therapeutic application.
Level of evidence
Clinical, Level II.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biologic solutions to address the pathologic process of osteoarthritis (OA) have been investigated as potential treatments for focal cartilage lesions, osteochondral lesions, and generalized OA [1]. Mesenchymal stem cells (MSCs) appear promising for the treatment of OA, but are subject to regulatory restrictions, and are not currently approved by the FDA or many other regulatory bodies for clinical use [6, 36]. Bone marrow concentrate (BMC) has been used to deliver MSCs to damaged cartilage [23], to augment microfracture [17, 26–28, 48], and as a direct joint injection method for cartilage repair and early OA [33]. BMC is generated by centrifugation of bone marrow aspirate (BMA) allowing for immediate administration to a patient, and many regulatory bodies have approved these products for clinical use. While BMC contains fewer MSCs than what can be obtained through culture expansion [11, 53], the number of MSC needed for effective therapy has yet to be determined. In addition to MSCs, BMC contains numerous bioactive molecules and cell types including lymphocytes, neutrophils, monocytes, and platelets in various stages of differentiation [21, 23, 34]. In orthopaedics, cytological analysis of BMC is rarely performed. In one study, a fourfold increase in platelets, total nucleated cells, and CD34+ cells in BMC compared to BMA was reported [34]. The concentration of growth factors in BMC has not been quantified, despite their role in the regenerative potential of BMC [40, 47].
Platelet-rich plasma (PRP) is gaining popularity as a biologic treatment for focal cartilage defects and OA [13, 20]. PRP is generated by centrifugation of peripheral blood resulting in increased platelet concentration. The platelets provide multiple growth factors with known roles in target cell activation, cell recruitment, cartilage matrix production, and modulation of the inflammatory response [22]. In current orthopaedic practice, it is commonly regarded that BMC is essentially PRP with additional stem cells; however, this has not been fully elucidated. Therefore, one aim of this study was to determine how BMC differs from PRP. There is known variability in PRP due to differences between patients and manufacturers, and the same premise is assumed for BMC, resulting in the second aim of this study: to compare BMCs generated from two different commercial systems.
Materials and methods
This study was prospective and supervised by the biologics committee as a quality assessment project. Between 11/2013 and 07/2014, 29 consecutive patients, within an age range of 18–70 years, consented for participation. All patients were under the care of a single surgeon (JGK). Patients were excluded if there was a history of blood disorders, haematological malignancy, use of immunosuppressive drugs, or drugs with bone marrow-suppressive effects.
Blood and bone marrow aspirate collection
Blood (25 mL) was drawn into a syringe containing 4 mL acid citrate dextrose (ACD). One millilitre was removed for the study and the remainder was processed to generate 3 mL PRP (Magellan®, Arteriocyte Medical Systems Inc., Hopkinton, MA). One millilitre of PRP was removed for study purposes. Bone marrow was aspirated from the iliac crest into a 30-mL syringe containing 4 mL ACD. The needle was advanced 1 cm and rotated 90° after each 5 mL aspirate until a total of 120 mL was aspirated in four syringes. The total aspirate was mixed, 1 mL was retained as the BMA sample for the study, and the remainder was separated into two 60 mL samples and processed in two systems; Magellan® (BMC-A) (Arteriocyte Medical Systems Inc.) and SmartPrep® 2 (BMC-B; Harvest Technologies Corp., Plymouth, MA). All aspirations were performed by the same surgeon (JGK). One millilitre of each BMC-A and BMC-B was retained for the study. Representatives from both BMC companies observed aspiration and concentration techniques. Samples were shipped de-identified to prevent bias in sample processing and analysis. All samples were processed within 24 h of collection.
Cytology
Automated counts were performed on whole blood (WB), PRP, BMA, BMC-A, and BMC-B samples to assess platelets, red blood cells (RBC), and nucleated cell counts (NCC), which included myeloid precursors as well as white blood cells (WBC: neutrophils, monocytes, lymphocytes, eosinophils, basophils). Cytological smears were evaluated of all samples to verify automated counts.
Colony-forming units and flow cytometry
Equal volumes of BMCs were used for CFU assays, rather than a known number of nucleated cells. This reflects clinical practice where volume, as opposed to cell count, would be the unit of BMC application. Samples of BMC were cultured in duplicate with DMEM containing 10 % FBS, penicillin/streptomycin, and 1 ng/mL basic fibroblastic growth factor [50]. Media were changed every 48 h. After 2 weeks, CFUs were circled with a 1.8-cm-diameter self-inking marker. A colony was counted if it was ≥1 mm in diameter. This adapted technique [24, 33] allowed for retention of viable cells and further analyses.
Cells were then passaged once and then lifted with Accumax (Sigma-Aldrich, St. Louis, MO) when confluent at passage 1 for flow cytometry of MSCs markers [16], including positive (CD146, CD73, CD271) and negative markers (CD34, CD45). Conjugated primary monoclonal antibodies and isotype controls were used as recommended by the manufacturer (BD Biosciences, San Jose, CA). Cells were analysed on a FACS Canto II (BD Immunocytometry Systems) flow cytometer and using FlowJo software (TreeStar Inc., Ashland, OR).
Growth factor and cytokine analyses
Multiplex assays were performed according to manufacturer directions to measure vascular endothelial growth factor (VEGF), interleukin 1 beta (IL-1β), interleukin 6 (IL-6), interleukin 8 (IL-8), tumour necrosis factor-α (TNF-α), interleukin 1 receptor antagonist protein (IL-1ra), and interferon-γ (IFN-γ; Fluorokine MAP Multiplex Human Cytokine Panel A, R&D Systems, Minneapolis, MN). Transforming growth factor beta 1 (TGF-β1), TGF-β2, and TGF-β3 were measured using the Fluorokine MAP Multiplex TGF-beta 3-plex Kit (R&D Systems). Platelet-derived growth factor-BB (PDGF-BB) and acidic fibroblast growth factor (FGF-1) were quantified using the Fluorokine MAP Multiplex Human Angiogenesis Panel A (R&D Systems). Multiplex assays were performed using the Luminex 200 instrument (Luminex Corp., Austin, TX).
Institutional Review Board approval
This study was approved by the Hospital for Special Surgery’s Institutional Review Board (ID #29055).
Statistical analyses
BMA was obtained from 29 patients. Blood was also obtained from the last 14 of the 29 patients. In BMC-B from the first six patients, no MSCs were observed in the cultures. The company was contacted, and they replaced the machine, which resulted in growth of MSCs in all subsequent samples. The authors regarded this as mechanical failure and elected to exclude these patients from the study. Four BMA and BMC samples were excluded because they were not processed within 24 h of collection due to a shipping delay as the result of inclement weather. Further inclusion/exclusion criteria for statistical analysis were set to confirm that BMC and PRP were generated. For BMC, this was defined as an increase in NCC or CFU compared to BMA, resulting in exclusion of 1 sample. For PRP, inclusion was defined as an increase in platelet or PDGF concentration compared to that measured in whole blood, resulting in exclusion of two patients [13]. To account for the individual variability inherent in biologics, paired statistical methods were used. Data were tested for normality using a Shapiro–Wilk test. A two-sided paired t test was used for normally distributed differences between groups; otherwise, a Wilcoxon Signed Rank test was used, with an α of 0.05. There were no available studies using a multiplex of BMA/BMC data to perform a power analysis.
Results
The mean age of patients was 48.2 years with a range of 23–68. Ten were female and the remainder (9) male. There were no reported complications associated with collection of bone marrow or blood.
Cytology and platelet concentration in PRP compared to BMC
BMC-A was used for comparison to PRP because both were generated from the same system, thus allowing for a comparison based on biologic differences rather than preparation methods. The concentration of total WBCs and all subtypes of WBCs were greater in BMC-A compared to PRP (Table 1). There was an 11.8-fold increase in WBC concentration in BMC-A compared to PRP. Neutrophils were increased in BMC-A by 19-fold, monocytes by 11-fold, and lymphocytes by sevenfold compared to PRP. The platelet concentration in PRP was increased by 2.5-fold compared to WB; however, there was no difference in platelet concentration between BMC-A and PRP.
Cytology and platelet concentration in BMA compared to BMC
The NCC in both BMC-A and BMC-B was increased compared to BMA verifying that both systems concentrated nucleated cells (Table 2). The NCC in BMC-A was increased by 3.3-fold, and by 4.1-fold in BMC-B compared to BMA. There was no difference in NCC between BMC-A and BMC-B. The total WBC concentration was increased in both BMC-A and BMC-B compared to BMA, but there was no difference between BMC-A and BMC-B. All subtypes of WBCs were increased in BMC-A and BMC-B compared to BMA. The average neutrophil concentration in BMC-B was 1.6-fold greater than BMC-A. In contrast, the average concentration of lymphocytes was 1.3-fold greater in BMC-A compared to BMC-B. However, there was no significant difference between lymphocyte concentration in BMC-A and BMC-B. There were no statistically significant differences in monocyte, eosinophil, or basophil concentrations between BMC-A and BMC-B. Platelet concentration was increased by 4.8-fold in BMC-A and 3.5-fold in BMC-B compared to BMA, resulting in a significantly greater platelet concentration in BMC-A compared to BMC-B.
Colony-forming units
The number of CFUs was not different between duplicates (p = 0.44), so duplicates were averaged for analyses. The mean number of CFUs was 7.8 ± S.D. 12.3 (range 0–46) in BMA, 41.4 ± 27.4 (range 9–90) in BMC-A, and 32.7 ± 30.3 (range 2–90) in BMC-B. CFUs were significantly increased in both BMCs compared to BMA (p < 0.0001), and BMC-A and BMC-B were not significantly different (p = 0.079).
Flow cytometry of MSCs from BMC
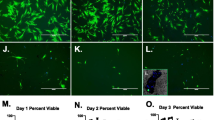
The minimum number of 2.5 × 105 cells needed for flow cytometry of each molecule limited the ability to measure all five MSC markers in every sample, so CD45 and CD271 were prioritized based on the literature [14, 45]. There were insufficient CFUs in BMA samples for comparison to BMC. All MSCs from BMC-A and BMC-B were negative for the lymphocyte marker CD45 (Fig. 1). The majority of BMC-A and BMC-B-derived MSCs were positive for CD271, and only a small percentage were positive for the haematopoietic stem cell marker CD34. All MSCs were positive for CD73 and variably positive for CD146.
Cell surface marker expression based on flow cytometry of cell samples cultured from bone marrow concentrate from commercial system A (BMC-A) and bone marrow concentrate from commercial system B (BMC-B). CD271, CD73, and CD146 are markers expressed on mesenchymal stem cells (MSCs). MSCs are negative for CD34, a haematopoietic stem cell marker, and CD45, a leucocyte marker
Growth factors and cytokines
PDGF-BB
The concentration of PDGF was not significantly different between PRP and BMC-A (Table 3). As expected, PDGF was increased (1.7-fold) in PRP compared to WB. PDGF was also increased in both BMC-A and BMC-B compared to BMA, with concentrations greater in BMC-A compared to BMC-B (Table 4).
TGF-β1, 2, 3
There was no significant difference in TGF-β1 concentration between BMC-A and PRP (Fig. 2). Not surprisingly, TGF-β1 was increased in PRP compared to WB and in both BMC-A and BMC-B compared to BMA. BMC-A had significantly more TGF-β1 compared to BMC-B. TGF-β2 was below the limit of detection (17.5 pg/mL) in all BMA and WB samples. TGF-β2 was present in 15 out of 18 BMC-A samples, 4 out of 19 BMC-B samples, and 9 out of 12 PRP samples. The concentration of TGF-β2 was greater in BMC-A compared to PRP (Table 3) and BMC-B (Table 4). TGF-β3 concentrations were below the limit of detection (62.2 pg/mL) in all samples.
Transforming growth factor beta 1 in BMA, BMC-A, BMC-B, whole blood (WB), and platelet-rich plasma (PRP). *Significant difference (p < 0.05) between two groups based on a two-sided paired T test. Data are presented in an outlier plot with whiskers extending to the farthest point within 1.5 interquantile range lengths
VEGF
VEGF concentrations were lowest in PRP, WB, and BMA samples (Tables 3, 4). In BMC-A, VEGF was increased compared to PRP (Table 3). Compared to BMA, VEGF concentration was increased fourfold in BMC-A and sevenfold in BMC-B (Table 4).
IL-8
IL-8 concentrations were below the limit of detection (3.5 pg/mL) in WB and PRP samples (Table 3). IL-8 was increased threefold in BMC-A and fivefold in BMC-B samples compared to BMA (Table 4). There was no difference in IL-8 concentration between the two BMC groups.
IL-1β
There was no measureable IL-1β in WB, PRP, or BMA (Tables 3, 4). Additionally, IL-1β was undetectable in nine BMC-A and six BMC-B samples. In samples that were above the lower limit of detection (1.5 pg/mL), IL-1β was greater in BMC-B compared to BMC-A (Table 4).
IL-1ra
All samples contained measurable IL-1ra. IL-1ra was significantly increased in BMC-A compared to PRP (Fig. 3). In WB, IL-1ra was greater than in PRP. Compared to BMA, IL-1ra was significantly increased in both BMC-A (threefold) and BMC-B (fivefold), and BMC-B was significantly greater than BMC-A. The ratios of IL-1ra to IL-1β were calculated for all samples with detectable concentrations of IL-1β (Fig. 4). The average IL-1ra/IL-1β ratio for the six patients with BMC-A and BMC-B samples with detectable IL-1β was not significantly different (p = 0.09).
Ratio of interleukin 1 receptor antagonist to interleukin 1 beta in BMC-A and BMC-B. Ratios represented are from the n = 9/18 BMC-A samples and n = 13/19 BMC-B samples that had detectable levels of IL-1b. The samples with IL-1b levels below the limit of detection (1.5 pg/mL) would have an even greater positive biological effect on competing with endogenous IL-1b in clinical use. *Significant difference (p < 0.05) between two groups based on a two-sided paired T test. Data are presented in an outlier plot with whiskers extending to the farthest point within 1.5 interquantile range lengths
TNF-α, IL-6, IFN-γ, FGF-1
All four of these cytokines were undetectable in all samples. Minimum detectable concentrations for the assays were: TNF-α (5.1 pg/mL), IL-6 (5.2 pg/mL), IFN-γ (2.9 pg/mL), and FGF-1 (5.7 pg/mL).
Discussion
The most important finding of this study is the discovery that bone marrow-derived biologics contain clinically relevant concentrations of IL-1ra. This study also shows several significant differences in the cellular and molecular composition of PRP and BMC (Table 5), and differences in cytology and bioactive molecules between BMC manufacturing systems. Both BMC manufacturing systems effectively concentrated BMA as demonstrated by an increased total NCC in most BMC samples. BMC-B initially failed to generate BMC due to a malfunction of the centrifuge. Further, there was one failure to generate BMC in BMC-A and two failures to generate PRP from blood with no known machine malfunction. This might be expected based on similar results when generating PRP [10], but it has not been previously documented. Failure to generate BMC or PRP would not be visually recognized by the surgical team which underscores the importance of counting cells and platelets in biologics when reporting outcome data.
Although there was an increase in NCC in the BMC-B group compared to BMC-A, CFUs in BMC-A and BMC-B were not significantly different. This suggests that NCC alone may not be predictive of MSC concentration. In a previous study, higher NCCs and CFUs were found in BMC-B compared to BMC-A [33]. These conflicting results are likely explained by different study methodologies. In the current study, a uniform volume of BMC was used for culture rather than a defined number of nucleated cells [33]. This method was chosen to reflect clinical practice where a doctor would administer a specified volume of BMC rather than a defined number of nucleated cells. All MSCs had flow cytometry markers consistent with MSCs. The heterogeneity in surface markers of MSCs observed in this study has been previously reported and is not surprising given the lack of cell selection processes and the short culture duration (analysis at passage 1) as some of the characteristic markers are not uniformly expressed until later passages [3, 38].
Several growth factors have positive effects on cartilage repair and in the treatment of OA. The role of PDGF in cartilage repair and homoeostasis has been extrapolated from its function during chondrogenesis [2]. PDGF induces MSC proliferation [22, 25] and inhibits IL-1β-induced chondrocyte apoptosis and inflammation [42]. PDGF was present in all samples in varying concentrations and corresponding to platelet concentrations. All three TGF-β isoforms examined have roles in chondrogenesis [7, 35, 39, 43, 44, 51, 56]. TGF-β1 has been shown to stimulate chondrogenesis of synovium and bone marrow-derived MSCs [18, 37], inhibit IL-1β-mediated inflammation [9], and enhance cartilage healing [12]. TGF-β1 was increased in PRP and BMC, paralleling increased platelet concentrations. PRP and BMC-A did not differ in TGF-β1 concentration; however, increased TGF-β1 was present in BMC-A compared to BMC-B. These findings are likely related to platelet, monocyte, and neutrophil concentrations [31]. TGF-β2 was present in most BMC-A and PRP samples, but only a few BMC-B samples. In OA chondrocytes, TGF-β2 decreases collagen type 2 cleavage and chondrocyte hypertrophy through inhibition of IL-1β and TNF-α [49]. Many cell types secrete TGF-β2, thus differences in cellular distribution may be the source of the divergent TGF-β2 concentrations.
In balance with growth factors, PRP and BMC products contain pro-inflammatory cytokines. IL-1β and IL-8 are secreted by monocytes, neutrophils, and MSCs [19, 29, 52]. In WB and PRP, IL-1β and IL-8 were undetectable, but they were present in BMA and increased in both BMC groups. This is likely a result of concentration of neutrophils, monocytes, and MSCs during centrifugation [4]. IL-8 is a potent chemoattractant for neutrophils, which secrete IL-1, and in turn, can further stimulate monocytes to produce IL-8 [4, 8, 32]. This might suggest that PRP would be a less inflammatory, more anabolic biologic. However, the surprising and significant concentration of IL-1ra found in BMC and its absence in PRP needs to be taken into consideration when choosing between PRP and BMC for any therapeutic modality.
Interleukin 1 receptor antagonist (IL-1ra) is a naturally occurring antagonist that competitively binds to IL-1β and IL-1α cell surface receptors, thereby inhibiting IL-1 catabolism. IL-1ra is thought to be responsible for the beneficial effects of the biologic autologous conditioned serum (ACS) [41, 54]. Two randomized control trials in knee OA reported superiority of ACS over hyaluronan or saline injection [5, 55]. ACS is not approved for use in the USA, thus its implementation has been confined primarily to Europe. The original reported concentration of IL-1ra in ACS was 10,254 pg/mL [41], and subsequent studies documented concentrations of 2015 pg/mL [54] and <2000 pg/mL [46]. In the present study, the average concentration of IL-1ra in BMA was 4510 pg/mL, which was then increased threefold in BMC-A and fivefold in BMC-B. For effective therapy, an IL-1ra/IL-1 ratio of 10:1 to 100:1 [15, 30] has been reported as sufficient to block IL-1. The IL-1ra/IL-1β ratio in BMC samples ranged from 249:1 to 17,568:1, indicating a net inhibitory effect on IL-1. The use of BMC products provides a patient-side method for generation of IL-1ra that would be allowed in regions such as the USA where ACS is restricted by regulatory agencies. While this study did not seek to correlate clinical outcomes with biologic components, due to the heterogeneity of surgical procedures involved, the variability of cellular and molecular components described can serve as a guide to design appropriately powered studies. Although PRP, BMC, and ACS are purported to alter the healing response through the actions of specific growth factors, stem cells, and IL-1ra, respectively, it is likely that the overall net effect of the various components will determine the clinical impact. Different tissues likely have different requirements for biologic agents, and the acute versus chronic disease progression will require distinct approaches. The ideal formulation for each tissue and disease state can only be determined through comparative clinical studies.
Conclusion
Differences in the cellular and molecular composition of PRP and BMC should be considered in light of the pathology being treated. BMC provides a patient-side method to generate the potent anti-inflammatory therapy, IL-1ra, and deliver stem cells in addition to growth factors commonly found in PRP to patients where they might not be otherwise available due to regulatory restrictions.
References
Anz AW, Hackel JG, Nilssen EC, Andrews JR (2014) Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg 22:68–79
Ataliotis P (2000) Platelet-derived growth factor A modulates limb chondrogenesis both in vivo and in vitro. Mech Dev 94:13–24
Baer PC, Geiger H (2012) Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int 2012:812693
Baggiolini M, Clark-Lewis I (1992) Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett 307:97–101
Baltzer AW, Moser C, Jansen SA, Krauspe R (2009) Autologous conditioned serum (Orthokine) is an effective treatment for knee osteoarthritis. Osteoarthr Cartil 17:152–160
Barry F, Murphy M (2013) Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol 9:584–594
Barry F, Boynton RE, Liu B, Murphy JM (2001) Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res 268:189–200
Bazzoni F, Cassatella MA, Rossi F, Ceska M, Dewald B, Baggiolini M (1991) Phagocytosing neutrophils produce and release high amounts of the neutrophil-activating peptide 1/interleukin 8. J Exp Med 173:771–774
Blaney Davidson EN, van der Kraan PM, van den Berg WB (2007) TGF-beta and osteoarthritis. Osteoarthr Cartil 15:597–604
Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA (2012) Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy 28:429–439
Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D (2008) Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician 11:343–353
Chen J, Chen H, Li P, Diao H, Zhu S, Dong L, Wang R, Guo T, Zhao J, Zhang J (2011) Simultaneous regeneration of articular cartilage and subchondral bone in vivo using MSCs induced by a spatially controlled gene delivery system in bilayered integrated scaffolds. Biomaterials 32:4793–4805
Cole BJ, Seroyer ST, Filardo G, Bajaj S, Fortier LA (2010) Platelet-rich plasma: where are we now and where are we going? Sports Health 2:203–210
Cuthbert R, Boxall SA, Tan HB, Giannoudis PV, McGonagle D, Jones E (2012) Single-platform quality control assay to quantify multipotential stromal cells in bone marrow aspirates prior to bulk manufacture or direct therapeutic use. Cytotherapy 14:431–440
Dinarello CA, Thompson RC (1991) Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today 12:404–410
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317
Enea D, Cecconi S, Calcagno S, Busilacchi A, Manzotti S, Kaps C, Gigante A (2013) Single-stage cartilage repair in the knee with microfracture covered with a resorbable polymer-based matrix and autologous bone marrow concentrate. Knee 20:562–569
Fan J, Ren L, Liang R, Gong Y, Cai D, Wang DA (2010) Chondrogenesis of synovium-derived mesenchymal stem cells in photopolymerizing hydrogel scaffolds. J Biomater Sci Polym Ed 21:1653–1667
Fernandes JC, Martel-Pelletier J, Pelletier JP (2002) The role of cytokines in osteoarthritis pathophysiology. Biorheology 39:237–246
Filardo G, Kon E, Roffi A, Di Matteo B, Merli ML, Marcacci M (2013) Platelet-rich plasma: why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg Sports Traumatol Arthrosc 23:2459–2474
Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M, Kon E (2013) Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc 21:1717–1729
Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ (2011) The role of growth factors in cartilage repair. Clin Orthop Relat Res 469:2706–2715
Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, Stokol T, Cheetham J, Nixon AJ (2010) Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am 92:1927–1937
Galotto M, Berisso G, Delfino L, Podesta M, Ottaggio L, Dallorso S, Dufour C, Ferrara GB, Abbondandolo A, Dini G, Bacigalupo A, Cancedda R, Quarto R (1999) Stromal damage as consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Exp Hematol 27:1460–1466
Gharibi B, Hughes FJ (2012) Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem Cells Transl Med 1:771–782
Gigante A, Cecconi S, Calcagno S, Busilacchi A, Enea D (2012) Arthroscopic knee cartilage repair with covered microfracture and bone marrow concentrate. Arthrosc Tech 1:e175–e180
Gobbi A, Karnatzikos G, Scotti C, Mahajan V, Mazzucco L, Grigolo B (2011) One-step cartilage repair and bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-year follow-up. Cartilage 2:286–299
Gobbi A, Karnatzikos G, Sankineani SR (2014) One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med 42:648–657
Goldring MB (2000) Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep 2:459–465
Granowitz EV, Clark BD, Mancilla J, Dinarello CA (1991) Interleukin-1 receptor antagonist competitively inhibits the binding of interleukin-1 to the type II interleukin-1 receptor. J Biol Chem 266:14147–14150
Grotendorst GR, Smale G, Pencev D (1989) Production of transforming growth factor beta by human peripheral blood monocytes and neutrophils. J Cell Physiol 140:396–402
Hattar K, Fink L, Fietzner K, Himmel B, Grimminger F, Seeger W, Sibelius U (2001) Cell density regulates neutrophil IL-8 synthesis: role of IL-1 receptor antagonist and soluble TNF receptors. J Immunol 166:6287–6293
Hegde V, Shonuga O, Ellis S, Fragomen A, Kennedy J, Kudryashov V, Lane JM (2014) A prospective comparison of 3 approved systems for autologous bone marrow concentration demonstrated nonequivalency in progenitor cell number and concentration. J Orthop Trauma 28:591–598
Johnson RG (2014) Bone marrow concentrate with allograft equivalent to autograft in lumbar fusions. Spine (Phila Pa 1976) 39:695–700
Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU (1998) In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238:265–272
Koh YG, Choi YJ, Kwon OR, Kim YS (2014) Second-look arthroscopic evaluation of cartilage lesions after mesenchymal stem cell implantation in osteoarthritic knees. Am J Sports Med 42:1628–1637
Kurth T, Hedbom E, Shintani N, Sugimoto M, Chen FH, Haspl M, Martinovic S, Hunziker EB (2007) Chondrogenic potential of human synovial mesenchymal stem cells in alginate. Osteoarthr Cartil 15:1178–1189
Lv FJ, Tuan RS, Cheung KM, Leung VY (2014) Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells 32:1408–1419
Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF (1998) Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng 4:415–428
McCarrel T, Fortier L (2009) Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res 27:1033–1042
Meijer H, Reinecke J, Becker C, Tholen G, Wehling P (2003) The production of anti-inflammatory cytokines in whole blood by physico-chemical induction. Inflamm Res 52:404–407
Montaseri A, Busch F, Mobasheri A, Buhrmann C, Aldinger C, Rad JS, Shakibaei M (2011) IGF-1 and PDGF-bb suppress IL-1beta-induced cartilage degradation through down-regulation of NF-kappaB signaling: involvement of Src/PI-3K/AKT pathway. PLoS ONE 6:e28663
Mueller MB, Tuan RS (2008) Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum 58:1377–1388
Mwale F, Stachura D, Roughley P, Antoniou J (2006) Limitations of using aggrecan and type X collagen as markers of chondrogenesis in mesenchymal stem cell differentiation. J Orthop Res 24:1791–1798
Poloni A, Maurizi G, Rosini V, Mondini E, Mancini S, Discepoli G, Biasio S, Battaglini G, Felicetti S, Berardinelli E, Serrani F, Leoni P (2009) Selection of CD271(+) cells and human AB serum allows a large expansion of mesenchymal stromal cells from human bone marrow. Cytotherapy 11:153–162
Rutgers M, Saris DB, Dhert WJ, Creemers LB (2010) Cytokine profile of autologous conditioned serum for treatment of osteoarthritis, in vitro effects on cartilage metabolism and intra-articular levels after injection. Arthritis Res Ther 12:R114
Schnabel LV, Mohammed HO, Miller BJ, McDermott WG, Jacobson MS, Santangelo KS, Fortier LA (2007) Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res 25:230–240
Skowronski J, Rutka M (2013) Osteochondral lesions of the knee reconstructed with mesenchymal stem cells—results. Ortop Traumatol Rehabil 15:195–204
Tchetina EV, Antoniou J, Tanzer M, Zukor DJ, Poole AR (2006) Transforming growth factor-beta2 suppresses collagen cleavage in cultured human osteoarthritic cartilage, reduces expression of genes associated with chondrocyte hypertrophy and degradation, and increases prostaglandin E(2) production. Am J Pathol 168:131–140
Tsutsumi S, Shimazu A, Miyazaki K, Pan H, Koike C, Yoshida E, Takagishi K, Kato Y (2001) Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun 288:413–419
Tuli R, Tuli S, Nandi S, Huang X, Manner PA, Hozack WJ, Danielson KG, Hall DJ, Tuan RS (2003) Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem 278:41227–41236
Van Lent PL, Van De Loo FA, Holthuysen AE, Van Den Bersselaar LA, Vermeer H, Van Den Berg WB (1995) Major role for interleukin 1 but not for tumor necrosis factor in early cartilage damage in immune complex arthritis in mice. J Rheumatol 22:2250–2258
Veronesi F, Giavaresi G, Tschon M, Borsari V, Nicoli Aldini N, Fini M (2013) Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cells Dev 22:181–192
Wehling P, Moser C, Frisbie D, McIlwraith CW, Kawcak CE, Krauspe R, Reinecke JA (2007) Autologous conditioned serum in the treatment of orthopedic diseases: the orthokine therapy. BioDrugs 21:323–332
Yang KG, Raijmakers NJ, van Arkel ER, Caron JJ, Rijk PC, Willems WJ, Zijl JA, Verbout AJ, Dhert WJ, Saris DB (2008) Autologous interleukin-1 receptor antagonist improves function and symptoms in osteoarthritis when compared to placebo in a prospective randomized controlled trial. Osteoarthr Cartil 16:498–505
Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, Johnstone B (1998) The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am 80:1745–1757
Acknowledgments
Arteriocyte Medical Systems Inc (JGK) and the Harry M. Zweig Memorial Fund (LAF) provided funding for the study. Personnel support (KAR, EJF) was provided by the Ohnell family foundation and the Michel Levitt family foundation. Stipend support (JMC) was provided by the Morris Animal Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author (JGK) is a consultant for Arteriocyte Medical Systems Inc. Author (LAF) is a consultant for Arthrex Inc. All samples were de-identified prior to shipment to prevent any potential biases from being introduced into the study analyses.
Ethical standards
All procedures performed in studies involving human participants were approved by the institutional research review board and in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Cassano, J.M., Kennedy, J.G., Ross, K.A. et al. Bone marrow concentrate and platelet-rich plasma differ in cell distribution and interleukin 1 receptor antagonist protein concentration. Knee Surg Sports Traumatol Arthrosc 26, 333–342 (2018). https://doi.org/10.1007/s00167-016-3981-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-016-3981-9