Abstract
Purpose
To compare the clinical outcomes of osteoarthritis indices (WOMAC and Lequesne scores) and adverse events in the treatment of osteoarthritis (OA) of the knee with platelet-rich plasma (PRP) versus hyaluronic acid (HA) or placebo.
Methods
A systematic review and meta-regression were performed to compare outcomes between PRP injections versus HA or placebo. Relevant randomized control trials were identified from Medline and Scopus from date of inception to 13 August 2015.
Results
Nine of 551 studies were eligible; 6, 5, 5, 5, 2, 2, 2 and 7 studies were included in pooling of WOMAC total, pain, stiffness and function scores, Lequesne score, IKDC score, EQ-VAS score and adverse events in OA knee patients, respectively. The PRP injections had −15.4 (95 % CI −28.6, −2.3, p = 0.021), lower mean WOMAC total scores, and 8.83 (95 % CI 5.88, 11.78, p < 0.001), 7.37 (95 % CI 4.33, 10.05, p = 0.021) higher mean IKDC and EQ-VAS scores when compared to HA injections. However, PRP injections had no significant differences in WOMAC pain, stiffness and function scores, as well as Lequesne score and adverse events when compared to HA or placebo.
Conclusion
In short-term outcomes (≤1 year), PRP injection has improved functional outcomes (WOMAC total scores, IKDC score and EQ-VAS) when compared to HA and placebo, but has no statistically significant difference in adverse events when compared to HA and placebo. This study suggests that PRP injection is more efficacious than HA injection and placebo in reducing symptoms and improving function and quality of life. It has the potential to be the treatment of choice in patients with mild-to-moderate OA of the knee who have not responded to conventional treatment.
Level of evidence
I.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is a degenerative joint disease that is common in the elderly population [9, 22]. Treatment goals include pain relief, improvement in knee function, improved quality of life and reduction in disability. Unfortunately, there are currently no pharmacologic agents available that can halt OA progression and reverse any existing damage. Current therapeutic approaches focus on developing less invasive procedures and applying interventions earlier in the disease progression, when the structural changes of OA may still be prevented or delayed [30, 34]. Recent developments in biologic research have highlighted the importance of growth factors in maintenance of normal tissue structure and tissue lesion repair [17, 31]. Several studies describe the use of biological therapies such as platelet-rich plasma (PRP) as effective and safe methods in the treatment of pain and joint dysfunction caused by knee OA. There is an increasing amount of evidence supporting the potential benefits of plasma that is rich in growth factors, which is an autologous PRP that is characterized by leucocytes (rich or poor) [28], pro-inflammatory cytokines and the presence of a specific dose of platelets and growth factors [4]. The use of this autologous biological therapy has been shown to enhance tissue repair and reduce tissue inflammation [3, 29]. Several randomized controlled trials [7, 12, 24, 26, 27, 30, 33] have shown favourable results of intra-articular PRP injections when compared to hyaluronic acid (HA) [7, 12, 26, 30, 33] and placebo injections [24, 27] in patients with cartilage damage and OA of the knee. However, the results also displayed negative outcomes. Five network meta-analyses [6, 8, 16, 18, 19] have been published recently. Four of these meta-analyses [8, 16, 18, 19] that pooled RCTs and comparative studies were inconclusive regarding the efficacy of PRP. The most recently published meta-analysis [6] was a systematic review of overlapping meta-analyses, and this meta-analysis found that although PRP injection improves knee symptoms for up to 12 months, there appears to be an increased risk of adverse reaction associated with its use. All of the meta-analyses did not strictly pool outcomes from studies of high methodological quality (RCTs) as there were very few RCTs available for review at the time. Sources of heterogeneity (e.g. grade of OA, age, sex, BMI and type of PRP) were also not assessed. Additional RCTs [11, 13, 26, 27] have since been published. Therefore, we conducted a systematic review and meta-analysis comparing clinical outcomes when treating osteoarthritis of the knee by injecting intra-articular PRP as compared to hyaluronic acid (HA) or placebo. The clinical outcomes of interest were osteoarthritis indices (WOMAC and Lequesne scores) and adverse events.
Materials and methods
Search strategy
The Medline and Scopus databases were used to identify relevant studies published in English from the date of inception to 13 August 2015. The PubMed and Scopus search engines were used to locate studies using the following search terms: [(osteoarthritis knee OR gonarthrosis OR elderly) AND (platelet rich plasma OR platelet concentrate OR PRP OR platelet derived growth factors OR PRGF) AND (visual analog score OR WOMAC score OR Lequesne score OR pain OR function OR radiographic grading OR X-ray) AND (clinical trial OR RCT OR randomized controlled trial)]. Search strategies for Medline and Scopus are described in the (Appendix in Electronic Supplementary Material). Relevant studies from the reference lists of identified studies and previous systematic reviews were also explored.
Selection of studies
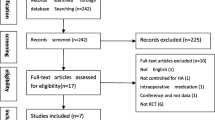
Identified studies were selected by two authors (W.K. and A.A.) and randomly checked by (J.K.). Titles and abstracts were initially screened; full papers were then retrieved if a decision could not be made from the abstracts. The reasons for ineligibility or exclusion of studies were recorded and described (Fig. 1).
Inclusion criteria
Randomized controlled trials or quasi-experimental designs comparing clinical outcomes between treatments in primary OA patients knee were eligible if they met the following criteria:
-
Compared clinical outcomes between platelet-rich plasma (PRP) with hyaluronic acid, normal saline solution or placebo (no treatment).
-
Compared at least one of following outcomes: range of motion, adverse events, function score, osteoarthritis indices including WOMAC total score, WOMAC sub-scores Lequesne algofunctional index (Lequesne scores), IKDC subjective score and EQ-VAS.
-
Had sufficient data to extract and pool, i.e. reported mean, standard deviation (SD) and numbers of subjects according to treatments for continuous outcomes; number of patients according to treatment for dichotomous outcomes.
Data extraction
Two reviewers (W.K. and A.A.) independently performed data extraction using standardized data extraction forms. General characteristics of the study (e.g. mean age, gender, body mass index (BMI), duration of OA, type of PRP, pain score, functional scores, osteoarthritis index at baseline) were extracted. The number of subjects, mean and SD of continuous outcomes (i.e. pain by visual analogue score (VAS), WOMAC total and sub-scores, Lequesne scores) between groups were extracted. Cross-tabulated frequencies between treatment and adverse events were also extracted. Any disagreements were resolved by discussion and consensus with a third party (J.K.).
Risk of bias assessment
Two authors (W.K. and A.A.) independently assessed risk of bias for each study. Six study quality domains were considered. These included sequence generation, allocation concealment, blinding (participant, personnel and outcome assessors), incomplete outcome data, selective outcome reporting and other sources of bias [21]. Disagreements between two authors were resolved by consensus and discussion with a third party (J.K.).
Outcomes
The outcomes of interests were WOMAC total and sub-scores (i.e. pain, stiffness and function), Lequesne score, EuroQol visual analogue scale (EuroQol-VAS), IKDC subjective scores and adverse events. Methods of measure for these outcomes were used according to the original studies. Briefly, this includes the VAS pain scale from 0 to 10; the WOMAC score that consists of pain (0–20), stiffness (0–8) and function (0–68) with total scores of 0 to 96 [5]; and the Lequesne algofunctional index that measures pain (0–10); maximum distance walked (0–6); and activities of daily living (0–8) with total scores of 0–24 [5, 20]. The EuroQol-VAS is a simple validated and commonly used patient-administered method that assesses pain intensity (0–100). The IKDC subjective evaluation form is commonly used and detects improvement in function and symptoms for knee disorders. The form has three domains: knee symptoms with seven items; sports and daily activities with ten items; and current knee function with one item. The total score ranges at 0–100, where 100 means the absence of symptoms and no limitation for daily or sporting activities [15]. The adverse events and patient satisfaction levels were recorded as well. Adverse events were considered as composite and separate outcomes of the following: injected site pain, infection and other local complications.
Statistical analysis
Direct comparisons of continuous outcomes were measured at the end of each study between PRP versus HA and PRP versus placebo and were then pooled using an unstandardized mean difference (UMD). Heterogeneity of the mean difference across studies was checked using the Q statistic, and the degree was quantified using the I 2 statistic. If heterogeneity was present (p < 0.10 or the I 2 > 25 %), the UMD was estimated using a random effect model; otherwise, a fixed effect model was applied.
For dichotomous outcomes, relative risks (RR) of the adverse reactions of treatment comparisons at the end of each study were estimated and pooled. Heterogeneity was assessed using the same method as mentioned previously. If heterogeneity was present, the Dersimonian and Laird method [1] was applied for pooling; otherwise, the fixed effect model by inverse variance method was applied. Meta-regression was applied to explore the source of heterogeneity [e.g. mean age, percentage of females, body mass index (BMI), OA grading, PRP formulation (injection time, spin approach, leucocyte rich or leucocyte poor) or duration of OA] if data were available. Subgroup or sensitivity analysis was then performed according to the results of meta-regression. Publication bias was assessed using contour-enhanced funnel plots [23, 25] and Egger tests [10]. Asymmetry of the funnel plot may be due to missing data in some studies in which the results that were negative might not have been published and thus could not be identified. The metatrim and fill method was used to estimate the number of studies that might be missing and to adjust the pooled estimate [1]. All analyses were performed using STATA version 13.0 [32]. A p value <0.05 was considered statistically significant, except for the test of heterogeneity where p < 0.10 was used.
Results
Fifty-six and 510 studies were identified from Medline and Scopus, respectively (Fig. 1). Fifteen of the studies were duplicates, leaving 551 studies for review of titles and abstracts. Of these, 8 full papers plus 1 study from hand searching were reviewed, leaving a total of 9 studies for data extraction. Characteristics of the 9 studies [7, 11–13, 24, 26, 27, 30, 33] are described in Table 1. Seven studies [7, 11–13, 26, 30, 33] compared PRP with HA. Two studies [24, 27] compared PRP with placebo. The osteoarthritis indices were reported using the WOMAC total score in 6 studies [7, 24, 26, 27, 30, 33], WOMAC sub-scores in 5 studies [24, 26, 27, 30, 33], Lequesne scores in 2 studies [30, 33], IKDC scores in 2 studies [11, 13] and EQ-VAS in 2 studies [11, 13]. Adverse events (composite outcomes of injected site pain, infection and other local complications) were reported in 7 studies [7, 12, 13, 24, 27, 30, 33]. Mean age, BMI and mean follow-up of participants varied from 52.7 to 66.4 years, 26 to 30.9 kg/cm2 and 6 to 12 months, respectively. Percentages of female gender ranged from 37.6 to 93.5 %. Percentages of patients with osteoarthritis graded by Kellgren–Lawrence (KL) I–II ranged from 50 to 90 %. The PRP formulations that were used by each trial (platelet concentration, leucocytes, activation method and injective protocol) were as follows. The mean platelet counts in all studies were more than 150,000/ul. Four studies were leucocyte-poor (LP) PRP and 5 studies were leucocyte-rich (LR) PRP. Four of the studies were single-spinning preparations of PRP and 5 studies were double-spinning preparations. From the 9 studies [7, 11–13, 24, 26, 27, 30, 33], 3 studies [24, 26, 27] had injected PRP twice, 5 studies [11–13, 30, 33] had injected PRP 3 times, and only one study [7] had injected PRP 4 times. One study [24] compared single injection and double injection with placebo injection. Results showed no statistical or clinical differences between single and double injection groups.
Risk of bias in included studies
Risk of bias assessment is described in Table 2.
Outcomes
WOMAC total scores were compared among 6 studies [7, 24, 26, 27, 30, 33] for PRP injection versus placebo and 4 studies [7, 26, 30, 33] for HA injection versus placebo with a total of 184 and 268 patients in each study, respectively. The pooled unstandardized mean difference (UMD) varied highly across studies (χ 2 = 87.96, d.f. = 3, p < 0.05, I 2 = 96.6 %) and was −15.4 (95 % CI −28.6, −2.3, p = 0.021), indicating that the PRP group had statistically significantly improved OA symptoms when compared to the HA group. The PRP group had a minimal clinically significant improvement in WOMAC total score by 12 % when compared to the HA group (Fig. 2; Table 3). None of the co-variables could explain the heterogeneity. There was no evidence of publication bias on Egger’s test or contour funnel plot (coefficient = −15.07, SE = 6.89, n.s.). Two studies [24, 27] with a total of 56 and 54 patients compared WOMAC total scores in PRP injection versus placebo in treatment of OA of the knee. The pooled UMD varied highly across studies (χ 2 = 12.56, d.f. = 1, p < 0.001, I 2 = 93.6 %) and had a −11.44 (95 % CI −32.81, 9.94, n.s.) lower WOMAC total score in PRP injection when compared to placebo (Fig. 3; Table 3).
WOMAC sub-scores among 5 studies [24, 26, 27, 30, 33] and 3 studies [24, 30, 33] with 224 and 208 patients compared WOMAC pain, stiffness and function scores in PRP versus HA. Two studies [24, 27] compared PRP versus placebo with a total number of 56 and 54 patients in each study.
Mean difference varied highly across studies (I 2 = 90.5, 92.9, 95.8 %) with an UMD of −1.95 (95 % CI −4.06, 0.17, n.s.), −0.99 (−2.09, 0.11, n.s.) and −8.02 (−17.45, 1.41, n.s.) showing lower WOMAC pain, stiffness and function scores in PRP when compared to HA, but with no statistically significant results (Table 3). There was no evidence of publication bias by Egger’s test for all pooled effects.
Mean difference varied highly across studies (I 2 = 85.5, 94.2 %) with an UMD of −2.81 (95 % CI −6.47, 0.84, n.s.) and −8.02 (95 % CI −17.45, 1.41, n.s.) showing lower WOMAC pain and function scores in PRP when compared to placebo, but with no statistically significant results (Table 3). The UMD was homogeneous (I 2 = 0) with a value of −0.09 (95 % CI −0.70, 0.53, n.s.), showing that WOMAC stiffness scores were lower in the PRP than the placebo groups, but this was also insignificant. There was no evidence of publication bias by Egger’s test and contour funnel plot.
Lequesne score
Two studies [30, 33] with 137 and 135 patients compared the mean Lequesne score between PRP and HA groups (Table 3). Mean difference varied highly across studies (χ 2 = 33.40, d.f. = 1, p < 0.05, I 2 = 97 %) with an unstandardized mean difference of −2.82 (95 % CI −8.01, 2.38, n.s.).
IKDC subjective scores
Two studies [11, 13] with 133 and 128 patients compared the mean IKDC subjective scores between PRP and HA groups (Table 3; Fig. 4). Mean difference varied highly across studies (d.f. = 1, p < 0.001, I 2 = 90.7 %) with an unstandardized mean difference of 8.83 (95 % CI 5.88, 11.78, p < 0.001), indicating that the PRP group had statistically significantly improved activity post-treatment when compared to the HA group.
EQ-VAS score
Two studies [11, 13] with 133 and 128 patients compared the mean EQ-VAS score between PRP and HA groups (Table 3; Fig. 4). Mean difference varied highly across studies (d.f. = 1, p < 0.05, I 2 = 79.9 %) with an unstandardized mean difference of 7.37 (95 % CI 4.33, 10.05, p = 0.021), indicating that the PRP group had statistically significantly better quality of life than the HA group.
Adverse events
Among 7 studies [7, 11, 12, 24, 27, 30, 33], 5 studies [7, 11, 12, 30, 33] compared risk of adverse events in PRP versus HA groups (Table 4; Fig. 5). The remaining two studies [24, 27] compared PRP with placebo groups. The pooled RR of the PRP groups was 0.85 (95 % CI 0.57, 1.28) (n.s.), which showed no statistically significantly lower risk of adverse events when compared to the HA groups. No heterogeneity (I 2 = 0) was present. Compared with the placebo groups, the pooled RR of PRP was 6.30 (95 % CI 0.34, 117.48) (n.s.). Neither contour funnel nor Egger’s test suggested evidence of publication bias.
Discussion
The most important finding of the present study is that PRP injection for treatment of osteoarthritis of the knee has a statistically significant improvement in outcomes for WOMAC total score, IKDC score and EQ-VAS score when compared to HA injection. In terms of WOMAC pain, stiffness, function scores and Lequesne scores, the PRP group had no statistically significant improvement when compared to both HA and placebo groups. Occurrence of adverse events was not significantly different across all three groups, but the PRP group did have a lower chance of adverse events when compared to the HA group. None of the co-variables [age, sex, BMI, OA grade, PRP formulation (single or double spin, LR or LP, injection protocol)] were sources of heterogeneity. The high heterogeneity may be associated with the varied cellular composition of commercially available PRP preparations; special attention has been devoted to varied leucocyte concentrations in different types of PRP. After subgroup analysis was applied for leucocyte concentration (LP/LR), it was seen that the functional outcome scores and the incidence of adverse events in PRP injections were not affected by leucocyte concentration. We have additional evidence with good methodological quality (RCT) that PRP injection has improved functional outcomes (WOMAC total scores, IKDC score and EQ-VAS) when compared to HA and placebo, but there is no difference in terms of adverse events when comparing PRP to HA or placebo. According to this study, PRP injection can be considered as a safe and useful treatment of choice in select patients with mild-to-moderate degrees of OA who fail to respond to other current treatments such as lifestyle modification, exercise and physical modalities.
From previous systematic reviews [2, 18, 19, 28], it has been concluded that PRP reduces pain and improves the osteoarthritis indices (WOMAC total score, WOMAC sub-score and Lequesne score) in osteoarthritis knee patients. Two meta-analyses were done from four systematic reviews, in which one [28] compared clinical outcomes and rates of adverse events between LP-PRP and LR-PRP. However, some of the outcomes had only one or two studies pooled, and non-RCT studies were included in the reviews.
This study has several strengths. First of all, 9 RCTs were included in the pooling of relevant clinical outcomes (i.e. WOMAC total score and sub-scores, Lequesne index, IKDC score, EQ-VAS score and adverse events) of PRP injection versus HA injection or placebo. Secondly, possible causes of heterogeneity were explored if covariate data at baseline [e.g. mean age, percentage of females, follow-up times, OA grading, times and type of PRP injection (single- or double-spinning approach, leucocyte poor or leucocyte rich)] were available. Publication bias for each outcome was also assessed.
There are some limitations in this study. When PRP injection was compared to placebo, the results of the PRP group were better than the placebo group in the WOMAC total and sub-scores, but this was not statistically significant. This was also true for the sub-WOMAC scores in PRP compared to HA. In order to reach statistical significance, the number of subjects that compared PRP to HA or placebo should be increased. All studies had a mean follow-up time of approximately 6 months to 1 year. Therefore, long-term effects of PRP and HA are still unknown. The quality of evidence was also assessed for each outcome [14] (Table 5) and showed intermediate strength for all outcomes.
Conclusion
For short-term outcomes (≤1 year), PRP injection has improved functional outcomes (WOMAC total scores, IKDC score and EQ-VAS) when compared to HA and placebo, but no difference in adverse events when compared to HA or placebo. This study suggests that PRP injection is more efficacious than HA injection and placebo in reducing symptoms, improving function and improving quality of life in patients with mild-to-moderate OA of the knee who have not responded to conventional treatment and therefore can be considered as a treatment of choice.
References
Altman DG, Bland JM (2003) Interaction revisited: the difference between two estimates. BMJ 326:219
Anitua E, Sanchez M, Aguirre JJ, Prado R, Padilla S, Orive G (2014) Efficacy and safety of plasma rich in growth factors intra-articular infiltrations in the treatment of knee osteoarthritis. Arthroscopy 30:1006–1017
Anitua E, Sanchez M, Orive G (2010) Potential of endogenous regenerative technology for in situ regenerative medicine. Adv Drug Deliv Rev 62:741–752
Anitua E, Sanchez M, Orive G, Andia I (2007) The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials 28:4551–4560
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15:1833–1840
Campbell KA, Saltzman BM, Mascarenhas R, Khair MM, Verma NN, Bach BR Jr, Cole BJ (2015) Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy. doi:10.1016/j.arthro.2015.03.041
Cerza F, Carnì S, Carcangiu A, Di Vavo I, Schiavilla V, Pecora A, De Biasi G, Ciuffreda M (2012) Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med 40:2822–2827
Chang KV, Hung CY, Aliwarga F, Wang TG, Han DS, Chen WS (2014) Comparative effectiveness of platelet-rich plasma injections for treating knee joint cartilage degenerative pathology: a systematic review and meta-analysis. Arch Phys Med Rehabil 95:562–575
Dieppe PA, Lohmander LS (2005) Pathogenesis and management of pain in osteoarthritis. Lancet 365:965–973
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Filardo G, Di Matteo B, Di Martino A, Merli ML, Cenacchi A, Fornasari P, Marcacci M, Kon E (2015) Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med 43:1575–1582
Filardo G, Kon E, Di Martino A, Di Matteo B, Merli ML, Cenacchi A, Fornasari PM, Marcacci M (2012) Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord 13:229
Gormeli G, Gormeli CA, Ataoglu B, Colak C, Aslanturk O, Ertem K (2015) Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-015-3705-6
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ, Group GW (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336:924–926
Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, Richmond JC, Shelborne KD (2001) Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med 29:600–613
Khoshbin A, Leroux T, Wasserstein D, Marks P, Theodoropoulos J, Ogilvie-Harris D, Gandhi R, Takhar K, Lum G, Chahal J (2013) The efficacy of platelet-rich plasma in the treatment of symptomatic knee osteoarthritis: a systematic review with quantitative synthesis. Arthroscopy 29:2037–2048
Kon E, Mandelbaum B, Buda R, Filardo G, Delcogliano M, Timoncini A, Fornasari PM, Giannini S, Marcacci M (2011) Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy 27:1490–1501
Lai LP, Stitik TP, Foye PM, Georgy JS, Patibanda V, Chen B (2015) Use of platelet rich plasma in intra-articular knee injections for osteoarthritis: a systematic review. PMR. doi:10.1016/j.pmrj.2015.02.003
Laudy AB, Bakker EW, Rekers M, Moen MH (2015) Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med 49:657–672
Lequesne MG (1997) The algofunctional indices for hip and knee osteoarthritis. J Rheumatol 24:779–781
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6:e1000100
Murray CJ, Vos T, Lozano R et al (2012) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2197–2223
Palmer TMPJ, Sutton AJ, Moreno SG (2008) Contour-enhanced funnel plots in meta-analysis. STATA J 8:242–254
Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A (2013) Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med 41:356–364
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L (2008) Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 61:991–996
Raeissadat SA, Rayegani SM, Hassanabadi H, Fathi M, Ghorbani E, Babaee M, Azma K (2015) Knee osteoarthritis injection choices: platelet-rich plasma (PRP) versus hyaluronic acid (A one-year randomized clinical trial). Clin Med Insights Arthritis Musculoskelet Disord. doi:10.4137/cmamd.s17894
Rayegani SM, Raeissadat SA, Taheri MS, Babaee M, Bahrami MH, Eliaspour D, Ghorbani E (2014) Does intra articular platelet rich plasma injection improve function, pain and quality of life in patients with osteoarthritis of the knee? A randomized clinical trial. Orthop Rev 6:5405
Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ (2015) Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. doi:10.1177/0363546515580787
Sanchez M, Anitua E, Azofra J, Aguirre JJ, Andia I (2008) Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: a retrospective cohort study. Clin Exp Rheumatol 26:910–913
Sánchez M, Fiz N, Azofra J, Usabiaga J, Aduriz Recalde E, Garcia Gutierrez A, Albillos J, Gárate R, Aguirre JJ, Padilla S, Orive G, Anitua E (2012) A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy 28:1070–1078
Spaková T, Rosocha J, Lacko M, Harvanová D, Gharaibeh A (2012) Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil 91:411–417
StataCorp. (2013) Stata 13 base reference manual. Stata Press, College Station
Vaquerizo V, Plasencia MÁ, Arribas I, Seijas R, Padilla S, Orive G, Anitua E (2013) Comparison of intra-articular injections of plasma rich in growth factors (PRGF-Endoret) versus durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy 29:1635–1643
Zhang W, Moskowitz RW, Nuki G et al (2007) OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthr Cartil 15:981–1000
Acknowledgments
All authors declare no funding sources or sponsor involvement in the study design, collection, analysis and interpretation of the data, writing of the manuscript and in submission of the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kanchanatawan, W., Arirachakaran, A., Chaijenkij, K. et al. Short-term outcomes of platelet-rich plasma injection for treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 24, 1665–1677 (2016). https://doi.org/10.1007/s00167-015-3784-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-015-3784-4