Abstract
Background
Within the past 20 years, knee ligament injuries have been increasingly reported in the literature to be treated with anatomic reconstructions over soft tissue advancements or sling-type procedures to recreate the native anatomy and restore knee function. Historically, early clinician scientists published on the qualitative anatomy of the knee, which provided a foundation for the initial knee biomechanical studies in the nineteenth and twentieth centuries. Similarly, the work of early sports medicine orthopaedic clinician scientists in the late twentieth century formed the basis for the quantitative anatomic and functional robotic biomechanical studies found currently in the sports medicine orthopaedic literature. The development of an anatomic reconstruction first requires an appreciation of the quantitative anatomy and function of each major stabilizing component of the knee.
Purpose
This paper provides an overview of the initial qualitative anatomic studies from which the initial knee ligament surgeries were based and expands to recent detailed quantitative studies of the major knee ligaments and the renewed recent focus on anatomic surgical reconstructions.
Conclusions
Anatomic repairs and reconstructions of the anterior cruciate ligament, posterior cruciate ligament, medial collateral ligament and posterolateral corner attempt to restore knee function by rebuilding or restoring the native anatomy. The basis of anatomic reconstruction techniques is a detailed understanding of quantitative knee anatomy. Additionally, an appreciation of the function of each component is necessary to ensure surgical success.
Level of evidence
V.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While orthopaedic procedures increase in complexity and new devices continually develop, the treatment of complex knee injuries has returned to the basics: the anatomy. The study of human anatomy traces its early origins to the “vivisections” of Galen in the second century AD [94] and Da Vinci’s anatomic drawings of the ideal human [7, 76]. As scientists’ understanding of human anatomy increased, anatomic theatres, such as the theatre at the University of Padua in Italy, became an integral portion of medical training and famous anatomists, such as Nicolaes Tulp and John Hunter, taught up-and-coming physicians the intricacies of the human body [80, 90]. However, to understand the meaning of an anatomic reconstruction requires an additional level of detail: the function. As Mueller has stated, “Anatomic structures are never an end in themselves but are, by virtue of their size and strength, the expression of a corresponding function” [79, 81, 85, 86].
In the late nineteenth and early twentieth century, attention turned to understanding the function of ligaments and tendons of the knee. Scientists such as the Weber brothers provided some of the earliest biomechanical studies on the knee [23, 35, 37, 42, 49, 77, 78, 81, 84, 97, 105]. Additionally, Mueller developed the concept of the “four-bar linkage,” [81] providing a greater understanding of the cruciate ligaments and their roles in the rotational and translational movement of the femur and tibia.
The long history of the study of human anatomy provided a foundation for the initial biomechanical studies of the knee by clinical scientists in the nineteenth and twentieth centuries. Similarly, the work of the early orthopaedic clinician scientists formed the basis for the quantitative anatomic and functional robotic biomechanical studies reported in the modern sports medicine orthopaedic literature. The purpose of anatomic reconstructions is to restore knee function by recreating the native anatomy. However, the development of an anatomic reconstruction first requires an appreciation of the quantitative anatomy and function of each component of the knee. This paper provides an overview of the initial qualitative anatomic studies from which the initial knee ligament surgeries were based and expands to recent detailed quantitative studies of the major knee ligaments and the renewed recent focus on anatomic surgical reconstructions.
The anterior cruciate ligament
Our understanding of the structure and function of the anterior cruciate ligament (ACL) has evolved over time through biomechanical and clinical orthopaedic research. Sectioning studies have elucidated the complex structure of the ACL in order to improve surgical techniques and re-establish the native kinematics with an anatomic reconstruction [24, 51, 109, 110]. While initial surgical procedures focused on sling-type procedures, a renewed focus on anatomic-based reconstructions has evolved over the past decade.
A qualitative understanding of the ACL dates back to Galen in the second century, who first described the anatomy of the ACL [27]. Further studies describing the ACL were not published until the nineteenth century, when Eduard and Wilhelm Weber reported that the ACL consisted of two separate intertwined fibre bundles and sectioning the ACL led to anterior tibial translation [105].
Anterior cruciate ligament research and surgical techniques continued in the late nineteenth and early twentieth century, with the first ACL repair reported by Battle in 1900 [6], Fick’s report on the reciprocal function of both cruciate ligaments in 1911 [22], a cadaveric analysis on the mechanism of ACL rupture by Goetjes in 1913 [32], and a description of the pivot shift test by Jones and Smith in 1913 [47].
Current research has validated early qualitative findings and demonstrated that the ACL is essentially comprised of two bundles: the anteromedial (AM) and posterolateral (PL) bundles, named for their relative tibial insertions (Fig. 1) [3]. The femoral ACL attachment is located on the posterior aspect of the medial surface of the lateral femoral condyle. Quantitative anatomic studies have reported that the AM bundle originates on the most proximal portion of the femoral ACL footprint, while the PL bundle originates on the distal portion of this footprint [89]. The femoral ACL footprint has been reported to have a semicircular shape, with a 16–24 mm diameter and a surface area of 113 ± 27 mm2. The tibial ACL insertion has been reported to be approximately 11 mm wide and 17 mm long in the anterior–posterior direction, with a total attachment area of 136 mm ± 33 mm2 [17, 31, 40].
Lateral view of a left knee showing the anteromedial bundle (AMB) and posterolateral bundle (PLB) of anterior cruciate ligament (ACL) and their pertinent osseous landmarks (from [111], with permission)
Biomechanical studies have long reported the primary role of the ACL in resisting anterior tibial translation [2, 10, 26, 75, 87, 91]. The AM and PL bundles contribute differing amounts to anterior tibial restraint as the knee moves from extension to increasing degrees of flexion [2, 48]. The ACL has also been reported to be a secondary rotatory restraint to external and internal tibial rotation [83]. One of the first studies which described the function of the ACL was by Girgis et al. in 1975 [31]. They reported that sectioning the ACL resulted in increased anterior tibial translation, increased internal rotation and increased external rotation in extension and flexion [31]. Additionally, studies by Noyes in Cincinnati and Kennedy in London, Ontario expanded our understanding of the ACL by determining the effect of age, stress and strain on the physical properties of the ACL [52, 85, 93].
Hey Groves is attributed with performing the first ACL reconstruction in 1916, although operative notes from E. Hesse and Major Zur Verth in 1914 reported on their own ACL reconstruction techniques [87, 93]. While Hey Groves’ technique was based on a qualitative understanding of the knee joint, he recognized the importance of recreating the native anatomy with his reconstruction [93]. Throughout the twentieth century, reconstruction techniques advanced as biomechanical research provided a greater understanding of the ACL. Extraarticular-based techniques controlling rotatory stability were first popularized by Lemaire in France in 1967 and refined later by MacIntosh and Galway in 1971, Trillat in 1972, Ellison in 1975 and Losee in 1978 [19, 28, 72–74, 99].
Additionally, harvesting techniques for reconstruction grafts emerged and evolved: fascia lata grafts were popularized in the early twentieth century, Edwards first reported the use of a hamstring tendon graft in a cadaver in 1926 [18], Jones published the use of a patella graft in 1963 [46], and England reported the use of a quadriceps tendon graft in 1976 [20].
Since then, quantitative anatomic and functional biomechanical studies have led to the current anatomic ACL reconstruction techniques. Two anatomic techniques have emerged: single-bundle (SB) and double-bundle (DB) ACL reconstructions. Both techniques attempt to recreate the function of the ACL through anatomic graft placement [3, 33, 43, 101, 111]. While an anatomic DB ACL reconstruction recreates the two bundle anatomy of the ACL, an anatomic SB ACL reconstruction is a simpler procedure and has been reported to result in similar postoperative anterior tibial translation, internal and external tibial rotation, and pivot shift as an anatomic DB ACL reconstruction [33, 82]. Quantitative studies have improved SB and DB ACL graft placement by measuring the precise distances between relative anatomic landmarks and the ACL footprints [58, 111]. Additionally the close proximity of the anterior meniscal roots to the tibial ACL footprint has emerged as a potential complication of anatomic ACL reconstructions (Fig. 2). The anterolateral meniscal root has been reported to overlap with the ACL tibial footprint, and an anatomic ACL reconstruction may decrease the anterolateral root attachment area and ultimate failure strength [58–60, 104]. Additional research is needed to investigate the anatomic relationships and interactions of the anterior meniscal roots and the tibial ACL footprint.
Axial view of a right knee showing the anterior cruciate ligament (ACL) tibial attachment footprint, anteromedial bundle (AM), posterolateral bundle (PL) and its relation with the anterior roots of the menisci. AM root, anterior meniscal root; AL root, anterolateral root; AC, articular cartilage; LTE, lateral tibial eminence; MTE, medial tibial eminence; PL root, posterior lateral meniscal root; PM root, posterior meniscal root; TT, tibial tuberosity; SFs, supplemental fibres (from [57], with permission)
The posterior cruciate ligament
The posterior cruciate ligament (PCL) is the largest intra-articular knee ligament [40] and provides significant stability to the knee. Early qualitative anatomic studies by the Weber brothers in the twentieth century and Fick in 1911 described the intertwined bundles of the PCL and the PCL’s reciprocal function to the ACL [22, 105]. Early biomechanical studies reported that the primary role of the PCL was as a restraint against posterior tibial translation [11, 15, 25, 31, 34]. However, the decreased incidence of PCL tears compared to ACL tears [4] has historically led to relatively less biomechanical and anatomic PCL research.
Within the past 20 years, there has been a growing interest in understanding the anatomy and kinematics of the PCL. The PCL is composed of two bundles, the anterolateral bundle (ALB) and the posteromedial bundle (PMB), named for their relative tibial insertions (Fig. 3) [1]. Quantitative anatomic and radiographic studies have elucidated the femoral and tibial attachment sites of each bundle. On the femur, the ALB has been reported to attach 12.1 ± 1.3 mm proximally and medial relative to the PMB [4], and 34.1 mm lateral to the medial femoral condyle [45]. The distal edge of the PMB has been reported to be 5.8 ± 1.7 mm proximal to the femoral articular cartilage margin, and on the tibia, the ALB and PMB are separated by a bundle ridge, with a reported mean distance of 8.9 ± 1.2 mm between the centres of the two bundles [4].
a Anterior and b posterior views of a right knee showing the posterior cruciate ligament (PCL) and the relation of the anterolateral bundle (ALB) and the posteromedial bundle (PMB) with osseous landmarks: the trochlear point, the medial arch point, the bundle ridge and the champagne glass drop-off. ACL, anterior cruciate ligament; aMFL, anterior meniscofemoral ligament (ligament of Humphrey); FCL, fibular collateral ligament; PFL, popliteofibular ligament; pMFL, posterior meniscofemoral ligament (ligament of Wrisberg); POL, posterior oblique ligament (from [55], with permission)
In addition to a greater understanding of the quantitative anatomy of the PCL, an improved understanding of PCL kinematics has evolved. Recent robotic cadaveric studies have elucidated the role of the PCL in rotation and the roles of the PCL at higher degrees of knee flexion. Kennedy et al. reported that sectioning the PCL resulted in increased posterior tibial translation, tibial internal rotation and tibial external rotation. Additionally, the PMB was noted to have a larger role in internal rotation [55].
With the improved understanding of PCL anatomy and biomechanical function, anatomic SB and DB PCL reconstruction techniques have emerged. A SB PCL reconstruction has historically been the more common technique [107]. However, biomechanical studies have reported that an anatomic DB PCL reconstruction technique more closely restores native PCL kinematics compared to an anatomic SB PCL reconstruction, including less posterior translation and internal rotation [38, 53, 107]. Additionally for a DB PCL reconstruction, fixing the PMB at 0°–15° and the ALB at 75°–105° of flexion has been reported to reduce overall knee laxity without leading to graft overconstraint [53]. Quantitative anatomic studies on the posterior meniscal roots have reported the additional risk of damaging the posterior meniscal root attachments when reconstructing the PCL (Fig. 4). The centre of the posterior medial meniscal root attachment is reported to be 8.2 ± 0.7 mm from the nearest PCL edge [45], and a single PCL reconstruction tunnel formed in the ALB footprint can lead to potential damage of the posterior medial meniscal root [54, 60].
Axial view of a right knee showing the posterior cruciate ligament (PCL) tibial attachment footprint, anterolateral bundle (AL), posteromedial bundle (PM) and its relation with the posterior roots of the menisci. LPRA, lateral posterior root attachment; MPRA, medial posterior roots attachment; ACL, anterior cruciate ligament; LTE, lateral tibial eminence; MTE, medial tibial eminence; SWF, shiny white fibres (from [60], with permission)
The medial side of the knee
The medial side of the knee is an important arrangement of ligamentous, capsular and tendinous attachments, including the superficial medial collateral ligament (sMCL) which is the most frequently injured ligament in the knee [61]. As with the other components of the knee, the anatomy of the medial side of the knee was first described qualitatively [9, 88, 95, 103]. Palmer in 1938 reported on the tibial collateral ligament in his anatomic dissections [88]. Slocum and Larson in 1968 [95] reported that the medial side of the knee included the tibial collateral ligament (sMCL) and a “sleeve” of anterior, middle and posterior capsular ligaments. Recently, the main medial knee structures have been further defined as the sMCL, the deep medial collateral ligament (dMCL) and the posterior oblique ligament (POL) [106].
The anatomy of the medial side of the knee has been further defined quantitatively with exact attachment locations and relative distances between structures. LaPrade and colleagues reported that the sMCL has one femoral attachment, 3.2 mm proximal and 4.8 mm posterior to the medial epicondyle, and two tibial attachments, a proximal attachment connecting to soft tissue and a distal attachment blending in with the pes anserinus bursa 61.2 mm distal to the knee joint. The dMCL has two portions attached to the medial meniscus: a meniscofemoral and a meniscotibial portion. The POL attaches on the femur 7.7 mm distal and 6.4 mm posterior to the adductor tubercle and 1.4 mm distal and 2.9 mm anterior to the recently reported gastrocnemius tubercle [61].
The quantitative anatomy described previously has led to new biomechanical studies based upon the newly described quantitative anatomy of the medial knee structures. The sMCL has been reported to provide resistance to external rotation in addition to being the main valgus stabilizer, while the POL stabilizes the knee in internal rotation when in extension [36, 92].
The quantitative anatomy and functional understanding of the medial structures of the knee have led to improved surgical techniques, including anatomic augmented repairs and anatomic medial knee reconstructions (Fig. 5). Biomechanical cadaveric studies have validated an anatomic augmented sMCL repair and an anatomic sMCL reconstruction, reporting <2 mm of medial joint gapping at 0° and 20° of knee flexion for both techniques [14, 108]. Development of anatomic-based reconstructions has led to more aggressive postoperative rehabilitation programs, with knee motion initiated within 24 h of surgery, which has led to a decrease in the rates of reported arthrofibrosis without the risk of postsurgical laxity [69]. While there is minimal difference biomechanically, further clinical outcome studies are needed to investigate whether there is any difference between the two anatomic techniques for patients.
Anatomy of the medial aspect of the left knee: a native superficial medial collateral ligament (sMCL); b anatomic augmented repair of the sMCL in a left knee with distal tibial fixation of the semitendinosus sutured to the sMCL remnant 6 cm distal to the joint line; c an anatomic sMCL reconstruction with femoral and distal tibial interference screw fixation. VMO, vastus medial obliquus (from [108], with permission)
The posterolateral corner
The posterolateral corner of the knee has long been described using differing nomenclature by many different centres in large part due to the complexity of the ligamentous, tendinous and capsular structures. Early dissection studies reported accounts of a long external lateral ligament, a short external lateral ligament and a posterior lateral collateral ligament [71, 79, 100]. These early accounts evolved into various studies describing the fibular (lateral) collateral ligament, the popliteus muscle, the arcuate ligament, the short lateral ligament, the mid-third lateral capsular ligament and the oblique popliteal ligament [5, 8, 21, 41, 50, 56, 71].
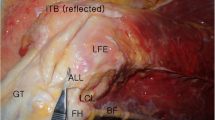
Within the past 20 years, extensive anatomic and biomechanical studies have provided a more detailed understanding of the important structures of the posterolateral corner and their functional contributions [39, 65, 67, 68, 70, 96, 102]. The main three static stabilizers of the posterolateral corner are the fibular collateral ligament (FCL), the popliteus tendon and the popliteofibular ligament. The FCL attaches proximally to the femur 1.4 mm proximal and 3.1 mm posterior to the lateral epicondyle and attaches distally to the lateral aspect of the fibular head with additional fibres extending into the peroneus longus fascia [65]. The popliteus tendon originates on the anterior fifth of the popliteal sulcus, 18.5 mm anterior and distal to the FCL femoral insertion and attaches distally to the posteromedial tibia (Fig. 6) [30, 65]. The popliteofibular ligament, which used to be called the arcuate ligament, consists of two divisions that originate at the popliteus musculotendinous junction. The anterior division attaches on the fibular head 2.8 mm distal to the fibular styloid process on the anteromedial downslope, and the posterior division attaches 1.6 mm distal to the fibular styloid process on the posteromedial downslope [65].
Lateral aspect of the knee demonstrating the 18.5-mm distance between the femoral fibular collateral ligament (FCL) insertion and the femoral popliteus tendon (PLT) insertion (from [65], with permission)
Functionally, biomechanical studies have reported that the FCL is the primary stabilizer to varus stress. Additionally, the FCL provides stability against external rotation at lower degrees of knee flexion [13]. The popliteus and the popliteofibular ligament stabilize against external rotation, especially at higher knee flexion angles, and act as secondary stabilizers to varus stress [64, 67, 68, 70].
An anatomic reconstruction of the posterolateral corner has evolved from the quantitative anatomic and functional biomechanical studies. The anatomic technique involves reconstructing the FCL, the popliteus and the popliteofibular ligament with two grafts (Fig. 7). Biomechanical studies have validated the anatomic approach, reporting reduced varus laxity and external rotation [13, 64, 66, 68, 70], and clinical outcome studies have reported improved patient outcomes after anatomic posterolateral reconstruction [29, 62, 63, 66].
Anatomic posterolateral corner reconstruction technique. Posterior (a) and lateral (b) views of a right knee. FCL, fibular collateral ligament; PLT, popliteus tendon; PFL, popliteofibular ligament (from [64], with permission)
Additionally, renewed interest has occurred in the anterolateral ligament. Recent biomechanical studies have reported the importance of the anterolateral ligament in rotatory stability and its association with ACL tears [12, 16].
Conclusions
Anatomic repairs and reconstructions of the ACL, PCL, MCL and PLC attempt to restore knee function by rebuilding or restoring the native anatomy. The basis of anatomic reconstruction techniques is a detailed understanding of quantitative knee anatomy. Understanding the spatial relationship of attachment sites relative to surrounding structures is imperative to restore the native anatomy. Additionally, an appreciation of the function of each component is necessary to ensure surgical success. Even though the anatomy has now been described and characterized in detail, the biomechanical function of anatomic-based reconstructions (i.e. forces seen by the grafts after reconstructions and the ideal fixation angles for anatomic-based ligament reconstructions) has not been recreated to the same extent. Consequently, anatomic reconstructions will continue to improve in the future as biomechanical research improves our understanding of the anatomy and function of the knee.
References
Amis AA, Gupte CM, Bull AM, Edwards A (2006) Anatomy of the posterior cruciate ligament and the meniscofemoral ligaments. Knee Surg Sports Traumatol Arthrosc 14(3):257–263
Amis AA, Dawkins GP (1991) Functional anatomy of the anterior cruciate ligament. Fibre bundle actions related to ligament replacements and injuries. J Bone Joint Surg Br 73(2):260–267
Anderson CJ, Westerhaus BD, Pietrini SD, Ziegler CG, Wijdicks CA, Johansen S, Engebretsen L, LaPrade RF (2010) Kinematic impact of anteromedial and posterolateral bundle graft fixation angles on double bundle anterior cruciate ligament reconstructions. Am J Sports Med 38(8):1575–1583
Anderson CJ, Ziegler CG, Wijdicks CA, Engebretsen L, LaPrade RF (2012) Arthroscopically pertinent anatomy of the anterolateral and posteromedial bundles of the posterior cruciate ligament. J Bone Joint Surg Am 94(21):1936–1945
Basmajian JV, Lovejoy J (1971) Functions of the popliteus muscle in man. A multifactorial electromyographic study. J Bone Joint Surg Am 53:557–562
Battle WH (1900) A case after open section of the knee-joint for irreducible traumatic dislocation. Trans Clin Soc Lond 33:232–233
Boas M (1962) The scientific renaissance 1450–1630. Fontana, New York
Bousquet G, Charmion L, Passot JP, Girardin P, Relave M, Gazielly D (1986) Stabilization of the external condyle of the knee in chronic anterior laxity. Importance of the popliteal muscle. Rev Chir Orthop Reparatrice Appar Mot 72(6):427–434
Brantigan OC, Voshell AF (1943) The tibial collateral ligament: its function, its bursae, and its relation to the medial meniscus. J Bone Joint Surg 25:121–131
Butler DL, Noyes FR, Grood ES (1980) Ligamentous restraints to anterior–posterior drawer in the human knee. A biomechanical study. J Bone Joint Surg Am 62(2):259–270
Chambat P (1978) Les ruptures isolees du LCP chirurgie du genou. 3ème Journees Simep 47–50
Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J (2013) Anatomy of the anterolateral ligament of the knee. J Anat 223(4):321–328
Coobs BR, LaPrade RF, Griffith CJ, Nelson BJ (2007) Biomechanical analysis of an isolated fibular (lateral) collateral ligament reconstruction using an autogenous semitendinosus graft. Am J Sports Med 35:1521–1527
Coobs BR, Wijdicks CA, Armitage BM, Spiridonov SI, Westerhaus BD, Johansen S, Engebretsen L, LaPrade RF (2010) An in vitro analysis of an anatomical medial knee reconstruction. Am J Sports Med 38(2):339–347
Dejour H, Walch G, Peyrot J, Eberhard P (1988) The natural history of rupture of the posterior cruciate ligament. Rev Chir Orthop Reparatrice Appar Mot 74(1):35–43
Dodds AL, Halewood C, Gupte CM, Williams A, Amis AA (2014) The anterolateral ligament: anatomy, length changes and association with the segond fracture. Bone Joint J 96-B(3):325–331
Dodds JA, Arnoczky SP (1994) Anatomy of the anterior cruciate ligament: a blueprint for repair and reconstruction. Arthroscopy 10(2):132–139
Edwards AH (1926) Operative repair of cruciate ligaments in severe trauma of knee. Br J Surg 13:432–438
Ellison AE (1975) A modified procedure for the extra-articular replacement of the anterior cruciate ligament. In: Presented at annual meeting of the American Orthopaedic Society for Sport Medicine. New Orleans, 28–30 July
England RL (1976) Repair of the ligaments about the knee. Orthop Clin North Am 7:195–204
Fabbriciani C, Oransky M, Zoppi U (1982) The popliteal muscle: an anatomical study. Arch Ital Anat Embriol 87(3):203–217
Fick R (1911) Handbuch der Anatomie und Mechanik der Gelenke. 3 Teil: Spezielle Gelenk- und Muskelmechanik. Gustav Fischer, Jena
Frenkel VH (1971) Biomechanics of the knee. Orthop Clin North Am 2:175–190
Fu FH, Shen W, Starman JS, Okeke N, Irrgang JJ (2008) Primary anatomic double-bundle anterior cruciate ligament reconstruction: a preliminary 2-year prospective study. Am J Sports Med 36(7):1263–1274
Fukubayashi T, Torzilli PA, Sherman MF, Warren RF (1982) An in vitro biomechanical evaluation of anterior-posterior motion of the knee. Tibial displacement, rotation, and torque. J Bone Joint Surg Am 64(2):258–264
Gabriel MT, Wong EK, Woo SL, Yagi M, Debski RE (2004) Distribution of in situ forces in the anterior cruciate ligament in response to rotatory loads. J Orthop Res 22(1):85–89
Galen C (1968) On the usefulness of parts of the body. May T. Cornell University Press, New York
Galway RB, Beaupre A, MacIntosh DL (1972) Pivot shift: a clinical sign of symptomatic anterior cruciate insufficiency. J Bone Joint Surg 54-B:763–764
Geeslin AG, LaPrade RF (2011) Outcomes of treatment of acute grade-III isolated and combined posterolateral knee injuries: a prospective case series and surgical technique. J Bone Joint Surg Am 93:1672–1683
Geiger D, Chang E, Pathria M, Chung CB (2013) Posterolateral and posteromedial corner injuries of the knee. Radiol Clin North Am 51:413–432
Girgis FG, Marshall JL, Monajem A (1975) The cruciate ligaments of the knee joint. Anatomical, functional and experimental analysis. Clin Orthop Relat Res 106:216–231
Goetjes H (1913) Über verletzungen der ligamenta cruciata des kniegelenks. Dtsch Z Chir 123:221–289
Goldsmith MT, Jansson KS, Smith SD, Engebretsen L, LaPrade RF, Wijdicks CA (2013) Biomechanical comparison of anatomic single and double-bundle anterior cruciate ligament reconstructions: an in vitro study. Am J Sports Med 41(7):1595–1604
Gollehon DL, Torzilli PA, Warren RF (1987) The role of the posterolateral and cruciate ligaments in the stability of the human knee. A biomechanical study. J Bone Joint Surg Am 69(2):233–242
Goodfellow J, O’Connor J (1978) The mechanics of the knee and prosthesis design. J Bone Joint Surg Br 60:358–369
Griffith CJ, Wijdicks CA, LaPrade RF, Armitage BM, Johansen S, Engebretsen L (2009) Force measurements on the posterior oblique ligament and superficial medial collateral ligament proximal and distal divisions to applied loads. Am J Sports Med 37(1):140–148
Groh W (1955) Kinematische untersuchungen des menschilichen kniegelenkes und einige prothesenkniekonstruktionen, die als “physiologische” kniegelenke bezeichnet warden. Arch Orthop Unfallchir 47:637–645
Harner CD, Janaushek MA, Kanamori A, Yagi M, Vogrin TM, Woo SL (2000) Biomechanical analysis of a double-bundle posterior cruciate ligament reconstruction. Am J Sports Med 28(2):144–151
Harner CD, Vogrin TM, Höher J, Ma CB, Woo SL (2000) Biomechanical analysis of a posterior cruciate ligament reconstruction. Deficiency of the posterolateral structures as a cause of graft failure. Am J Sports Med 28(1):32–39
Harner CD, Baek GH, Vogrin TM, Carlin GJ, Kashiwaguchi S, Woo SL (1999) Quantitative analysis of human cruciate ligament insertions. Arthroscopy 15(7):741–749
Hughston JC, Andrews JR, Cross MJ, Moschi A (1976) Classification of the knee ligaments instabilities. Part II. The lateral compartment. J Bone Joint Surg Am 58(2):173–179
Huson A (1974) Biomechanische probleme des kniegelenks. Ortopaede 3:119–126
Järvelä T (2007) Double-bundle versus single-bundle anterior cruciate ligament reconstruction: a prospective, randomize clinical study. Knee Surg Sports Traumatol Arthrosc 15(5):500–507
Johannsen AM, Anderson CJ, Wijdicks CA, Engebretsen L, LaPrade RF (2013) Radiographic landmarks for tunnel positioning in posterior cruciate ligament reconstructions. Am J Sports Med 41(1):35–42
Johannsen AM, Civitarese DM, Padalecki JR, Goldsmith MT, Wijdicks CA, LaPrade RF (2012) Qualitative and quantitative anatomic analysis of the posterior root attachments of the medial and lateral menisci. Am J Sports Med 40(10):2342–2347
Jones KG (1963) Reconstruction of the anterior cruciate ligament. J Bone Joint Surg 45-A:925–932
Jones R, Smith A (1913) On rupture of the crucial ligaments of the knee and on fractures of the spine of the tibia. Br J Surg 1:70–89
Jordan SS, DeFrate LE, Nha KW, Papannagari R, Gill TJ, Li G (2007) The in vivo kinematics of the anteromedial and posterolateral bundles of the anterior cruciate ligament during weightbearing knee flexion. Am J Sports Med 35(4):547–554
Kapandji IA (1970) The physiology of the Joint, vol II. Churchill Livingstone, Edinburgh
Kaplan EB (1962) Some aspects of functional anatomy of the human knee joint. Clin Orthop 23:18–29
Kopf S, Musahl V, Tashman S, Szczodry M, Shen W, Fu FH (2009) A systematic review of the femoral origin and tibial insertion morphology of the ACL. Knee Surg Sports Traumatol Arthrosc 17(3):213–219
Kennedy JC, Fowler PJ (1971) Medial and anterior instability of the knee: an anatomical and clinical study using stress machines. J Bone Joint Surg 53-A:1257–1270
Kennedy NI, LaPrade RF, Goldsmith MT, Faucett SC, Rasmussen MT, Coatney GA, Engebretsen L, Wijdicks CA (2014) Posterior cruciate ligament graft fixation angles, part 2: biomechanical evaluation for anatomic double-bundle reconstruction. Am J Sports Med 42(10):2346–2355
Kennedy NI, Michalski MP, Engebretsen L, LaPrade RF (2014) Iatrogenic meniscus posterior root injury following reconstruction of the posterior cruciate ligament. JBJS Case Connect 4(1):e20
Kennedy NI, Wijdicks CA, Goldsmith MT, Michalski MP, Devitt BM, Årøen A, Engebretsen L, LaPrade RF (2013) Kinematic analysis of the posterior cruciate ligament, part 1: the individual and collective function of the anterolateral and posteromedial bundles. Am J Sports Med 41(12):2828–2838
Lahlaidi A (1971) Valeur morphologique des insertions postérieures du ménisque externe dans le genou humain. Rev Chir Orthop 57:593–600
LaPrade CM, Ellman MB, Rasmussen MT, James EW, Wijdicks CA, Engebretsen L, LaPrade RF (2014) Anatomy of the anterior root attachments of the medial and lateral menisci: a quantitative analysis. Am J Sports Med 42(10):2386–2392
LaPrade CM, James EW, Engebretsen L, LaPrade RF (2014) Anterior medial meniscal root avulsions due to malposition of the tibial tunnel during anterior cruciate ligament reconstruction: two case reports. Knee Surg Sports Traumatol Arthrosc 22(5):1119–1123
LaPrade CM, Smith SD, Rasmussen MT, Hamming MG, Wijdicks CA, Engebretsen L, Feagin JA, LaPrade RF (2015) Consequences of tibial tunnel reaming on the meniscal roots during cruciate ligament reconstruction in a cadaveric model, part 1: the anterior cruciate ligament. Am J Sports Med 43(1):200–206
LaPrade CM, Smith SD, Rasmussen MT, Hamming MG, Wijdicks CA, Engebretsen L, Feagin JA, LaPrade RF (2015) Consequences of tibial tunnel reaming on the meniscal roots during cruciate ligament reconstruction in a cadaveric model, part 2: the posterior cruciate ligament. Am J Sports Med 43(1):207–212
LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L (2007) The anatomy of the medial part of the knee. J Bone Joint Surg Am 89(9):2000–2010
LaPrade RF, Griffith CJ, Coobs BR, Geeslin AG, Johansen S, Engebretsen L (2014) Improving outcomes for posterolateral knee injuries. J Orthop Res 32:485–491
LaPrade RF, Johansen S, Agel J, Risberg MA, Moksnes H, Engebretsen L (2010) Outcomes of an anatomic posterolateral knee reconstruction. J Bone Joint Surg Am 92:16–22
LaPrade RF, Johansen S, Wentorf FA, Engebretsen L, Esterberg JL, Tso A (2004) An analysis of an anatomical posterolateral knee reconstruction: an in vitro biomechanical study and development of a surgical technique. Am J Sports Med 32:1405–1414
LaPrade RF, Ly TV, Wentorf FA, Engebretsen L (2003) The posterolateral attachments of the knee: a qualitative and quantitative morphologic analysis of the fibular collateral ligament, popliteus tendon, popliteofibular ligament, and lateral gastrocnemius tendon. Am J Sports Med 31:854–860
LaPrade RF, Spiridonov SI, Coobs BR, Ruckert PR, Griffith CJ (2010) Fibular collateral ligament anatomical reconstructions: a prospective outcomes study. Am J Sports Med 38(10):2005–2011
LaPrade RF, Terry GC (1997) Injuries to the posterolateral aspect of the knee: association of anatomic injury patterns with clinical instability. Am J Sport Med 25:433–438
LaPrade RF, Tso A, Wentorf FA (2004) Force measurements on the fibular collateral ligament, popliteofibular ligament, and popliteus tendon to applied loads. Am J Sports Med 32:1695–1701
LaPrade RF, Wijdicks CA (2012) Surgical technique: development of an anatomic medial knee reconstruction. Clin Orthop Relat Res 470(3):806–814
LaPrade RF, Wozniczka JK, Stellmaker MP, Wijdicks CA (2010) Analysis of the static function of the popliteus tendon and evaluation of an anatomic reconstruction: the “fifth ligament” of the knee. Am J Sports Med 38:543–549
Last RJ (1948) Some anatomical details of the knee joint. J Bone Joint Surg Br 30B(4):683–688
Lemaire M (1967) Ruptures anciennes du ligament croisé antérieur. Fréquence-Clinique-Traitement. J Chir 93:311–320
Losee RE, Johnson TR, Southwick WO (1978) Anterior subluxation of the lateral tibial plateau: a diagnostic test and operative repair. J Bone Joint Surg 60-A:1015–1030
MacIntosh DL, Darby TA (1976) Lateral substitution reconstruction. J Bone Joint Surg 58-b:142
Markolf KL, Gorek JF, Kabo JM, Shapiro MS (1990) Direct measurement of resultant forces in the anterior cruciate ligament. An in vitro study performed with a new experimental technique. J Bone Joint Surg Am 72(4):557–567
Mason SF (1962) A history of the sciences. Collier, New York
Menschik A (1974) Mechanics of the knee joint, part I. Z Orthop Ihre Grenzgeb 112(3):481–495
Menschik A (1975) Mechanics of the knee joint, part II, the final rotation. Z Orthop Ihre Grenzgeb 113(3):388–400
von Meyer H (1853) Die mechanik des kniegelenks. Arch Anat Physiol Wiss Med 1:497–547
Moore W (2005) The knife man: blood, body-snatching and the birth of modern surgery. Bantam Press, London
Mueller W (1983) The knee: form, function and ligament reconstruction. Springer, Berlin
Musahl V, Citak M, O’Loughlin PF, Choi D, Bedi A, Pearle AD (2010) The effect of medial versus lateral meniscectomy on the stability of the anterior cruciate ligament-deficient knee. Am J Sports Med 38(8):1591–1597
Musahl V, Plakseychuk A, VanScyoc A, Sasaki T, Debski RE, McMahon PJ, Fu FH (2005) Varying femoral tunnels between the anatomical footprint and isometric positions: effect on kinematics of the anterior cruciate ligament-reconstructed knee. Am J Sports Med 33(5):712–718
Nietert M (1975) Untersuchungen zur Kinematik des Menschlichen Kniegelenkes im Hinblick auf ihre Approximation in der Protetik. Dissertation, Technische Universitat, Berlin
Noyes FR, DeLucas J, Torvik PJ (1974) Biomechanics of anterior cruciate ligament failure: an analysis of strain rate sensitivity and mechanism of failure in primates. J Bone Joint Surg Am 56:236–253
Noyes FR, Torvik PJ, Hide WB, De Lucas JL (1974) Biomechanics of ligament failure. An analysis of immobilization, exercises and reconditioning effects in primates. J Bone Joint Surg Am 56:1406–1518
Paessler HH, Michel D (1992) How new is the lachman test? Am J Sports Med 20(1):95–98
Palmer I (2007) On the injuries to the ligaments of the knee joint: a clinical study. 1938. Clin Orthop Relat Res 454:17–22
Petersen W, Zantop T (2007) Anatomy of the anterior cruciate ligament with regard to its two bundles. Clin Orthop Relat Res 454:35–47
Rosner L (2011) The anatomy murders. being the true and spectacular history of edinburgh’s notorious burke and hare and of the man of science who abetted them in the commission of their most heinous crimes. University of Pennsylvania Press, Philadelphia
Sakane M, Fox RJ, Woo SL, Livesay GA, Li G, Fu FH (1997) In situ forces in the anterior cruciate ligament and its bundles in response to anterior tibial loads. J Orthop Res 15(2):285–293
Sakane M, Livesay GA, Fox RJ, Rudy TW, Runco TJ, Woo SL (1999) Relative contribution of the ACL, MCL, and bony contact to the anterior stability of the knee. Knee Surg Sports Traumatol Arthrosc 7(2):93–97
Schindler OS (2012) Surgery for anterior cruciate ligament deficiency: a historical perspective. Knee Surg Sports Traumatol Arthrosc 20:5–47
Singer C (1957) A short history of anatomy & physiology from greeks to harvey. Dover, New York
Slocum DB, Larson RL (1968) Rotatory instability of the knee: its pathogenesis and a clinical test to demonstrate its presence. J Bone Joint Surg Am 50(2):211–225
Stäubli HU, Birrer S (1990) The popliteus tendon and its fascicles at the popliteal hiatus: gross anatomy and functional arthroscopic evaluation with and without anterior cruciate ligament deficiency. Arthroscopy 6(3):209–220
Strasser H (1917) Lehrbuch der Muskel- und Gelenkmechanik, Bd III: Die untere Extremitat. Springer, Berlin
Terry GC, LaPrade RF (1996) The posterolateral aspect of the knee. Anatomy and surgical approach. Am J Sports Med 24:732–739
Trillat A, Ficat P (1972) Laxités post-traumatiques du genou. Rev Chir Orthop 58:32–114
Vallois HV (1914) Etudes Anatomiques de l’Articulation du Genou Ches les Primates. Thesis, Universite de Montpellier, No. 63
van Eck CF, Schreiber VM, Liu TT, Fu FH (2010) The anatomic approach to primary, revision and augmentation anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 18(9):1154–1163
Vogrin TM, Höher J, Arøen A, Woo SL, Harner CD (2000) Effects of sectioning the posterolateral structures on knee kinematics and in situ forces in the posterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc 8(2):93–98
Warren LF, Marshall JL (1979) The supporting structures and layers on the medial side of the knee: an anatomical analysis. J Bone Joint Surg Am 61(1):56–62
Watson JN, Wilson KJ, LaPrade CM, Kennedy NI, Campbell KJ, Hutchinson MR, Wijdicks CA, LaPrade RF (2014) Iatrogenic injury of the anterior meniscal root attachments following anterior cruciate ligament reconstruction tunnel reaming. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-014-3079-1
Weber W, Weber E (1836) Mechanik der menschlichen Gehwerkzeuge. Dieterichsche Buchhandlung, Göttingen
Wijdicks CA, Griffith CJ, Johansen S, Engebretsen L, LaPrade RF (2010) Injuries to the medial collateral ligament and associated medial structures of the knee. J Bone Joint Surg Am 92(5):1266–1280
Wijdicks CA, Kennedy NI, Goldsmith MT, Devitt BM, Michalski MP, Årøen A, Engebretsen L, LaPrade RF (2013) Kinematic analysis of the posterior cruciate ligament, part 2: a comparison of anatomic single- versus double-bundle reconstruction. Am J Sports Med 41(12):2839–2848
Wijdicks CA, Michalski MP, Rasmussen MT, Goldsmith MT, Kennedy NI, Lind M, Engebretsen L, LaPrade RF (2013) Superficial medial collateral ligament anatomic augmented repair versus anatomic reconstruction: an in vitro biomechanical analysis. Am J Sports Med 41(12):2858–2866
Yagi M, Wong EK, Kanamori A, Debski RE, Fu FH, Woo SL (2002) Biomechanical analysis of an anatomic anterior cruciate ligament reconstruction. Am J Sports Med 30(5):660–666
Zantop T, Petersen W, Sekiya JK, Musahl V, Fu FH (2006) Anterior cruciate ligament anatomy and function relating to anatomical reconstruction. Knee Surg Sports Traumatol Arthrosc 14(10):982–992
Ziegler CG, Pietrini SD, Westerhaus BD, Anderson CJ, Wijdicks CA, Johansen S, Engebretsen L, LaPrade RF (2011) Arthroscopically pertinent landmarks for tunnel positioning in single-bundle and double-bundle anterior cruciate ligament reconstructions. Am J Sports Med 39(4):743–752
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
LaPrade, R.F., Moulton, S.G., Nitri, M. et al. Clinically relevant anatomy and what anatomic reconstruction means. Knee Surg Sports Traumatol Arthrosc 23, 2950–2959 (2015). https://doi.org/10.1007/s00167-015-3629-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-015-3629-1