Abstract
Purpose
Anterior knee pain is a common symptom after intramedullary nailing in tibia shaft fracture. Moreover, patellofemoral malalignment is also known to be a major reason for anterior knee pain. Patellofemoral malalignment predisposes to increased loading in patellar cartilage. In the previous study, we have demonstrated the quadriceps atrophy and patellofemoral malalignment after intramedullary nailing due to tibia shaft fracture. In this study, our aim was to clarify the effects of quadriceps atrophy and patellofemoral malalignment with the pathologic loading on the joint cartilage.
Methods
Mesh models of patellofemoral joint were constructed with CT images and integrated with soft tissue components such as menisci and ligaments. Physiological and sagittal tilt models during extension and flexion at 15°, 30° and 60° were created generating eight models. All the models were applied with 137 N force to present the effects of normal loading and 115.7 N force for the simulation of quadriceps atrophy. Different degrees of loading were applied to evaluate the joint contact area and pressure value with the finite element analysis.
Results
There was increased patellofemoral contact area in patellar tilt models with respect to normal models. The similar loading patterns were diagnosed in all models at 0° and 15° knee flexion when 137 N force was applied. Higher loading values were obtained at 30° and 60° knee flexions in sagittal tilt models. Furthermore, in the sagittal tilt models, in which the quadriceps atrophy was simulated, the loadings at 30° and 60° knee flexion were higher than in the physiological ones.
Conclusions
Sagittal malalignment of the patellofemoral joint is a new concept that results in different loading patterns in the patellofemoral joint biomechanics. This malalignment in sagittal plane leads to increased loading values on the patellofemoral joint at 30° and 60° of the knee flexions. This new concept should be kept in mind during the course of diagnosis and treatment in patients with anterior knee pain. Definition of the exact biomechanical effects of the sagittal tilting will lead to the development of new treatment modalities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anterior knee pain is the most common complication of intramedullary nail application after tibial shaft fracture [17]. Although the aetiology has not been clarified due to the concept of ambiguity, the presence of a nail prominence, iatrogenic intra-articular harm, saphenous nerve damage, patellar tendon and/or infra-patellary fat pad, injuries are supposed to be causative factors for anterior knee pain [18, 21].
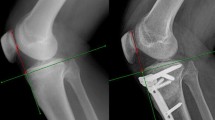
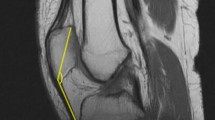
A commonly encountered reason for anterior knee pain is the malalignment of the patellofemoral joint [19]. There are previously defined patellofemoral malalignment terms such as patella alta, patella baja and patellar tilt [15, 22]. However, the malalignment of the patellofemoral joint in the sagittal plane is a new concept. We had previously demonstrated 15.5 % quadriceps atrophy and 8° sagittal patellar tilt (increase in patella/patellar tendon angle) in patients with tibial shaft fractures [3, 4]. However, the effects of this situation on the patellofemoral joint biomechanics are unknown.
Non-physiological positioning of the patella associates with the pathological loadings on the patellofemoral joint. If the effects of quadriceps atrophy and sagittal plane malpositioning on patellofemoral joint biomechanics could be clarified, it would be possible to have better results by improving different physical and surgical treatment modalities.
Finite element analysis (FEA) is a novel method to qualify joint kinematics, contact areas and normal or pathologic loading in cartilage. Nowadays, this method is used to understand the biomechanics of the patellofemoral joint [7, 12].
In the present study, the effect of alteration in the patellofemoral alignment in the sagittal plane and quadriceps atrophy on patellofemoral joint loading and its distribution were evaluated using finite element analyses. It was hypothesized that there would be significant changes in patellofemoral loading and its distribution in models where sagittal patellar tilt and quadriceps atrophy were simulated.
Materials and methods
The study was performed with three-dimensional static linear finite element analyses. “Visible human project” data were used for three-dimensional bone modelling [1]. The data of multiple axial slices derived from the visible human project were converted to three-dimensional models by using 3D-Doctor software (Able Software Corp., Lexington, MA). To create three-dimensional mesh models, Intel Xeon ® R CPU 3.30 GHz processor, 500 GB Hard disk, 14 GB RAM hardware, Windows 7 Ultimate Version Service Pack 1 operation system and Rhinoceros 4.0 (3670 Woodland Park Ave N., Seattle, WA, 98103 USA) three-dimensional modelling software, VRMesh Studio (VirtualGrid Inc, Bellevue City, WA, USA) mesh editing and fixing software were used.
Models
The joint cartilage, medial and lateral menisci, anterior and posterior cruciate ligaments, medial and lateral collateral ligaments, medial and lateral patellofemoral ligaments and patellar tendon were modelled after bone modelling to simulate knee joint structure according to data in the literature (Fig. 1) [20]. Quadriceps muscle vectors were designed as vastus lateralis (VL), rectus femoris–vastus intermedius (RF–VIM) and vastus medialis (VM) obliques. The values of elastic modulus and Poisson’s index for bone, cartilage and meniscus were utilized as 11,000, 6 and 10 Gpa and 0.3, 0.47 and 0.45, respectively [5, 20]. Tendons were modelled as spring elements with a stiffness of 2000.
After modelling the knee joint at full extension, flexion models of the knee at 15°, 30° and 60° flexion were constructed. Sagittal tilt models were modelled by taking the mean sagittal tilt which was 8° in our previous report into consideration (3). With the tilting of the patella by 8° between the cranio-caudal line in sagittal plane, eight models were created (Fig. 2). Sliding and rotation movements of bony structures during knee flexion were taken into consideration [11].
Distribution of force analyses
Finite element analysis was performed with Algor Fempro (ALGOR, Inc., 150 Beta Drive Pittsburgh, PA, 15238-2932, USA) software. Models were 3D mesh modelled with Algor Fempro to bricks and tetrahedral elements. In bricks and tetrahedral modelling, Algor Fempro uses 8 node elements as much as it can and 7, 6, 5 and 4 node elements as needed. Vectors of quadriceps muscle were positioned as follows: RF–VIM parallel to frontal femoral axis, vastus medialis obliques 41° medial and VL 22° lateral. RF/VIM was oriented 4° anterior to the femoral axis in the sagittal plane, whereas VMO and VL were oriented parallel to it.
In all models, totally 137 N force was applied along VL, RF–VMI and VMO vectors. The loading values of VL, RF–VMI and VMO were 50 N, 60 N and 40 N according to muscle dimensions [20]. To simulate 15.5% quadriceps atrophy, force vectors were lowered to 115.7 N. The contact area between the distal femur and the patella was calculated. Then, total loading in this area was measured.
Results
With the increasing degree of knee flexion, there were generally increments in surface contact area in both the physiological group and the sagittal tilt models. Contact areas were increased between full extension and 60° flexion from 175.5 to 623.6 mm2 in physiological models and from 404 to 701 mm2 in sagittal tilt models, respectively.
In all sagittal tilt models at the same flexion degrees, much more surface contact area was established than in the physiological group (Fig. 3).
When the loading parameters were analysed, there were increments from extension towards 15° flexion and decrements from 30° towards 60° flexion. There were similar pressure values on patellar cartilage in all physiological and sagittal tilt models during extension and 15° flexion under 137 N loading. However, higher pressure values were obtained at 30° and 60° flexion in the sagittal tilt models (Fig. 4).
There were higher loading values in the physiological models during full extension and 15° flexion when 137 and 115.7 N were applied to physiological and the sagittal tilt models, respectively. There were also slightly increased pressure values in sagittal tilt models at 30° and 60° flexion (Fig. 5).
Discussion
The main finding of our study was the increase in patellofemoral loadings in 30° and 60° knee flexion in models where sagittal tilt was simulated. The quadriceps hypotrophy and the position of patella in the sagittal plane affected patellofemoral joint loadings. Moreover, the patellofemoral contact area in the sagittal tilt models was found to be increased.
Anterior knee pain is a common situation after intramedullary nailing in patients with tibial shaft fractures. In spite of its controversial aetiology, patellar malalignment or maltracking is known as a reason for anterior knee pain. We had previously reported that patellar malalignment in the sagittal plane causes anterior knee pain in patients treated with intramedullary nailing for tibial shaft fractures [3, 4]. Furthermore, quadriceps atrophy was found to correlate with patellar malalignment in sagittal plane [4].
Patellofemoral malalignment causes increased loading on articular cartilage [10], and increased cartilage loading leads to increased stress on subchondral bony tissue that results in pain and patellar chondromalacia or cartilage destruction [5].
Anterior knee pain and patellofemoral loading were evaluated in the literature with cadaveric models [2, 16]. The controversial aspects of these studies are their demonstrations of non-physiological loadings. FEA is a novel method that is used in the evaluation of patellofemoral joint pathologies with the demonstration of complex knee structure with its menisci, cartilage, bone, muscle and ligamentous tissues [10].
In a study that compares ten patients with anterior knee pain and controls with FEA, increased shear stress was seen on the patellar cartilage and femur [10]. Loading in the patellofemoral joint depends on the magnitude and direction of quadriceps force vectors, the shape of joint surface, cartilage thickness, the biomechanical properties of bony and soft tissues, passive soft tissue constraint and flexion degrees of the knee. There are arguments in the literature related to the joint’s contact area and magnitude of tension [6, 10, 11]. It is also indicated that decreased surface area and increased tension in the articular surface would lead to cartilage degeneration [13].
In a study that evaluates surface area of the knee in 0°, 30° and 60° flexion in 16 healthy individuals, surface areas were reported to be 210, 414 and 520 mm2 in males and 210, 269 and 396 mm2 in females, respectively [5]. Moreover, in another study in which different knee biomechanics were evaluated with respect to different quadriceps loading, increased surface area and contact force were recognized in consequence to the increased degrees of knee flexion [20]. In the present study, increased contact area was demonstrated with respect to increased flexion degrees of the knee in normal and sagittal tilt models. Increased surface area was reported in the sagittal tilt models with respect to normal models at the same flexion degrees. Altered intra-articular surface geometry might be a reason for increased surface area in sagittal tilt models.
It has been reported in the literature that maximal pressure values were reached between 15° and 30° flexion on patellofemoral cartilage [8, 23]. We obtained the same pressure increments during 15°–30° flexion in our study. Moreover, we have demonstrated a similar pattern in sagittal tilt models. The patellofemoral joints in the sagittal tilt models were demonstrated to have increased surface pressure with respect to normal models under the influence of the same quadriceps force vectors. In spite of the increased surface areas in the patellar tilt models compared with the normal ones, it can be claimed that this change in magnitude or orientation of force vectors might be a reason for pressure increments.
Quadriceps mass is related to force in patellofemoral joints [20]. Due to the relationship between sagittal tilt and quadriceps atrophy, pressure values were simulated in accordance with the severity of quadriceps atrophy. While sagittal tilt models were subjected to 15.5% decreased force due to quadriceps atrophy with respect to normal models with exact force, increased loading was recorded in normal models with 0° and 15° flexion and 30° and 60° flexion in sagittal tilt models. Increased pressure values at 30° and 60° flexion in sagittal tilt models, in spite of increased surface area and 15.5% decreased force merits further analysis (Fig. 6).
It is known that daily activities such as stair climbing are a risk factor for patellofemoral pain formation due to increased articular loading especially in early flexion movements [9, 14]. It should be kept in mind that increased patellofemoral loading at 30° and 60° knee flexion in the sagittal tilt group might affect daily activities and be an issue throughout the rehabilitation process.
There are some limitations in this study. The main limitation is the absence of the model simulations by dynamic MRI evaluations. Furthermore, the quadriceps atrophy was allocated equally to the quadriceps vectors, which might be unequal in a clinical setting. On the other hand, if the simulation of quadriceps atrophy were to be simulated by data that could be attributed by the isokinetic muscle evaluation of surgically treated patients, it would give more certain data about the relationship between quadriceps atrophy and patellofemoral pressure. To the best of our knowledge, this study is the first to demonstrate the effect of sagittal patellar tilt on patellofemoral loading and distribution. The inclusion of all the structures within the knee anatomy by FEA increases the power of this study. Further studies analysing the presence of the sagittal patellar tilt in all patients with patellofemoral pain syndrome will help to reveal the pathophysiology underlying the patellofemoral pain.
Conclusions
We believe that the sagittal plane tilt concept will be a cornerstone of patellofemoral pain management. The finite elements analysis revealed that the presence of quadriceps atrophy and sagittal plane malalignment on the patellofemoral joint leads supra-physiological loading patterns on the patellofemoral joint. Clarification of the exact biomechanical aspects of the sagittal tilting would lead to the development of new surgical and conservative treatment algorithms.
References
Ackerman MJ (1998) The visible human project: a resource for anatomical visualization. Stud Health Technol Inform 52(Pt 2):1030–1032
Ahmed AM, Burke DL, Yu A (1983) In-vitro measurement of static pressure distribution in synovial joints—part II: retropatellar surface. J Biomech Eng 105(3):226–236
Aksahin E, Ganal I, Dogan Ö, Hapa O, Yüksel HY, Deren T, Bicimoglu A (2013) Sa4. 13 Anterior knee pain after tibial nailing: What is the role of patellofemoral joint kinematics? Injury 44:S26–S27
Aksahin E, Karasoy I, Hapa O, Dogan Ö, Duran S, Yüksel HY, Yildirim A, Bicimoglu A (2013) Sa4. 12 Does the change in the mass of parapatellar muscle influence the patellofemoral kinematics in the sagittal plane following the surgical treatment of tibial shaft fractures? Injury 44:S26
Besier TF, Gold GE, Beaupre GS, Delp SL (2005) A modeling framework to estimate patellofemoral joint cartilage stress in vivo. Med Sci Sports Exerc 37(11):1924–1930
Besier TF, Gold GE, Delp SL, Fredericson M, Beaupre GS (2008) The influence of femoral internal and external rotation on cartilage stresses within the patellofemoral joint. J Orthop Res 26(12):1627–1635
Bischoff JE, Hertzler JS, Mason JJ (2009) Patellofemoral interactions in walking, stair ascent, and stair descent using a virtual patella model. J Biomech 42(11):1678–1684
Cohen ZA, Roglic H, Grelsamer RP, Henry JH, Levine WN, Mow VC, Ateshian GA (2001) Patellofemoral stresses during open and closed kinetic chain exercises. An analysis using computer simulation. Am J Sports Med 29(4):480–487
Dye SF (2005) The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res 436:100–110
Farrokhi S, Keyak JH, Powers CM (2011) Individuals with patellofemoral pain exhibit greater patellofemoral joint stress: a finite element analysis study. Osteoarthr Cartil 19(3):287–294
Fitzpatrick CK, Baldwin MA, Laz PJ, FitzPatrick DP, Lerner AL, Rullkoetter PJ (2011) Development of a statistical shape model of the patellofemoral joint for investigating relationships between shape and function. J Biomech 44(13):2446–2452
Fitzpatrick CK, Rullkoetter PJ (2012) Influence of patellofemoral articular geometry and material on mechanics of the unresurfaced patella. J Biomech 45(11):1909–1915
Fulkerson JP, Shea KP (1990) Disorders of patellofemoral alignment. J Bone Joint Surg Am 72(9):1424–1429
Goudakos IG, Konig C, Schottle PB, Taylor WR, Singh NB, Roberts I, Streitparth F, Duda GN, Heller MO (2009) Stair climbing results in more challenging patellofemoral contact mechanics and kinematics than walking at early knee flexion under physiological-like quadriceps loading. J Biomech 42(15):2590–2596
Grelsamer RP (2000) Patellar malalignment. J Bone Joint Surg Am 82-A(11):1639–1650
Huberti HH, Hayes WC (1984) Patellofemoral contact pressures. The influence of q-angle and tendofemoral contact. J Bone Joint Surg Am 66(5):715–724
Katsoulis E, Court-Brown C, Giannoudis PV (2006) Incidence and aetiology of anterior knee pain after intramedullary nailing of the femur and tibia. J Bone Joint Surg Br 88(5):576–580
Leliveld MS, Verhofstad MH (2012) Injury to the infrapatellar branch of the saphenous nerve, a possible cause for anterior knee pain after tibial nailing? Injury 43(6):779–783
Luyckx T, Didden K, Vandenneucker H, Labey L, Innocenti B, Bellemans J (2009) Is there a biomechanical explanation for anterior knee pain in patients with patella alta?: influence of patellar height on patellofemoral contact force, contact area and contact pressure. J Bone Joint Surg Br 91(3):344–350
Mesfar W, Shirazi-Adl A (2005) Biomechanics of the knee joint in flexion under various quadriceps forces. Knee 12(6):424–434
Song SY, Chang HG, Byun JC, Kim TY (2012) Anterior knee pain after tibial intramedullary nailing using a medial paratendinous approach. J Orthop Trauma 26(3):172–177
Tecklenburg K, Dejour D, Hoser C, Fink C (2006) Bony and cartilaginous anatomy of the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc 14(3):235–240
Wilson DR, Apreleva MV, Eichler MJ, Harrold FR (2003) Accuracy and repeatability of a pressure measurement system in the patellofemoral joint. J Biomech 36(12):1909–1915
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aksahin, E., Kocadal, O., Aktekin, C.N. et al. The effects of the sagittal plane malpositioning of the patella and concomitant quadriceps hypotrophy on the patellofemoral joint: a finite element analysis. Knee Surg Sports Traumatol Arthrosc 24, 903–908 (2016). https://doi.org/10.1007/s00167-014-3421-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-014-3421-7