Abstract
Purpose
Graft healing following anterior cruciate ligament (ACL) reconstruction is a complex process characterized by phases of healing that lead to ACL remodelling. Our hypothesis is that fibrin clot addition to ACL reconstruction will result in advanced graft remodelling and healing when compared to a control group at 12 weeks as observed by histology, immunohistochemistry and magnetic resonance imaging (MRI).
Methods
Eleven Spanish Boar goats underwent double-bundle ACL reconstruction: 8 were analysed and 3 were excluded. Group 1 was treated with DB ACL reconstruction utilizing autologous fibrin clots (n = 4), and group 2 was treated with standard DB ACL-R (n = 4). Histological and radiographic analysis was performed at 12 weeks. Each animal underwent 3-T MRI immediately after euthanization for evaluation of graft signal intensity utilizing the signal noise quotient (SNQ). Specimens were then sectioned and stored for standard histological and immunohistochemistry testing.
Results
The mean ligament tissue maturity index score was significantly higher for group 1 (15 ± 2.3) compared with group 2 (7.7 ± 5.2) (p < 0.05). The mean vascularity (cell/mm2) for group 1 was 7.1 ± 1.3 and 9.3 ± 3.1 for group 2 (n.s.). The mean collagen type 1 (% 50× field) for group 1 was 35.8 ± 22.1 and 19.9 ± 20.5 for group 2 (n.s.). The mean SNQ for the AM bundle was 1.1 ± 0.7 for group 1 and 3.1 ± 1.8 for group 2 (n.s.). The mean SNQ for the total PL bundle was significantly lower for group 1 (1.1 ± 0.7) compared with group 2 (3.7 ± 1.3) (p < 0.05). There was a significant correlation between the vascularity and the ligament tissue maturity index score as well as between collagen type 1 and SNQ, both AM and PL bundles (p < 0.05).
Conclusion
The use of fibrin clot in ACL reconstruction in a caprine model demonstrated improved healing with respect to histological analysis of the intra-articular ACL reconstruction segment and decreased signal intensity on MRI. It may lead to improved graft healing and maturation. By accelerating the intra-articular healing and ligamentization, the outcome of patients after ACL-R can be improved with faster return to sports and daily activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The primary goal of anterior cruciate ligament (ACL) reconstruction is to replicate native anatomy and restore knee stability [1, 6, 35]. Early graft failure, including re-rupture or persistent instability, is reported in 2–3 % of the cases [15, 18, 29]. Biological factors play a major role in graft failure as evidenced by a high re-rupture rate during the first 12 months following ACL reconstruction [38]. This phenomenon is also detected in double-bundle (DB) ACL reconstruction where single-bundle re-ruptures have been observed [13].
ACL graft healing involves stages of inflammation, vascularization as well as cellular and fibrous remodelling [11]. The process of graft remodelling begins with an inflammatory response driven by recruitment of macrophages and cytokines in the first day following surgery [17]. This leads to progressive cellular repopulation of the tendon graft and formation of fibrous scar tissue at the bone-to-tendon junction. The intra-articular segment of the graft heals faster and undergoes a stage of early revascularization when compared to healing at the bone-to-tendon junction. At 20 weeks postoperatively, the intrinsic vasculature of the graft is matured [4]. Vascularization is followed by progressive remodelling and finally complete healing of the graft [11].
Efforts to improve ACL graft healing through biological enhancements have focused on the use of bone morphogenic protein, gene therapy and exogenous growth factors. Increased bone formation, decreased tunnel widening, increased collagen fibrils and increased load to failure have been described [7, 19, 33, 36]. Recent research has focused on platelet-derived therapies [16, 22, 27, 30, 32]. However, the use of platelet-rich plasma (PRP) alone was shown to be insufficient in improving primary ACL repair [21].
Murray et al. showed that the addition of a scaffold to PRP enhances primary ACL repair and ACL graft healing [12, 16]. Fibrin clots have been described for the enhancement of healing of musculoskeletal tissues, such as meniscus and cartilage lesions [5, 26, 28]. Fibrin clots are readily available and display properties necessary for structural support as well as a delivery mechanism of platelet-derived growth factors. The collagen matrix stabilizes the platelets and protects the clot from premature dissolution by the synovial fluid. Therefore, the objective of this study was to examine the effect of a fibrin clot on ACL healing following double-bundle (DB) ACL reconstruction in a caprine model. A DB reconstruction technique was chosen due to the fact that the native ACL consists of two functional bundles that are attached to each other histologically. Therefore, it would be necessary not only to improve the healing/ligamentization within a bundle but also to achieve improved healing between the two bundles. By accelerating the intra-articular healing and ligamentization, the outcome of patients after ACL reconstruction can be improved with faster return to sports and daily activity. It was hypothesized that the addition of a fibrin clot to ACL reconstruction would result in advanced healing when compared to a control ACL-R group. Analysis was performed by histology, immunohistochemistry and magnetic resonance imaging (MRI).
Materials and methods
ACL reconstruction in the goat model has been successfully established with a low rate of osteoarthritis and graft failure postoperatively [10, 39]. Eleven mature female Spanish Boar goats, between 3 and 4 years of age, were used in this study. Animals were obtained from a licensed US Department of Agriculture dealer and were housed in the animal facilities at our institution. Each animal underwent caprine arthritis encephalitis virus testing prior to boarding to ensure the absence of accelerated progression of knee osteoarthritis.
In each animal, DB ACL reconstruction was performed on the right hindlimb utilizing an Achilles tendon autograft technique. The left hindlimb was observed as a normal control. Group 1 (n = 5) underwent DB ACL reconstruction utilizing an autologous fibrin clot, and group 2 (n = 6) underwent standard DB ACL reconstruction. Animals were randomly assigned to each group. Both histological and MRI analysis were performed by blinded observers in the Department of Orthopaedic Surgery (DH, KI).
Surgical technique
Prior to induction of anaesthesia, the animals were clinically examined for signs of trauma or pathology to the knee that might interfere with the surgical procedure. A posterolateral incision was made over the Achilles tendon, and the middle third of the Achilles tendon was harvested yielding a graft of approximately 10 cm in length by 5–6 mm in width. After harvesting, the tendon was re-approximated and the tendinous sheath was repaired.
The graft was split longitudinally in line with the orientation of the fibres to form two separate grafts. Each individual graft was looped incorporating a whipstitch at the distal end and a suture through the looped portion, both utilizing Ultrabraid sutures (Smith and Nephew Endoscopy, Andover, MA). Each graft was sized to 5 mm diameter.
Double-bundle ACL reconstruction
A standard medial para-patellar incision was used, and after preparation, the patella was dislocated laterally. The native insertion site of the anteromedial (AM) and posterolateral (PL) bundles was identified, marked to allow for anatomic ACL reconstruction and then excised using a No. 15 scalpel. A guide pin was inserted into the centre of the PL insertion on the femur. Using a 5-mm reamer (Smith and Nephew Endoscopy, Andover, MA), the femoral PL tunnel was reamed through the lateral cortex (Fig. 1a). The AM position was identified and marked with a guide pin in identical fashion (Fig. 1b). The tibial PL and AM position were identified, and an ACL tip guide (Acufex, Smith & Nephew, Andover, MA) was used to place a guide wire in the PL and AM tibial insertion sites (Fig. 1c). Five-mm tibial tunnels were reamed for the PL and AM tunnels leaving a bone bridge between the tunnels. The two grafts were passed from their respective tibial tunnel to the femoral tunnel, starting with the PL graft (Fig. 1d). Femoral fixation was achieved with sutures tied over a titanium button (Smith and Nephew Endoscopy, Andover, MA). Tibial fixation was achieved with sutures tied over a 3.5-mm cortical screw and washer. Standard layered closure was performed, assuring adequate medial stabilization in order to prevent patellar dislocation. Postoperative lateral radiographs were obtained to document correct tunnels and hardware positioning.
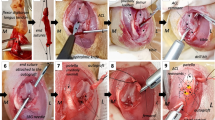
Intra-operative photographs of the DB ACL reconstruction. a Placement of the PL guide wire (downwards arrow) in the centre of PL footprint. b The AM (asterisk) and PL (downwards arrow) tunnels in their anatomic footprints. c Both the femoral and tibial AM and PL tunnels are shown. d Both AM and PL grafts are passed
Fibrin clot preparation
Ten minutes prior to drilling the tibial tunnels, 50 cc of venous whole blood was drawn from a peripherally located, commonly used permanent venous catheter and placed in a 250-ml sterile, borosilicate glass beaker. Using a sterile stir rod, the whole blood was gently stirred in a twisting clockwise fashion for 10–15 min until coagulation and formation of a fibrin clot was achieved. Once clotted, the fibrin clot was removed from the beaker and placed on sterile gauze. The clot was separated into two small portions and one larger portion. The two smaller portions were incorporated into the proximal (femoral) ends of the graft (Fig. 2a), with the larger portion of the fibrin clot placed between the two intra-articular grafts (Fig. 2b).
Double-bundle ACL reconstruction with fibrin clot
The DB ACL reconstruction was performed in the same manner as described above with the following additional steps: the AM and PL grafts had a fibrin clot incorporated into the proximal portion as described above. After passage of the grafts, a looped suture was placed between the AM and PL bundle in order to place gentle traction on the AM bundle in an anterior direction (Fig. 2b). This created adequate space for the placement of the fibrin clot, which was introduced between the AM and PL bundles, taking care to maximize the surface area of contact between the clot and grafts. Using a 2-0 vicryl, three simple stitches were placed through the AM bundle into the PL bundle to secure the clot in place.
Postoperatively, free cage activity was allowed and gait was documented as described previously utilizing a lameness score [39]. Vital signs and general animal health were monitored. Goats were killed after 12 weeks with an overdose injection of sodium pentobarbital.
Histology
The knee joints were harvested and assessed macroscopically for signs of graft failure, osteoarthritis, fibrous formation and any signs of abnormal pathology. The intra-articular ACL reconstruction segments were harvested from the femoral and tibial origins and immediately frozen in liquid nitrogen for cryosectioning. Serial sections of 8 μm thickness were followed by routine histological staining (haematoxylin and eosin [H&E]) for visualization with light microscopy. Specimens were sectioned in the axial and coronal planes. For histological evaluation, images were obtained at low- and high-power magnification with a Leica DFC 300 FX digital camera (Leica Microsystems, Wetzlar, Germany) coupled to a Leica DMLB light microscope. For histological evaluation, three central sections of every animal were analysed by two observers who were blinded to each other and to the assigned treatment for the animal. The ligament tissue maturity index, as described by Murray et al. [23], was used to quantify histological specimens. A native ACL, harvested from the contralateral knee, was used as a reference for these criteria.
Immunohistochemistry
For detailed immunohistochemical analysis of the neoligament vascularity, cryosections were stained with monoclonal antibodies against alpha-smooth muscle actin (α-SMA) and collagen type 1. After fixation with methanol, all samples were treated with 0.3 % hydrogen peroxide in methanol for 15 min to block endogenous peroxidase activity. Non-specific binding of primary antibodies was reduced by blocking with normal donkey serum. α-SMA antibody binding was detected with Alexaflour 488 Donkey, and counterstaining was performed using DAPI. As in histological analysis, three sections of every animal were analysed (counting of the blood vessels) and the median from these sections was used for statistical analysis. Photomicrographs were taken on three different slides treated with α-SMA. On these slides, vascularity was determined by counting all the vessels in a 6-mm2 area and the vessel count per mm2 was calculated. Collagen type 1 antibody binding was detected with Alexaflour 594 Donkey, and counterstaining was performed using DAPI. Photomicrographs were taken and the two best, most representative sections of every animal were analysed for the percentage of collagen type 1 staining per 100× magnification field, and the mean was used for statistical analysis.
Magnetic resonance imaging
Immediately after euthanization, the right hindlimb was harvested and imaged on a 3-T MRI (Siemens, Erlangen, Germany). Sagittal T2, TE-18, slice thickness 2-mm sequences were used for analysis. MRI signal intensity was determined utilizing the signal to noise quotient (SNQ) [3]. Using the OSIRIX free imaging software (version 3.7.1, Pixmeo Sari, Bernex, Switzerland), three positions on the AM and PL bundle were determined: proximal, midsubstance and distal. A 4-mm2 circle region of interest (ROI) was placed in the centre of the three locations. The ROI size was determined as the maximum circular area that could be used in all animals and stay within the limits of the graft in all three regions. The mean signal intensity and standard deviation were determined for each region. A single 4-mm2 circle ROI was determined in the tibialis anterior (TA) and on the background 5 mm anterior to the patella as a normal control (Fig. 3). SNQ was calculated as (1) AM SNQ = (AM signal − TA signal)/Background signal and (2) PL SNQ = (PL signal − TA signal)/Background signal.
The study protocol was approved by the Institutional Animal Care and Use Committee of our institution (Division of Laboratory Animal Resources, Protocol no. 0912030).
Statistical analysis
Data analysis began with the calculation of descriptive statistics including frequency counts and percents for categorical variables and measures of central tendency (means, medians) and dispersion [standard deviation, inter-quartile ranges (IQR)] for continuous variables. Because of the small sample sizes, the data are reported as medians and inter-quartile ranges, and we used nonparametric statistical methods to analyse the data. Independent sample Mann–Whitney U tests were used to compare the ligament tissue maturity index score, vascularity, collagen type 1 and the SNQ between the two groups. Spearman’s correlation coefficients were used to determine the relationships between the ligament tissue maturity index score, vascularity, collagen type 1 and the SNQ. All statistical analyses were performed using SPSS for Windows, version 16 (SPSS, Chicago, IL). Statistical significance was determined to be p < 0.05.
Results
Three animals were excluded from this study. One animal in the “control” group sustained systemic sepsis in the immediate postoperative period and was euthanized at 1 week. One animal in the “fibrin clot” group sustained a neurological insult due to a fall on postoperative day #2 and was euthanized at 1 week. One animal in the fibrin clot group developed septic arthritis. The animal was therefore excluded from further analysis. The remaining animals tolerated surgery well and displayed uneventful skin healing. Normal gait was achieved by 4 weeks postoperatively. Eight animals were included in the analysis for this study.
Macroscopic analysis
In both groups, newly formed scar tissue could be found adjacent to the pre-patellar fat pad and the neoligament. After removal of the tissue, it was observed that the fibrin clot group had more continuity between the AM and PL bundle; however, excessive macroscopic examination was not undertaken in order to preserve new tissue formation between the bundles for histological analysis. All grafts appeared intact with stable connections to femur and tibia (Fig. 4).
Histology
On H&E analysis, there was a consistently more organized and ligamentous-appearing tissue in the “fibrin clot” group when compared to the “control” group, although the morphology and microstructure of both groups contrasted with the native caprine ACL. More fusiform cells could be found in the “fibrin clot” group throughout the grafts, whereas ovoid cells were found in the “control” group. There was a more pronounced septum between the AM and PL bundles in the “fibrin clot” group (Fig. 5). However, the median and inter-quartile range (IQR) for the ligament tissue maturity index score were not significantly different (Table 1).
Hyphen indicates representative samples of histological staining of the graft (haematoxylin and eosin [H&E]). Notice the different appearance of the remodelling tissue between the two groups under polarized microscope. a Stained tissue appears more tendon-like (asterisk) with some areas of increased remodelling and higher cell density (hash). c On higher magnification, bundle-like structures and crimp can be noticed. d Remodelling tissue in the non-fibrin clot group appears inhomogeneous with the appearance of a large number of cells. e The fibrin clot group shows consistently more spindle cells (arrows). f In the non-fibrin clot group, more ovoid cells can be found indicating increased remodelling. g Area between the bundles (hash) showing dens scar tissue formation. h Shows loose tissue with less active ovoid cells. Bar size: a, b, g, h 100 μm; c–f 40 μm; longitudinal section: a–f, cross section: g + h
Immunohistochemistry (α-SMA)
In contrast to the native ACL, samples from both groups show increased vascularity in the central regions of the graft (Fig. 6). Vessels in the “fibrin clot” group were smaller and distributed more regularly than in the “control” group with their orientation along the bundles (Fig. 6c, d). Arterioles were noticed in 7 out of 8 goats in areas between the two bundles of the graft as well as in the adjacent newly formed tissue (Fig. 6b). There was no significance between the two groups in regard to vascularity content (Table 1). There was a significant negative correlation between the vascularity and the ligament tissue maturity index score (r = −0.9, p = 0.005).
Hyphen indicates representative immunohistochemistry, α-smooth muscle actin (α-SMA) where green is a positive stain, cross-sectional samples. a Native ACL: minimal vascularity inside the tendon (hash) and increased vascularity at the border of the ligament (red arrows). b Arterioles (asterisk) between bundles of a sample from the fibrin clot group. c, d Intraligamentous examples of fibrin clot group showing less vascularity compared to an representative sample from the non-fibrin clot group. Bar size in all samples: 40 μm
Immunohistochemistry (collagen type 1)
The native goat ACL had a collagen type 1 of 86.58 % and was orientated and organized in the axial section (Fig. 7a). Orientation and organization of collagen fibres and percentage per field were not significant between the groups (Figs. 7b, c, 8a–d, n.s.; Table 1).
Magnetic resonance imaging (Fig. 9)
There was a significant difference in the proximal AM, proximal PL and distal PL SNQ between the two groups (p < 0.05; Fig. 5; Table 2). There was a significant negative correlation between total AM SNQ and collagen type 1 (r = −0.8, p = 0.02), as well as total PL SNQ and collagen type 1 (r = −0.7, p = 0.05).
Sagittal T2 MRI. a One week following DB ACL-R without fibrin clot showing low signal intensity. b Twelve weeks following DB ACL-R with fibrin clot showing low signal intensity in AM and PL bundles. c Twelve weeks following DB ACL-R without fibrin clot showing high signal intensity in the AM and PL bundles
Discussion
The most important finding of the present study was that the addition of a fibrin clot resulted in evidence of advanced healing on histology and decreased signal intensity on MRI when compared to a control group at 12 weeks following DB ACL reconstruction in a caprine model. These results support the hypothesis that the addition of a fibrin clot to ACL-R would result in advanced healing when compared to standard DB ACL reconstruction without a fibrin clot.
The ligament maturity index in the fibrin clot group indicates, however, statistically not significant in the present study, that the remodelling tissue appears more ligamentous compared with the samples without a fibrin clot. Addition of a fibrin clot resulted in more fusiform cells indicating the capability of producing and organizing collagen [2, 8]. Although not statistically significant, the trend towards a higher collagen content in the fibrin clot group is supported by this. It is therefore likely that improved biomechanical properties after DB ACL reconstruction with a fibrin clot would be achieved [9, 25].
The histological and immunohistochemical results in both groups showed higher vascularity of the graft after 12 weeks compared with the native ACL. The addition of a fibrin clot resulted in decreased vascularity and more mature appearing vessels; however, the number of vessels between both groups was not significant. A recent study showed in a porcine model that the vascularity of remodelling tissue increases until 6 weeks postoperatively and then decreases until 12 weeks after surgery, indicating a more matured graft [16, 20]. Furthermore, higher vascularization correlates to decreased mechanical properties [34, 37]. The current study demonstrated that there was a significant negative correlation between vascularity and ligament tissue maturity index score, with a higher vascularity associated with a lower ligament tissue maturity index score.
The mean percentage of collagen type 1 per group was higher in the fibrin clot group than in the non-fibrin clot, although this was not statistically significant. As evident by the native ACL collagen type 1 content (86.58 %), a higher collagen type 1 concentration is associated with a more ligamentous-like structure and increased tensile properties when compared to lower collagen type 1 percentages. An increase in signal intensity on MRI (both and AM and PL bundles, Fig. 9) was shown to correlate with a decrease in collagen type 1 percentage. This correlation may prove to be clinical useful when evaluating patients with increased signal intensity in the ACL on MRI after reconstruction as this may be related to a decrease in collagen type 1 content and potentially decreased biomechanical properties. Previous literature has shown that the use of PRP in ACL-R resulted in lower signal intensity when compared to a control group [24, 27, 31]. This study showed that signal intensity on MRI of the “fibrin clot” group was significantly lower in two regions (one in AM and one in PL) and the PL bundle as a whole compared to the “control” group (Table 2). Studies have shown that high intensity on MRI is correlated with an increase in vascularity and a decrease in mechanical properties of the graft [37].
Complete healing and incorporation of the graft require the two bundles created with double-bundle ACL reconstruction to heal to one another and allow load sharing as in the native ACL. The current study found evidence that addition of a fibrin clot in between the AM and PL bundles resulted in increased tissue formation between the bundles as well as a more mature graft. This could aid in healing of the bundles and provide a basis for bundle synergy.
Limitations of this study include lack of biomechanical testing on the effect of the fibrin clot on ACL reconstruction. Furthermore, there is a small sample size in the present study. However, while the results indicate favourable effects of a fibrin clot, further studies with a larger number of animals in each group will be necessary to achieve significance. This study did not have a PRP control group, and therefore, a direct comparison of PRP over a fibrin clot could not be made. However, previous studies attempted to use PRP in the caprine model and found it to be unsuccessful due to the similarities in the caprine red blood cell and platelet [32]. The properties of the fibrin clot, such as type of growth factors and their concentration inside the clot as well as their release over time, were not investigated. These issues will also have to be investigated in further studies. The growth factor concentrations in the fibrin clot are also unknown and would be beneficial as studies have shown that delivery of platelet-derived growth factors alter the mechanical properties during graft remodelling [36].
This study showed that there is potential to improve healing and ligamentization of the graft and usage of a fibrin clot is one option; however, significant improvement could not be demonstrated.
Conclusion
Fibrin clots are readily available, require no additional cost and can be prepared in as little as 10 min [14]. Data from this study indicated that the addition of a fibrin clot to DB ACL reconstruction resulted in a slightly higher histological score of ligament and decreased MRI signal intensity indicating improved healing. There were also trends indicating that a fibrin clot increased collagen content and decreased vascularity although these were not statistically significant. Bundle-to-bundle healing in this study was improved providing the necessary basis for load sharing.
References
Abebe ES, Moorman CT 3rd, Dziedzic TS, Spritzer CE, Cothran RL, Taylor DC, Garrett WE Jr, DeFrate LE (2009) Femoral tunnel placement during anterior cruciate ligament reconstruction: an in vivo imaging analysis comparing transtibial and 2-incision tibial tunnel-independent techniques. Am J Sports Med 37(10):1904–1911
Aggeler J, Frisch SM, Werb Z (1984) Changes in cell shape correlate with collagenase gene expression in rabbit synovial fibroblasts. J Cell Biol 98(5):1662–1671
Ahn JH, Lee SH, Choi SH, Lim TK (2010) Magnetic resonance imaging evaluation of anterior cruciate ligament reconstruction using quadrupled hamstring tendon autografts: comparison of remnant bundle preservation and standard technique. Am J Sports Med 38(9):1768–1777
Arnoczky SP, Tarvin GB, Marshall JL (1982) Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg Am 64(2):217–224
Arnoczky SP, Warren RF, Spivak JM (1988) Meniscal repair using an exogenous fibrin clot. An experimental study in dogs. J Bone Joint Surg Am 70(8):1209–1217
Bedi A, Altchek DW (2009) The “footprint” anterior cruciate ligament technique: an anatomic approach to anterior cruciate ligament reconstruction. Arthroscopy 25(10):1128–1138
Chen CH, Chang CH, Su CI, Wang KC, Liu HT, Yu CM, Wong CB, Wang IC (2010) Arthroscopic single-bundle anterior cruciate ligament reconstruction with periosteum-enveloping hamstring tendon graft: clinical outcome at 2 to 7 years. Arthroscopy 26(7):907–917
Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE (1997) Geometric control of cell life and death. Science 276(5317):1425–1428
Doillon CJ, Dunn MG, Bender E, Silver FH (1985) Collagen fiber formation in repair tissue: development of strength and toughness. Coll Relat Res 5(6):481–492
Ekdahl M, Nozaki M, Ferretti M, Tsai A, Smolinski P, Fu FH (2009) The effect of tunnel placement on bone–tendon healing in anterior cruciate ligament reconstruction in a goat model. Am J Sports Med 37(8):1522–1530
Ekdahl M, Wang JH, Ronga M, Fu FH (2008) Graft healing in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 16(10):935–947
Fleming BC, Spindler KP, Palmer MP, Magarian EM, Murray MM (2009) Collagen–platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am J Sports Med 37(8):1554–1563
Fu FH, Shen W, Starman JS, Okeke N, Irrgang JJ (2008) Primary anatomic double-bundle anterior cruciate ligament reconstruction: a preliminary 2-year prospective study. Am J Sports Med 36(7):1263–1274
Illingworth KD, Musahl V, Lorenz S, Fu FH (2010) Use of fibrin clot in the knee. Oper Tech Orthop 20(2):90–97
Jarvela T (2007) Double-bundle versus single-bundle anterior cruciate ligament reconstruction: a prospective, randomize clinical study. Knee Surg Sports Traumatol Arthrosc 15(5):500–507
Joshi SM, Mastrangelo AN, Magarian EM, Fleming BC, Murray MM (2009) Collagen–platelet composite enhances biomechanical and histologic healing of the porcine anterior cruciate ligament. Am J Sports Med 37(12):2401–2410
Kawamura S, Ying L, Kim HJ, Dynybil C, Rodeo SA (2005) Macrophages accumulate in the early phase of tendon-bone healing. J Orthop Res 23(6):1425–1432
Lewis PB, Parameswaran AD, Rue JP, Bach BR Jr (2008) Systematic review of single-bundle anterior cruciate ligament reconstruction outcomes: a baseline assessment for consideration of double-bundle techniques. Am J Sports Med 36(10):2028–2036
Martinek V, Latterman C, Usas A, Abramowitch S, Woo SL, Fu FH, Huard J (2002) Enhancement of tendon-bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-2 gene transfer: a histological and biomechanical study. J Bone Joint Surg Am 84-A(7):1123–1131
Murray MM, Martin SD, Martin TL, Spector M (2000) Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am 82(10):1387–1397
Murray MM, Palmer M, Abreu E, Spindler KP, Zurakowski D, Fleming BC (2009) Platelet-rich plasma alone is not sufficient to enhance suture repair of the ACL in skeletally immature animals: an in vivo study. J Orthop Res 27(5):639–645
Murray MM, Spindler KP, Abreu E, Muller JA, Nedder A, Kelly M, Frino J, Zurakowski D, Valenza M, Snyder BD, Connolly SA (2007) Collagen–platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res 25(1):81–91
Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB (2007) Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen–platelet-rich plasma scaffold. J Orthop Res 25(8):1007–1017
Orrego M, Larrain C, Rosales J, Valenzuela L, Matas J, Durruty J, Sudy H, Mardones R (2008) Effects of platelet concentrate and a bone plug on the healing of hamstring tendons in a bone tunnel. Arthroscopy 24(12):1373–1380
Oxlund H, Barckman M, Ortoft G, Andreassen TT (1995) Reduced concentrations of collagen cross-links are associated with reduced strength of bone. Bone 17(4 Suppl):365S–371S
Paletta GA, Arnoczky SP, Warren RF (1992) The repair of osteochondral defects using an exogenous fibrin clot. An experimental study in dogs. Am J Sports Med 20(6):725–731
Radice F, Yanez R, Gutierrez V, Rosales J, Pinedo M, Coda S (2010) Comparison of magnetic resonance imaging findings in anterior cruciate ligament grafts with and without autologous platelet-derived growth factors. Arthroscopy 26(1):50–57
Ritchie JR, Miller MD, Bents RT, Smith DK (1998) Meniscal repair in the goat model. The use of healing adjuncts on central tears and the role of magnetic resonance arthrography in repair evaluation. Am J Sports Med 26(2):278–284
Salmon L, Russell V, Musgrove T, Pinczewski L, Refshauge K (2005) Incidence and risk factors for graft rupture and contralateral rupture after anterior cruciate ligament reconstruction. Arthroscopy 21(8):948–957
Sanchez M, Anitua E, Azofra J, Prado R, Muruzabal F, Andia I (2010) Ligamentization of tendon grafts treated with an endogenous preparation rich in growth factors: gross morphology and histology. Arthroscopy 26(4):470–480
Sanchez M, Anitua E, Lopez-Vidriero E, Andia I (2010) The future: optimizing the healing environment in anterior cruciate ligament reconstruction. Sports Med Arthrosc 18(1):48–53
Spindler KP, Murray MM, Carey JL, Zurakowski D, Fleming BC (2009) The use of platelets to affect functional healing of an anterior cruciate ligament (ACL) autograft in a caprine ACL reconstruction model. J Orthop Res 27(5):631–638
Tanaka Y, Yonetani Y, Shiozaki Y, Kanamoto T, Kita K, Amano H, Kusano M, Hirakawa M, Horibe S (2014) MRI analysis of single-, double-, and triple-bundle anterior cruciate ligament grafts. Knee Surg Sports Traumatol Arthrosc 22(7):1541–1548
Tohyama H, Yasuda K (2000) Extrinsic cell infiltration and revascularization accelerate mechanical deterioration of the patellar tendon after fibroblast necrosis. J Biomech Eng 122(6):594–599
van Eck CF, Lesniak BP, Schreiber VM, Fu FH (2010) Anatomic single- and double-bundle anterior cruciate ligament reconstruction flowchart. Arthroscopy 26(2):258–268
Weiler A, Forster C, Hunt P, Falk R, Jung T, Unterhauser FN, Bergmann V, Schmidmaier G, Haas NP (2004) The influence of locally applied platelet-derived growth factor-BB on free tendon graft remodeling after anterior cruciate ligament reconstruction. Am J Sports Med 32(4):881–891
Weiler A, Peters G, Maurer J, Unterhauser FN, Sudkamp NP (2001) Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging. A two-year study in sheep. Am J Sports Med 29(6):751–761
Wright RW, Huston LJ, Spindler KP, Dunn WR, Haas AK, Allen CR, Cooper DE, DeBerardino TM, Lantz BB, Mann BJ, Stuart MJ (2010) Descriptive epidemiology of the multicenter ACL revision study (MARS) cohort. Am J Sports Med 38(10):1979–1986
Zantop T, Ferretti M, Bell KM, Brucker PU, Gilbertson L, Fu FH (2008) Effect of tunnel–graft length on the biomechanics of anterior cruciate ligament-reconstructed knees: intra-articular study in a goat model. Am J Sports Med 36(11):2158–2166
Acknowledgments
This study received funding from a research grant provided by Smith and Nephew. The authors would like to acknowledge Hongshuai Li, Carola van Eck, Monica Linde-Rosen, Keenan Kim and the Department of Laboratory Animal Research at the University of Pittsburgh for their contributions to this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Daniel Hensler and Kenneth D. Illingworth have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Hensler, D., Illingworth, K.D., Musahl, V. et al. Does fibrin clot really enhance graft healing after double-bundle ACL reconstruction in a caprine model?. Knee Surg Sports Traumatol Arthrosc 23, 669–679 (2015). https://doi.org/10.1007/s00167-014-3380-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-014-3380-z