Abstract
Purpose
The aim of this study was to determine whether selective decontamination of the digestive tract (SDD) reduces in-hospital mortality in mechanically ventilated critically ill adults admitted to the intensive care unit (ICU) with acute brain injuries or conditions.
Methods
We carried out a post hoc analysis from a crossover, cluster randomized clinical trial. ICUs were randomly assigned to adopt or not to adopt a SDD strategy for two alternating 12-month periods, separated by a 3-month inter-period gap. Patients in the SDD group (n = 2791; 968 admitted to the ICU with an acute brain injury) received a 6-hourly application of an oral paste and administration of a gastric suspension containing colistin, tobramycin, and nystatin for the duration of mechanical ventilation, plus a 4-day course of an intravenous antibiotic with a suitable antimicrobial spectrum. Patients in the control group (n = 3191; 1093 admitted to the ICU with an acute brain injury) received standard care. The primary outcome was in-hospital mortality within 90 days. There were four secondary clinical outcomes: death in ICU, ventilator-, ICU- and hospital-free days to day 90.
Results
Of 2061 patients with acute brain injuries (mean age, 55.8 years; 36.4% women), all completed the trial. In patients with acute brain injuries, there were 313/968 (32.3%) and 415/1093 (38%) in-hospital deaths in the SDD and standard care groups (unadjusted odds ratio [OR], 0.76, 95% confidence interval [CI] 0.63–0.92; p = 0.004). The use of SDD was associated with statistically significant improvements in the four clinical secondary outcomes compared to standard care. There was no significant heterogeneity of treatment effect between patients with and without acute brain injuries (interaction p = 0.22).
Conclusions
In this post hoc analysis of a randomized clinical trial in critically ill patients with acute brain injuries receiving mechanical ventilation, the use of SDD significantly reduced in-hospital mortality in patients compared to standard care without SDD. These findings require confirmation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Improvement in clinically important outcomes associated with selective decontamination of the digestive tract (SDD), such as reduced mortality and duration of mechanical ventilation, may be restricted to specific high-risk populations such as those with acute brain injuries. SDD may have no benefit in patients without acute brain injuries |
Introduction
Selective decontamination of the digestive tract (SDD), consisting of an oral antibiotic paste and a gastric antibiotic suspension, combined with a short course of intravenous antibiotics has been extensively studied in critically ill patients [1]. In the recently published Selective Decontamination of the Digestive Tract in the Intensive Care Unit (SuDDICU) trial, hospital mortality was not significantly different for patients allocated to SDD and standard care without SDD [2]. When data from this and other randomized clinical trials were combined in a Bayesian meta-analysis, there was a 99.3% posterior probability that SDD was associated with reduced hospital mortality compared to standard care [1]. It remains uncertain whether the reduction in mortality associated with SDD [2] was driven by a benefit in a particular subgroup or subgroups.
Patients with acute brain injuries or conditions with reduced levels of consciousness and impaired airway reflexes are at risk of aspiration events that may progress to lower respiratory tract infections or ventilator-associated pneumonia [1, 3, 4]. Ventilator-associated pneumonia may result in fever, hypoxaemia and impaired ventilation that are recognized causes of secondary brain injury [4], potentially resulting in additional deaths. As SDD is an infection control strategy designed to prevent ventilator-associated pneumonia, it may be of particular benefit in this group of patients.
To evaluate the possibility that SDD benefits patients admitted to the ICU with acute brain injuries, a post hoc subgroup analysis using data from the SuDDICU trial was conducted.
Methods
Consent
Ethical approval for the SuDDICU Australia trial was obtained from Human Research Ethics Committees and Research Governance Offices at each site. As SDD was implemented as an ICU-wide intervention, a waiver of individual patient consent was obtained from each Lead Human Research Ethics Committee according to jurisdictional requirements. For patients in the standard care group, a waiver of consent was obtained as no intervention was provided. As no new data were obtained for this subgroup analysis, additional ethical approval was not required.
Study design and oversight
The study protocol and statistical analysis plan [5] and the primary manuscript [2] for the SuDDICU crossover, cluster randomized clinical trial have been published previously.
The SuDDICU trial was reported according to the CONSORT 2010 reporting guidelines [6] (electronic supplementary material, ESM).
Data were entered into an encrypted database for statistical analyses conducted at The George Institute for Global Health, Australia.
Details of the trial management, sponsorship, collaborations and committees are provided in the ESM.
Trial participants
The SuDDICU Australia trial was conducted in 19 ICUs in 17 hospitals in Australia from May 2017 to November 2021.
Eligible ICUs were general medical and surgical ICUs capable of treating mechanically ventilated adults and able to implement SDD in all eligible patients.
Eligible patients were mechanically ventilated (either on ICU admission or during ICU admission) and expected to remain ventilated until at least the second day after enrolment. Patients who were not initially expected to require 2 days of ventilation were rescreened and enrolled if eligibility criteria were subsequently met. SuDDICU trial site and participant eligibility criteria are shown in ESM.
For this post hoc subgroup analysis, patients with an acute brain injury at ICU admission were identified using the Acute Physiological And Chronic Health Assessment (APACHE)-III ICU admission diagnosis [7]. Patients with the following admission diagnoses were defined as having an acute brain injury: cardiac arrest; intracerebral hemorrhage; subarachnoid hemorrhage; stroke; brain infection; neurologic neoplasm; seizure; subdural haematoma; coma; traumatic brain injury; and epidural haematoma. All other patients were defined as not having an acute brain injury.
Randomization
ICUs were randomly assigned to adopt a SDD strategy or to continue standard care for two alternating 12-month periods, separated by a 3-month inter-period gap. Full details of randomization, that was stratified by the number of ICU beds in the study site, is outlined in the protocol5 and the SuDDICU trial manuscript [2].
Interventions
SDD comprised (i) a 6-hourly topical application of 0.5 g of oral paste containing 10 mg colistin, 10 mg tobramycin and 125,000 international units of nystatin applied to the buccal mucosa and oropharynx; (ii) a six-hourly administration of 10 mL of gastric suspension containing 100 mg colistin, 80 mg tobramycin and 2 × 106 international units of nystatin to the upper gastrointestinal tract via a gastric or post-pyloric tube; (iii) a 4-day course of an intravenous SDD-compliant antibiotic that included a third-generation cephalosporin or ciprofloxacin, unless already treated with specified antibiotics with activity against Gram-negative bacteria during the first 4 days after enrolment, in which case additional antibiotics were not administered.
The SDD paste and suspension were manufactured by Verita Pharma® (Sydney, Australia) under licence from The George Institute for Global Health in accordance with the standards for Good Manufacturing Practice approved by the Therapeutic Goods Administration of Australia. Details of the SDD drug preparations have been described previously [2].
The SDD oral paste and gastric suspension were administered as soon as possible after eligibility criteria were met and were continued until extubation or day 90, whichever came first.
All other treatments, including use of prophylactic or therapeutic antibiotics, were at the discretion of treating clinicians in accordance with respective institutional microbiological prescription policies.
Data and study management
Data collected at baseline included demographics, ICU admission diagnosis, APACHE score (a severity of illness score ranging from 0 to 71 [APACHE-II] [8] or 0 to 299 [APACHE-III] [9], with higher scores indicating an increased risk of death) and specific risk factors for infection including prior receipt of oral chlorhexidine and intravenous antibiotics.
For patients treated in ICUs during the SDD intervention period, daily data documenting the delivery of SDD oral paste and gastric suspension were collected for the duration of mechanical ventilation up to 90 days and administration of SDD-compliant antibiotics for 5 days. Adherence in administering the topical components of SDD was reported as the proportion of patients receiving at least one eligible dose of SDD on a daily basis for the duration of mechanical ventilation.
Doses of all intravenous antibiotics were collected for 28 days, presented as daily defined doses, as defined by the World Health Organisation [10]. Data recorded daily for 90 days included the duration of mechanical ventilation, ICU and hospital admission, all new organisms isolated from blood and non-blood cultures, any new positive test for Clostridioides difficile and new antibiotic resistant organisms from all cultures, as described previously [2].
Details of source data verification and monitoring are provided in the ESM.
Outcome measures
The primary outcome was all-cause in-hospital mortality within 90 days of enrolment during the index hospital admission.
Clinical secondary outcomes were ICU mortality and days alive and free of mechanical ventilation, ICU admission and hospitalization through 90 days.
Microbiological secondary outcomes were the results from all new blood cultures; the incidence of new positive Clostridioides difficile tests; the incidence of new pre-defined antibiotic resistant organisms from all blood, non-blood surveillance and clinical cultures, and total antibiotic use, defined in daily defined doses.
Statistical analysis
Although not pre-specified, this subgroup analysis was conducted using the same statistical analysis plan used in the main SuDDICU analysis [2]. Patients were analyzed in their randomization group, regardless of adherence, using all available data without imputation.
The primary outcome of death in the hospital within 90 days was analyzed using an individual-level hierarchical logistic regression model, including both a random cluster effect and a random cluster-period effect. The effect of the intervention was estimated as the odds ratio (OR) for death and the 95% confidence interval (CI) with degrees of freedom adjusted by the Kenward-Roger correction [11]. Absolute difference of event rate was also estimated from a linear regression at cluster level weighted proportionally to the inverse of the binomial variance for each cluster-period. ICU mortality was evaluated in a similar fashion.
The number of days alive and free of mechanical ventilation, ICU, and hospitalisation within 90 days were analyzed using a hierarchical linear regression model with the Kenward-Roger correction. Intervention effects were reported as adjusted mean differences and 95% CIs. Time to discharge alive from the ICU and the hospital was summarized by subgroup using cumulative incidence functions treating mortality as a competing risk, censored at day 90. Intervention effects were estimated as hazard ratios (HR) and 95% CIs obtained from a cause-specific Cox model, with a fixed effect of treatment and a random site effect.
In all analyses, heterogeneity in treatment response for patients with and without an acute brain injury was assessed by adding the subgroup variable as well as its interaction with the intervention to the main analysis model.
To evaluate for the possibility of heterogeneity of treatment effect on in-hospital mortality within the subgroup of patients with acute brain injuries, the subgroup was divided group into three mutually exclusive categories and fitted an interaction between treatment allocation and category. These categories were: (i) traumatic brain injury (defined as APACHE-III ICU admission diagnoses of head trauma, subdural hematoma and subdural/epidural hematoma; (ii) subarachnoid hemorrhage and stroke (defined as APACHE-III ICU admission diagnoses of intracerebral hemorrhage; subarachnoid hemorrhage; stroke; intracerebral hemorrhage) and (iii) other brain injuries (defined as APACHE-III ICU admission diagnoses of cardiac arrest; brain infection; neurologic neoplasm; seizure; other neurologic disease; coma). An additional within brain injury subgroup analysis compared heterogeneity of treatment effect on in-hospital mortality was based on whether or not patients were receiving intravenous antibiotics at baseline.
Microbiological outcomes were analyzed using the proportions of patients with at least one event in each cluster-period. These proportions were modelled using weighted linear regression where the weights are computed using the inverse of variance for each cluster-period. All these analyses were performed without any adjustment.
All statistical tests were performed using a two-sided level of 0.05.
As all analyses conducted in this study are post hoc, they were be considered to be exploratory.
Statistical analyses were conducted using SAS software (version 9.4) (Cary NC, USA).
Results
Study sites and patients
A total of 5982 mechanically ventilated adults were included with 2061 (mean age 55.8 years; 36.4% women) defined as having an acute brain injury or condition.
In the first intervention period, 1019 patients with an acute brain injury were recruited into the trial with 388 (38.1%) in the SDD group and 631 (61.9%) in standard care group. In the second intervention period, 1042 patients with an acute brain injury were recruited with 580 (55.7%) in the SDD group and 462 (44.3%) in standard care group.
The primary outcome was available for all 968 patients in the SDD group and all 1093 patients in the standard care group (Fig. 1).
Baseline characteristics of patients with acute brain injuries allocated to SDD and standard care groups were similar (Table 1) except that the time from ICU admission to enrolment was a median of 11.6 h (interquartile range [IQR] 1.3–29.6 h) in the SDD group and a median of 1.9 h (IQR 0–17.5 h) in the standard care group; oral chlorhexidine was used in 261/968 (27%) of patients in the SDD group and 198/1093 (18.1%) in the standard care group and systemic steroids were used in 51/968 (5.3%) in the SDD group and 103/1093 (9.4%) in standard care group.
Study treatments and process measures
Among patients with acute brain injuries in the SDD group, the proportion of days of mechanical ventilation where patients received both the SDD oral paste and gastric suspension was 93.1% (eFigure 1, ESM).
Data on administration of each component of SDD are shown in eTable 1, ESM and the proportions of patients receiving SDD-compliant antibiotics in the SDD and standard care groups are shown in eFigure2, ESM.
Primary outcome
At hospital discharge within 90 days of enrolment, in patients with acute brain injuries, 313 (32.3%) of 968 allocated to the SDD group and 415 (38%) of 1093 allocated to the standard care group had died (mean difference − 6.2%, 95% CI − 8.9 to − 3.5%; OR 0.76, 95% CI 0.63–0.92; p = 0.004). Findings were similar after adjusting for age at baseline, sex, APACHE II/III score and diagnosis (OR 0.74; 95% CI 0.57–0.97; p = 0.03) but not significant after adding time from ICU admission to enrolment, systemic steroids, oral chlorhexidine and receipt of intravenous antibiotics at the time of enrolment (OR 0.78; 95% CI 0.59–1.04; p = 0.08). (Table 2).
The respective hazard ratios for time to death with SDD vs. standard care were 0.83 (95% CI 0.71–0.96) and 1.01 (95% CI 0.89–1.15) for patients with and without acute brain injuries, respectively (Fig. 2a, b).
There was no significant heterogeneity in the effect of SDD on mortality for patients with (OR 0.78, 95% CI 0.59–1.04, p = 0.08) and without (OR 0.85, 95% CI 0.72–1.00, p = 0.05) acute brain injuries, respectively (interaction p = 0.22) (Fig. 3, eTable2, ESM).
Clinical outcomes for patients with and without acute brain injuries at ICU admission. All analysis are adjusted for baseline Age, Sex, APACHE II/III score, diagnosis (operative vs non-operative), time from ICU admission to enrolment, systemic steroids, oral chlorhexidine, IV antibiotics at time of enrolment, acute neurological injuries and interaction between treatment and acute neurological injuries. SDD selective decontamination of the digestive tract
There was no significant difference in mortality between the SDD and standard care groups between patients with traumatic brain injury vs. subarachnoid hemorrhage or between stroke vs. other brain injuries (eTable 3, ESM); or based on receipt or not of intravenous antibiotics at baseline (eTable 3, ESM).
Causes of death in patients with acute brain injuries by treatment group are shown in eTable 4, ESM.
Clinical secondary outcomes
Of four clinical secondary outcomes (death in ICU, days alive and free of mechanical ventilation, days alive and free of ICU admission and days alive and free of hospital admission), there were statistically significant differences in favor of the SDD group in patients with acute brain injuries (Table 2).
The respective hazard ratios for days alive and free of mechanical ventilation with SDD vs. standard care were 1.11 (95% CI 1.01–1.22) and 1.1 (95% CI 1.03–1.18) for patients with and without acute brain injuries, respectively (eFigure 3a and 3b, ESM).
The respective hazard ratios for alive and free of ICU with SDD vs. standard care were 1.1 (95% CI 0.99–1.22) and 1.03 (95% CI 0.96–1.11) for patients with and without acute brain injuries, respectively (eFigure 4a and 4b, ESM).
The respective hazard ratios for alive and free of hospital with SDD vs. standard care were 1.06 (95% CI 0.94–1.18) and 1 (95% CI 0.93–1.07) for patients with and without acute brain injuries, respectively (eFigure 5a and 5b, ESM).
There was no significant heterogeneity in the effect of SDD on the four secondary clinical outcomes for patients with and without acute brain injuries (eTable2 and eFigure 3, ESM).
Microbiological secondary outcomes
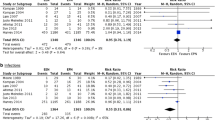
For the SDD group compared to the standard care group, there was a statistically significant reduction in the proportion of patients from whom new antibiotic resistant organisms were cultured (20.5% vs 34.2%; absolute difference − 13.9 percentage points; 95% CI − 17.5 to − 10.3); for new positive blood cultures excluding coagulase negative Staphylococcus aureus (2.8% vs 5.5%; absolute difference − 1.9 percentage points, 95% CI − 3.5 to − 0.4) and for new positive Clostridioides difficile tests (0.1% vs 0.8%; absolute difference − 0.6 percentage points, 95% CI − 1 to − 0.1) (Table 2).
Among the patients with acute brain injuries in the SDD and standard care groups, respectively, the number of patients with blood cultures collected was 552 (57%) vs. 713 (65.2%) and the number of patients with non-blood cultures collected was 229 (23.7%) vs. 411 (37.6%). Data on specific organisms cultured from blood specimens and for new antibiotic resistant organisms cultured from non-blood specimens from the SDD and standard care groups are shown in the eTable 5, ESM. New antibiotic resistant organisms were cultured from the respiratory tract in 115 of 968 (11.9%) and 275 of 1093 (25.2%) of patients allocated to SDD and standard care groups, respectively.
The mean cumulative daily defined doses over the first 28 days of all intravenous antibiotics and of intravenous antibiotics not administered as part of the SDD treatment regimen are shown in eFigure 6, ESM. The cumulative daily defined doses of each antibiotic class are also shown in eFigure 7, ESM. Among patients with acute brain injuries, daily defined doses of antibiotics administered over the first seven days following enrolment were significantly higher in the SDD group compared to the standard care group (eFigure 8, ESM) although daily defined doses over the first 28 days following enrolment were not significantly different between the two groups (eFigure 9, ESM).
Adverse events and protocol deviations
New positive Clostridioides difficile infections occurred in 1 of 968 (0.1%) patients in the SDD group and 9 of 1093 (0.8%) patients in the standard care group. Other adverse and serious adverse events were rare (eTable 6, ESM).
Discussion
In this post hoc analysis using data from a crossover, cluster randomized clinical trial, the use of SDD in the subgroup of mechanically ventilated critically ill adults with acute brain injuries or conditions was associated with a statistically significantly reduced in-hospital mortality compared with standard care without SDD. The use of SDD was associated with significantly reduced ICU mortality, duration of mechanical ventilation and duration of ICU and hospital admission and with lower rates of new blood stream infections and new cultures of antibiotic resistant organisms. In patients without acute brain injuries or conditions, who made up around two thirds of the SuDDICU trial population, no significant differences in any clinical outcome between SDD and standard care groups were observed.
Apart from one small trial [12], patients with acute brain injuries have not been a focus of clinical trials evaluating SDD [1]. Prior randomized clinical trials have suggested that intravenous antibiotic prophylaxis may reduce rates of ventilator-associated pneumonia in patients after cardiac arrest [13, 14] and with other brain injuries in the ICU [15, 16]. These trials of prophylactic antibiotics did not focus on patient-important outcomes such as mortality.
In this large subgroup of nearly 2000 patients with acute brain injuries from a pragmatic randomized clinical trial, SDD was associated with improvements in several patient-centred outcomes. The 5.7 percentage point reduction in mortality corresponds to a number need to treat of 18 to avoid 1 death. This is a clinically important effect size in a population of patients with a control mortality rate of 38 percentage points. Excellent protocol adherence was achieved with over 90 percentage points of eligible doses of commercial-standard SDD drug preparations administered for the duration of mechanical ventilation. In contrast to the overall SuDDICU trial population [2], patients with acute brain injuries who were allocated to SDD received significantly more daily defined doses of antibiotics in the first 7 days compared patients allocated to standard care.
Although SDD was associated with lower rates of new infections that may have mitigated potential contributors to secondary brain injury, data about brain process measures, such as intracranial pressure, types, duration and intensity of brain-specific therapies or assessments of longer term functional brain outcome, were not available to confirm or refute putative mechanisms of benefit. While ventilator-associated pneumonia is a common cause of ICU-acquired infection [17], specific data on the diagnosis or source of infection or the impact of ventilator-associated pneumonia on respiratory function were not available for analysis, although lower rates of new antibiotic resistant organisms were cultured from the respiratory tract in patients allocated to SDD.
This subgroup analysis has some limitations. First, the intervention was unblinded and, therefore, subject to ascertainment bias, although this was mitigated by the objective primary outcome and the adoption of SDD as standard care administered to all eligible patients during the intervention period. Second, we reported the primary ICU admission diagnoses defining the presence of an acute brain injury that may have miscategorized some patients. Third, while the primary outcome was reported using pre-specified unadjusted analyses, adjusted analyses accounting for baseline imbalances are equally important for a post hoc analysis. While statistically significant differences in the primary outcome were lost after adjustment for baseline imbalances, the magnitude and direction of the effect size were not substantially different. Fourth, although the modest reduction in mortality with SDD observed in patients with acute brain injuries is consistent with our study hypothesis and with the beneficial effects from SDD observed in the clinical secondary outcomes, a differential mortality treatment effect larger than we observed is likely to be implausible. Accordingly, the absence of statistically significant heterogeneity of treatment effect in our study may be due to low statistical power. Fifth, this analysis was hypothesis-driven and was not pre-specified before the primary trial.
While the cumulative evidence suggests that the use of SDD is associated with reduced hospital mortality [1], this post hoc analysis of data from the SuDDICU study suggests that this effect may be primarily driven by a benefit in patients with acute brain injuries and that other patients may have little or no benefit from SDD. Before implementation into clinical practice, these findings must be confirmed through analysis of patient-level data from other trials or through new randomized clinical trials.
Conclusion
Among critically ill patients receiving mechanical ventilation, SDD significantly reduced in-hospital mortality in patients with acute brain injuries compared to standard care without SDD. However, the findings from this post hoc analysis in this patient population require confirmation.
Data availability
JAM and LB had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. See ESM.
References
Hammond NE, Myburgh J, Seppelt I et al (2022) Association between selective decontamination of the digestive tract and in-hospital mortality in intensive care unit patients receiving mechanical ventilation: a systematic review and meta-analysis. JAMA 328(19):1922–1934. https://doi.org/10.1001/jama.2022.19709
The SuDDICU Investigators for the Australian and New Zealand Intensive Care Society Clinical Trials Group. Effect of selective decontamination of the digestive tract on hospital mortality in critically ill patients receiving mechanical ventilation: a randomized clinical trial. JAMA. 2022;328(19):1911–1921. https://doi.org/10.1001/jama.2022.17927
Bonten MJ, Kollef MH, Hall JB (2004) Risk factors for ventilator-associated pneumonia: from epidemiology to patient management. Clin Infect Dis 38(8):1141–1149. https://doi.org/10.1086/383039
Ewig S, Torres A, El-Ebiary M et al (1999) Bacterial colonization patterns in mechanically ventilated patients with traumatic and medical head injury. Incidence, risk factors, and association with ventilator-associated pneumonia. Am J Respir Crit Care Med 159(1):188–198. https://doi.org/10.1164/ajrccm.159.1.9803097
The SuDDICU Investigators (2021) Protocol summary and statistical analysis plan for the Selective Decontamination of the Digestive Tract in Intensive Care Unit Patients (SuDDICU) crossover, cluster randomised controlled trial. Crit Care Resusc 23(2):183–193
Campbell MK, Elbourne DR, Altman DG et al (2004) CONSORT statement: extension to cluster randomised trials. BMJ 328(7441):702–708. https://doi.org/10.1136/bmj.328.7441.702
ANZICS Centre for Outcome and Resource Evaluation. APD Data Dictionary. Version 6.1. April 2022 https://www.anzics.com.au/wp-content/uploads/2021/03/ANZICS-APD-Data-Dictionary.pdf.
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13(10):818–829
Knaus WA, Wagner DP, Draper EA et al (1991) The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 100(6):1619–1636. https://doi.org/10.1378/chest.100.6.1619
World Health Organization. Defined daily dose (DDD). Accessed 3rd July, 2023. https://www.who.int/tools/atc-ddd-toolkit/about-ddd.
Kenward MGRJH (2009) An improved approximation to the precision of fixed effects from restricted maximum likelihood. Comput Stat Data Anal 53:2583–2595
Korinek AM, Laisne MJ, Nicolas MH, Raskine L, Deroin V, Sanson-Lepors MJ (1993) Selective decontamination of the digestive tract in neurosurgical intensive care unit patients: a double-blind, randomized, placebo-controlled study. Crit Care Med 21(10):1466–1473. https://doi.org/10.1097/00003246-199310000-00013
Ribaric SF, Turel M, Knafelj R et al (2017) Prophylactic versus clinically-driven antibiotics in comatose survivors of out-of-hospital cardiac arrest—a randomized pilot study. Resuscitation 111:103–109. https://doi.org/10.1016/j.resuscitation.2016.11.025
Francois B, Cariou A, Clere-Jehl R et al (2019) Prevention of early ventilator-associated pneumonia after cardiac arrest. N Engl J Med 381(19):1831–1842. https://doi.org/10.1056/NEJMoa1812379
Sirvent JM, Torres A, El-Ebiary M, Castro P, de Batlle J, Bonet A (1997) Protective effect of intravenously administered cefuroxime against nosocomial pneumonia in patients with structural coma. Am J Respir Crit Care Med 155(5):1729–1734. https://doi.org/10.1164/ajrccm.155.5.9154884
Acquarolo A, Urli T, Perone G, Giannotti C, Candiani A, Latronico N (2005) Antibiotic prophylaxis of early onset pneumonia in critically ill comatose patients. A randomized study. Int Care Med 31(4):510–516. https://doi.org/10.1007/s00134-005-2585-5
Vincent JL, Rello J, Marshall J et al (2009) International study of the prevalence and outcomes of infection in intensive care units. JAMA 302(21):2323–2329. https://doi.org/10.1001/jama.2009.1754
Acknowledgements
The authors and investigators wish to acknowledge the patients who were enrolled in our study and their families; the health care workers who cared for these patients; the research co-ordinators at each participating hospital; members and executive of the Australian and New Zealand Intensive Care Society Clinical Trials Group; the international independent Data and Safety Monitoring Committee; manufacturing and support staff at Verita® Pharma, Sydney, who provided the SDD drug preparations; international collaborators in Canada and the United Kingdom; and the research and support teams at The George Institute for Global Health, Sydney. This paper was endorsed by the Australian and New Zealand Intensive Care Society Clinical Trials Group.
SuDDICU Australia Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group: Anthony Gordon, Brian Cuthbertson, Robert Fowler, Srinivas Murthy, Natalie Pattison, Jon Iredell, Colman Taylor, Duncan Young, Tom van der Poll, Ian Roberts, Catherine Boschert, Emma Broadfield, Timothy Chimunda, Jason Fletcher, Cameron Knott, Sanjay Porwal, Julie Smith, Deepak Bhonagiri, Monique Leijten, Sandhya Narayan, David Sanchez, Peta Saunders, Carli Sherriff, Jonathan Barrett, Gabrielle Hanlon, Sarah Jelly-Butterworth, Julie O’Donnell, Judith Watson, Shailesh Bihari, Julia Brown, Sharon Comerford, Russell Laver, JoAnne McIntyre, Tapaswi Shrestha, Jin Xia, Samantha Bates, Gerard Fennessy, Craig French, Sathyajith Kootayi, Fiona Marshall, Rebecca McEldrew, Forbes McGain, Rebecca Morgan, John Mulder, Anna Tippett, Miriam Towns, Ellie Barker, Shelley Donovan, Katrina Ellis, Atul Gaur, Hannah Gibbons, Rebecca Gregory, Eloise Hair, Mary Keehan, Jess Naumoff, Elisha Turner, Gail Brinkerhoff, Dustin Bush, Federica Cazzola, Joshua Davis, Ken Havill, Paul Healey, Amber Poulter, Krishna Sunkara, Anders Aneman, Rachel Choit, Kelsey Dobell-Brown, Kairui Guo, Jillian Lee, Monique Leijten, Lien Lombardo, Zachariah Manalil, Jennene Miller, Jordan Rogers, Antony Stewart, Jana Yanga, Rebecca Gresham, Julie Lowrey, Kristy Masters, Ian Seppelt, Christina Whitehead, Beverly Zaratan, Matthew Grigg, Meg Harward, Cassie Jones, Josephine Mackay, Jason Meyer, Emma Saylor, Ellen Venz, James Walsham, Krista Wetzig, Nerissa Brown, Marianne Chapman, Kathleen Glasby, Samuel Gluck, Tejaswini Murthy, Stephanie O'Connor, Eamon Raith, Justine Rivett, Joannies Yap, Angela Ashelford, Frances Bass, Simon Finfer, Emily Fitzgerald, Oliver Flower, Naomi Hammond, Bernard Hudson, Pierre Janin, Elizabeth Limbrey, Sharon Mar, Anne O'Connor, Melissa Owen, Naomi Pallas, Julia Pilowsky, Veronica Roach, Elizabeth Ruse, Wade Stedman, Miyuki Tokumitsu, Elizabeth Yarad, Deborah Inskip, Theresa Jacques, Adeline Kintono, Jennene Miller, Catherine Milner, John Myburgh, Rebecca Sidoli, Samantha Bates, Gerard Fennessy, Craig French, Sathyajith Kootayi, Fiona Marshall, Rebecca McEldrew, Forbes McGain, Rebecca Morgan, John Mulder, Anna Tippett, Miriam Towns, Catherine Kurenda, Sandra Peake, Tricia Williams, Jeremy Cohen, Amanda Davie, Amy Owens, Roslyn Purcell, Bala Venkatesh, Cartan Costello, Alan Davey-Quinn, Michael Davies, Ahmed Elgendy, Wenli Geng, Veerendra Jagarlamudi, Matthew Mac Partlin, Mahadev Patil, Adam Purdon, Martin Sterba, Andrea Marshall, Anthony Delaney, Simon Finfer, Naomi Hammond, John Myburgh, Ian Seppelt, Balasubramanian Venkatesh, Maryam Correa, Fiona Goodman, Marwa Abdel-All, Hayley Clark, Natalie Espinosa, Benjamin Finfer, Miranda Hardie, Sharon Micallef, Jennene Miller, Dijlah Moungatonga, Conrad Nangla, Anne O'Connor, Fiona Osbourne, Julia Pilowsky, Tina Schneider, Isabella Schoeler, Prakriti Shrestha, Anna Tippett, Elizabeth Wilson, Laurent Billot, Manuela Armenis, Dominic Byrne, Qiang Li, Jayanthi Mysore, Amrutha Nagarajaiah, Prakash Velappan, Parisa Glass, Kate Myburgh, Philippa Smith, Martina Bachmaier, Daryll Knowles, Michael Tattersall.
Funding
No specific funding for this post hoc analysis was obtained. The SuDDICU Australia trial was supported by a Project Grant from the National Health and Medical Research Council of Australia (Project Grant number 1084244); by a Leadership Fellowship from National Health and Medical Research Council of Australia (to JAM); by Practitioner Fellowships from the National Health and Medical Research Council of Australia (to SRF); by a Career Development Fellowship from National Health and Medical Research Council (to JSD); by an Emerging Leader Investigator Grant from the National Health and Medical Research Council of Australia (to NEH); by a Clinical Practitioner Research Fellowship from the Health Research Council of New Zealand (to PJY). The George Institute for Global Health, Australia was the Principal Sponsor for this trial. SDD drug preparations were purchased and manufactured under contract with the George Institute for Global Health by Verita Pharma® (Sydney, Australia). The sponsor, funders and drug manufacturer had no input into the design and conduct of the study, collection, management, analysis and interpretation of data; preparation, review or approval of the manuscript; and the decision to submit the manuscript for publication. The Sponsor had no right of veto to publish this trial or to control the decision regarding to which journal the paper was submitted.
Author information
Authors and Affiliations
Consortia
Contributions
Non-author collaborators: listed in the Acknowledgement section. Concept and design: LB, JSD, AD, AD, SRF, NEH, QL, JAM, IMS, BV, PJY. Acquisition, analysis and interpretation of data: LB, AD, AD, SRF, NEH, QL, SM, JAM, IMS, PJY. Drafting of the manuscript: PJY (wrote the first draft of the manuscript); LB, AD, SRF, QL, SM, JAM, IMS. Critical revision of the manuscript for important intellectual content: LB, JSD, AD, AD, SRF, NEH, QL, SM, JAM, IMS, BV, PJY. Statistical analysis: LB, AD, QL. Obtained funding: JSD, SRF, JAM, IMS, PJY. Administrative, technical or material support: LB, AD, SRF, NEH, QL, SM, JAM. Supervision: LB, SRF, NEH, QL, JAM.
Corresponding author
Ethics declarations
Conflicts of interest
The George Institute for Global Health holds all intellectual property rights related to the SuDDICU study drugs, including component drug acquisition, manufacturing, packaging and distribution. None of the SuDDICU investigators have any direct or indirect financial or commercial interests relating to the development of the SuDDICU study drugs.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The SuDDICU Australia Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group are listed in the Acknowledgement section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Young, P.J., Devaux, A., Li, Q. et al. Selective digestive tract decontamination in critically ill adults with acute brain injuries: a post hoc analysis of a randomized clinical trial. Intensive Care Med 50, 56–67 (2024). https://doi.org/10.1007/s00134-023-07261-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-023-07261-y