Abstract
Clinical and pathophysiological understanding of septic shock has progressed exponentially in the previous decades, translating into a steady decrease in septic shock-related morbidity and mortality. Even though large randomized, controlled trials have addressed fundamental aspects of septic shock resuscitation, many questions still exist. In this review, we will describe the current standards of septic shock resuscitation, but the emphasis will be placed on evolving concepts in different domains such as clinical resuscitation targets, adequate use of fluids and vasoactive drugs, refractory shock, and the use of extracorporeal therapies. Multiple research opportunities remain open, and collaborative endeavors should be performed to fill in these gaps.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Septic shock treatment standards have evolved in the past decades, alongside with deeper physiological and clinical understanding of the disease. In this review, we present both the current practice and evolving concepts related to different clinical domains of septic shock resuscitation. |
Introduction
A recent review addressed current and evolving standards of care for patients with acute respiratory distress syndrome [1], a condition where simple rules to avoid ventilatory-induced lung injury can be recommended. In the field of septic shock, there is a lack of solid evidence in almost every aspect of care. Several randomized, controlled trials (RCTs) have addressed the proper use and place of the pillars of hemodynamic resuscitation such as fluids, vasopressors, and inotropes, but no intervention was associated with a significant effect on survival [2]. This also holds true for monitoring devices as these by themselves cannot change outcome and thus, their effectiveness relies on the proper use of adequate protocols that deal with relevant clinical abnormalities. While the role of new vasopressors or inotropes is still uncertain, new concepts in fluid resuscitation have been slowly introduced.
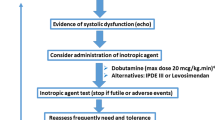
In this narrative review, we provide an update on different domains of septic shock resuscitation including the devices used to monitor the effect of different interventions. The basis is current practice with emphasis on evolving concepts in the monitoring and management of septic shock (Fig. 1).
Current practice
Current practice, although heterogenous, mostly follows Surviving Sepsis Campaign (SSC) guidelines [3], especially in the emergency department.
Clinical targets
Septic shock is characterized by an abnormal distribution of blood flow associated with vasodilation due to vasoplegia that is associated with the release of mediators due to the innate immune response to the infection, resulting in microvascular injury and inadequate tissue oxygen supply and abnormal cell metabolism. Several mechanisms may contribute to the decrease in venous return in these conditions. Most important is the venous pooling of blood that can be further complicated by poor fluid intake or increased losses during the early phase of illness. In addition, endothelial damage resulting in a capillary leak into the interstitium may further worsen venous return and, thus, macrocirculatory perfusion. The presence of cardiac dysfunction (due to sepsis-related myocardial depression, pulmonary hypertension, or a decreased myocardial perfusion in most severe cases) may further compromise hemodynamics.
The clinical picture of shock usually reveals a mixture of hypotension (usually requiring vasopressors), altered tissue perfusion (abnormal skin perfusion, altered mentation, decreased urine output), and increased lactate levels [4]. As resolution of these clinical signs indicates shock reversal, they may be used as resuscitation endpoints. Although lactate-based resuscitation has been shown effective [5], the complexity of using lactate levels and blood pressure as a target has been recently highlighted and this general approach needs individualization [6,7,8]. An overview of the different aspects of hemodynamic and perfusion monitoring is presented in Table 1.
Initial fluid resuscitation and fluid responsiveness
Fluid resuscitation in septic shock is an effective intervention to increase venous return, and thus cardiac output (CO) and oxygen transport [9]. The key element in fluid resuscitation is the adequacy of volume and timing in combination with the monitoring of the result to prevent fluid overload [10]. As the aim of fluid resuscitation in a shock state is to reverse tissue hypoperfusion, timely fluid resuscitation is critical. The SSC guidelines for adults recommend to start resuscitation immediately upon recognition and suggest to use at least 30 ml/kg to be completed within 3 h of recognition [3]. As fluids should only be used in patients likely to respond with an increase in CO (fluid responders) dynamic assessment of fluid responsiveness is important [11]. As only 50–60% of the patients in the early phase of septic shock are fluid responders [10, 12] and 25% may already be fluid unresponsive after an initial fluid resuscitation [13], over-treatment (and, thus, possible harm) is possible in a significant number of patients. This recommended dose of 30 ml/kg is only based on observational evidence and is controversial as individualization is warranted where continued fluid administration might be needed in patients with a favorable clinical and hemodynamic response [8, 14].
Testing for fluid responsiveness is not indicated where hypovolemia and low venous return is obvious (e.g., hemorrhage) and reaching a state of fluid unresponsiveness is used as an absolute stopping point for use of fluids rather than an endpoint of fluid resuscitation. Although assessing fluid responsiveness has limitations, a recent study in septic shock patients showed that testing for fluid responsiveness was possible in more than 80% of patients [13]. Three approaches have been advocated to assess fluid responsiveness. First, actually testing the system by infusing a given volume of fluid in a short period of time while monitoring CO and tissue perfusion [15]. A second approach is to assess fluid responsiveness where venous return is potentially recruited using a physical/mechanical intervention. Examples of these are the passive leg raising (PLR) test, end-expiratory hold or increasing tidal volume (6–8 ml/kg) in mechanically ventilated patients or a more continuous way using stroke volume variation (SVV) or pulse pressure variation (PPV). All of these techniques have been shown to be valuable in the right context but still have limitations [11, 16]. Finally, echo-derived variations in the superior vena cava represent a more reliable method, when compared to the inferior vena cava, with less limitations although this requires a more invasive trans-esophageal echocardiography [17].
As the effects of the infused fluids may wear of rapidly [18], the parameters used to assess the efficacy of the fluid resuscitation need to be closely monitored.
Fluid unresponsiveness on the other hand could be used to safely remove fluids in the hemodynamically stable patient [19].
Vasoactive drug use
Norepinephrine is currently the vasopressor of first choice [3]. Administration of norepinephrine not only increases mean arterial pressure (MAP) but also its beta-1 adrenergic effects together with an increase in venous return, it may increase CO and tissue perfusion [20,21,22,23].
Dobutamine is the inotropic agent of reference in the acutely ill patient given its clinical response and favorable onset and offset of action [24]. A limited dose of dobutamine reliably increases blood flow and microcirculatory perfusion, without significantly affecting arterial pressure [25]. Current use is mostly indicated when signs of altered tissue perfusion following fluid resuscitation persist [3]. Although tachycardia may complicate the use of dobutamine this usually is a marker of (relative) hypovolemia. Dobutamine should, thus, be avoided in patients with evident hypovolemia as the associated vasodilation may also induce profound hypotension.
The use of vasopressin is variable in usual practice despite its physiological rationale. Given the distribution of V1a receptors in the kidney, vasopressin may maintain renal perfusion better than norepinephrine [26]. This positive effect did not result in improved mortality in large clinical trials although it seemed to benefit patients with less severe shock [27, 28]. A meta-analysis showed that vasopressin use was associated with a decrease in the requirement for renal replacement therapy, a trend to lower 90-day mortality, but more complications like digital ischemia [29]. Currently, it is recommended to add vasopressin to a low-to-moderate dose of norepinephrine (0.25–0.5 mcg/kg min) when MAP is still inadequate instead of using it as a first-line drug [30]. Other beneficial effects expected of the use of vasopressin, that drive clinical use, are reduced rates of tachyarrhythmias.
Evolving concepts
New potential resuscitation targets
Peripheral perfusion
The skin territory lacks auto-regulatory blood flow control and therefore, sympathetic activation impairs skin perfusion during circulatory dysfunction, a phenomenon that can be evaluated by peripheral perfusion assessment. For long, indicators of abnormal peripheral perfusion have been associated with increased morbidity and mortality [31, 32]. A cold clammy skin, mottling or prolonged capillary refill time (CRT) has been suggested as a clinical trigger for fluid resuscitation in patients with septic shock [33]. Moreover, the excellent prognosis associated with CRT normalization, its rapid response time to fluid loading, its relative simplicity, its availability in resource-limited settings, and its capacity to change in parallel with perfusion of physiologically relevant territories such as the hepatosplanchnic region constitute strong reasons to consider CRT as an important target for fluid resuscitation in septic shock patients. A recent trial found that CRT-targeted resuscitation was associated with a lower mortality (34.9% vs. 43.4%, p = 0.06), beneficial effects on organ dysfunction, and less intensity of treatment [13], supported by a subsequent Bayesian analysis [34]. Although limited evidence of prospective studies is present to date, the recent SSC guideline [3] has also adopted the use of CRT to guide resuscitation in addition to the other parameters of tissue perfusion. Evolving markers of tissue perfusion/oxygenation like veno-arterial PCO2 difference and the central venous–arterial carbon dioxide to arterial–venous oxygen content ratio, have shown an association with outcome parameters [35, 36] but they still lack sufficient clinical evidence of benefit when used in early resuscitation.
The microcirculation
Over the past decade, the sublingual microcirculatory alterations during shock and its resuscitation have been shown to be closely related to outcome. In addition, parameters of microcirculatory perfusion amendable to the optimization of tissue perfusion have been identified [37]. The latter being the ultimate goal of resuscitation [4]. It would, thus, be meaningful to use these parameters as an endpoint for the use of fluids and vasoactive agents as has been shown in various small studies confirming the beneficial effects of fluids and vasoactives (norepinephrine, inotropes/inodilators and vasodilators) but also drugs targeting the endothelium more specifically [38]. An important element of these studies is the observation by Dubin et al. [25]. When analyzing all patients, norepinephrine did not improve microcirculatory blood flow; however, in patients with abnormal microcirculatory perfusion, norepinephrine did improve perfusion underscoring the fact that resuscitation should be individualized and recruitment of the microcirculation might contain targets beyond MAP and CO [39].
From a physiological point of view, it is clear that the hemoglobin level is an important determinant of tissue oxygenation [40], much more than convective flow, meaning that care should be taken to avoid dilutional anemia during fluid resuscitation [41]. The recent introduction of the microcirculatory parameter named tissue red blood cell perfusion (tRBCp), which is based on microcirculatory analysis and combines the diffusional capacity and the convective capacity of red blood cell flow and oxygen delivery in the microcirculation may provide an effective resuscitation end point [42]. Evolving concepts of resuscitation could, thus, be the use of blood transfusion and vasodilating agents. However, despite these physiological sound arguments, it is still unclear whether these interventions affect clinically relevant outcome measures.
Another aspect of using microcirculatory perfusion to guide resuscitation of septic shock patients is the objective interpretation of the images. In this respect, the availability of fast and accurate software could enhance the use of the sublingual microcirculation at the bedside [42, 43].
Evolving concepts in fluid resuscitation
Type of fluids
Crystalloids remain the mainstream fluid for sepsis resuscitation. Balanced (“low chloride”) solutions are increasingly used in clinical practice and may be more beneficial, especially when started in the very early phase (emergency department) of septic shock resuscitation [44, 45]. Several aspects of fluid resuscitation remain unresolved and will still evolve in the coming years. First, the effect of chloride in the fluids administered, potentially inducing hyperchloremia which can be harmful [46]. Although a recent trial failed to find a significant benefit in favor of a balanced solution when compared to high-chloride saline this was in the absence of induced hyperchloremia [47].
The use of albumin in early resuscitation also remains a topic of debate. Although the recent SSC guideline recommends using albumin when a large volume of crystalloids has been used during resuscitation the evidence is moderate as no study has shown a mortality benefit in one-size-fits-all studies [3]. Given the physiologic rationale and the limited benefit shown in RCTs (lower net fluid balance, higher blood pressure), future studies should focus on these aspects in selected patient groups. Besides the fluid composition other important questions remain, including the optimal rate of infusion and the temperature of the fluid [47].
SOSD concept
The salvage, optimization, stabilization and de-escalation (SOSD) concept has been introduced to describe the different stages of shock resuscitation [48]. In the salvage phase, the focus is on increasing blood pressure in hypotensive patients using fluid resuscitation and the start of vasopressors when the MAP or diastolic arterial pressure (DAP) is critically low. The optimization phase is focused on tissue perfusion and oxygen delivery. Stabilization and de-escalation are crucial periods in which decreases in fluid balances and weaning of vasoactive medication should be achieved while maintaining adequate tissue perfusion. However, studies determining how to best guide de-escalation and the effects on outcome parameters are lacking.
Restrictive fluid resuscitation and timely removal of excessive fluids
As true hypovolemia is often not present in septic shock patients, especially in those already hospitalized, fluid administration should be individualized, incorporating etiology, clinical context, existence of comorbidities and side effects, rather than using a fixed volume. Recent data suggest that starting vasopressors simultaneously with fluids or following a very limited fluid resuscitation is associated with beneficial effects (Table 2) while a delayed start has been associated with increased mortality [49]. The simultaneous start of fluids and vasopressors is associated with the use of less fluids, a lower net fluid balance, shortened duration of hypotension and lower incidence of pulmonary edema and new onset arrhythmias [50,51,52]. Although these possible beneficial effects did not result in decreased mortality in a RCT [51], a systematic review incorporating both observational and randomized trials showed decreased short-term mortality [52]. While sufficient clinical evidence favoring the early start of vasopressors is currently lacking, the possible beneficial hemodynamic effects should be considered in early septic shock resuscitation and warrant further interventional studies. Fluid resuscitation based on markers of peripheral perfusion seems to be safe and has been associated with a decreased net fluid balance [13, 33]. Cautious use of fluids is warranted and constant reassessment of the benefit/risk ratio should be part of the resuscitation protocol as rational and restrictive fluid administration may decrease fluid overload [53].
Fluid overload can be estimated using the change in body weight (or net fluid balance) and is frequently associated with evidence of organ disfunction (weaning related heart failure, organ edema, intraabdominal hypertension, acute kidney injury, etc.) resulting in increased morbidity (increased duration of mechanical ventilation (MV) and length of stay) and mortality [54].
Removal of excess fluid following stabilization of the patient, thus, seems rational. However, this is still an evolving concept as guidelines on how to execute this in clinical practice are lacking [54, 55]. Frequently used interventions include the use of diuretics [56] or renal replacement therapy [57]. As this may induce excessive fluid removal possibly resulting in hypovolemia and regional hypoperfusion [54] other more physiology driven approaches are needed. The diagnosis of fluid unresponsiveness in stabilized patients has been successfully used to start removal of fluids [19] and use of ultrasound to guide fluid removal showed improved efficiency [58], whereas the use of continuous fluid removal, using renal replacement therapy, has been associated with improved outcome [57]. Given these scarce data, further studies in both patients on continuous renal replacement therapy or without kidney injury are warranted.
Evolving concepts in vasopressor use
Use of norepinephrine according to vasomotor tone
Decreased vasomotor tone is a common characteristic of sepsis-related hypotension. A low diastolic blood pressure (DAP) frequently indicates a state of vasodilation and the use of the diastolic shock index (ratio between DAP and heart rate) [59], may optimize the early use of vasopressors.
The coupling between the pump and the vasculature (ventriculo-arterial coupling, VAC) assessed by the ratio between arterial elastance (Ea) and end-systolic elastance (Ees) [60] might be used to predict and monitor the effect of norepinephrine on myocardial performance [12] and VAC [21] and may detect drug-induced uncoupling [61]. At present, these parameters require complex monitoring and modeling and cannot be easily used at the bedside. However, the ratio between PPV and SVV reflecting a dynamic arterial elastance (Eadyn) can be obtained at the bedside using an arterial catheter and pulse contour analysis [62] and may help to identify patients who will increase MAP when CO is increased by a fluid challenge [63]. The Eadyn may also assist weaning from vasopressor support in the de-escalation phase [48] as it may identify patients who tolerate weaning off norepinephrine without developing hypotension [64]. Although variables of vasomotor tone and VAC have the potential to facilitate adequate use of fluids and vasopressors at the bedside further studies are required to develop protocols on ensuring efficacy in septic shock resuscitation.
The effects of norepinephrine also extend to the venous circulation by increasing mean systemic filling pressure, and thus venous return and CO [65]. The effect of norepinephrine on alpha and beta adrenergic receptors in the myocardium increases contractility facilitating the increase in venous return.
The extent to which the increased CO and MAP also contribute to improvement of microcirculatory perfusion is variable, depending on the balance between the improvement in organ perfusion pressure and a potential deterioration in driving pressure at the level of the microcirculation. This was shown in two studies. First, the effect of increasing MAP with norepinephrine on microcirculatory perfusion was dependent on the baseline state of the microcirculation [25]. Second, the optimal MAP varied between individuals and was independent of previous hypertension [66]. Given this variable effect of norepinephrine on important elements of tissue perfusion a challenge of the system by temporarily increasing the norepinephrine dose to reach a higher MAP could optimize resuscitation. In a RCT, subgroup analysis showed that previously hypertensive patients might benefit from a higher MAP [67]. This also applies to patients without previous hypertension [25, 66]. A large study in elderly patients (≥ 65 years) allocated to relative hypotension (MAP 60–65 mmHg) showed no overall significant effect on mortality when compared to usual care but a possible beneficial effect in a subgroup analysis of chronic hypertensive patients [68]. Therefore, a higher MAP target might not benefit all chronic hypertensive patients.
Therefore, a vasopressor test, whereby the MAP is increased and the effect on tissue perfusion is monitored (CRT, urine output, lactate, limb/cardiac ischemia, arrhythmias, etc.), seems a logical step in the resuscitation of patients who have not improved with initial treatment. This concept was first introduced in the ANDROMEDA-SHOCK study in previously hypertensive patients without improvement of peripheral perfusion or decrease of lactate levels following initial treatment targeted to a MAP of 65 mmHg [13]. Following the results of the recent study on the effects of maintaining a higher MAP [67] in septic shock patients, the norepinephrine dose was increased to reach a MAP 80–85 mmHg (depending on the randomization group) while monitoring the effect on peripheral perfusion (1 h later) or lactate levels (2 h later). When the target was reached the higher MAP level was maintained, if the target could not be met, the MAP was returned to baseline level.
Although the tools used in these clinical interventions are not new the context in which these are now increasingly used in clinical practice have evolved. However, as the effects on outcome parameters have not been established, these are not part of current guidelines and, thus, require further studies.
Use of vasopressin analogs in septic shock
The current use of vasopressin is mainly as an adjunct to norepinephrine when a pre-defined dose is reached. Vasopressin is usually added at a pre-defined dose [3], aiming to further increase MAP when required while sparing additional adrenergic burden.
Other vasopressin analogs have been studied. Given the relative advantages of terlipressin (greater V1a-receptor selectivity) and even the possibility to use intermittent bolus treatment (given the long half-life), it has been compared to norepinephrine [69]. However, the study did not find a difference in mortality but a higher incidence of adverse events (30% terlipressin, 12% norepinephrine) like digital ischemia [69]. Selepressin is an even more selective V1a agonist than terlipressin. Its use in experimental models and in a phase II randomized study was associated with reduced fluid requirements and edema formation [70]. These benefits did not translate into clinical benefit as selepressin was similarly effective (mortality and vasopressor- and ventilator free days) compared to placebo in a Phase 2b/3 randomized study; however, adverse ischemic events were higher in the selepressin group when compared to norepinephrine alone [71]. Currently, selepressin is not yet approved for clinical use and given the outcome of the studies using terlipressin, its use is currently not recommended.
Use of other vasopressors
In the renin–angiotensin–aldosterone system, angiotensin II (ATII) has vasoconstrictive effects. Relative deficiency of ATII has been associated with worse outcomes in septic shock. The use of ATII in septic shock increases blood pressure and decreases the need of standard vasopressors without an effect on outcome [72]. However, as many as 30% of the patients did not respond to ATII. Interestingly in a post hoc analysis, patients with acute kidney injury requiring renal replacement therapy and elevated renin levels had a significant mortality benefit even if MAP did not respond to angiotensin II [73, 74]. Additional studies are required for clear recommendation on clinical use.
Methylene blue (MB) improves hemodynamics by reducing excessive production of nitric oxide by blocking guanylate cyclase. However, only small-sized studies have been conducted with conflicting results; thus, its use cannot be recommended currently [75, 76].
The use of these agents in patients requiring high-dose adrenergic agents to maintain MAP should be further evaluated in clinical studies.
Evolving concepts in inotrope use
Dobutamine
Cardiomyopathy in septic shock is frequently present and whether this leads to inadequate tissue perfusion depends on the interplay between the effect of contractility and state of vasoplegia (afterload) both induced by the septic process [77]. The use of repeated echocardiography combined with clinical, hemodynamic, and biological variables is, therefore, crucial to detect, monitor and treat septic cardiomyopathy were dobutamine is the current recommended first-line inotrope for its treatment [24]. A recent study using hemodynamic profiling identified characteristics of patients with septic cardiomyopathy who might benefit from inotropic treatment [78]. Dobutamine benefitted 18% of these patients, characterized by a low left ventricular ejection fraction (LVEF) (< 40%) and low aortic velocity time integral (VTI) as a measure of stroke volume [78]. Effective dobutamine treatment is characterized by improvement in clinical status together with an increase in LVEF and VTI, and without an increase or even a decrease in heart rate [79].
So, adequately selecting patients likely to benefit or trialing dobutamine treatment and monitoring its effects may represent a better approach and could facilitate individualization. A recent network meta-analysis found dobutamine to be significantly associated with reduced mortality in patients with severe sepsis or septic shock (OR for dobutamine vs placebo 0.30; 95% CI 0.09–0.99) [80]. More evidence is expected from an ongoing multicenter trial (ClinicalTrials.gov ID: NCT04166331) studying the effectiveness of dobutamine in improving tissue hypoperfusion and its associated organ dysfunctions in patients with septic shock and septic cardiomyopathy.
Outside of the cardiac dysfunction context, improving CO may result in improved tissue perfusion. Earlier studies have shown that adding low dose (5 mcg/kg min) dobutamine to current treatment showed not only improvements in macro- but also in microcirculatory flow, the latter even being independent of the effect on CO [81, 82]. The latter could have been related to the vasodilatory actions of dobutamine. However, the effects may vary and there is no single agent that has been shown to reproducibly lead to improved macro/microvascular flow [83, 84], and side effects may complicate the use of dobutamine, underscoring the trialing approach [84].
This trialing approach using a limited dose of dobutamine was recently introduced in septic shock patients (similar to the vasopressor test described earlier) [13] as an inodilator test. It involved administering a 5 mcg/kg min infusion for a limited time (see previous description) after which the effect on the target parameter was assessed. If beneficial, the infusion would be maintained.
It is clear that while dobutamine may have beneficial effects on tissue perfusion its timing, dosing and effect on outcome parameters in specific hemodynamic profiles in septic shock have not yet been clarified.
Use of alternative inotropes
Calcium sensitizers (e.g., levosimendan) and phosphodiesterase-3 inhibitors (e.g., milrinone and enoximone) exhibit inotropic and vasodilatory effects.
In general, addition of levosimendan to standard treatment without clear impairment in cardiac function is not associated with improved morbidity or mortality even when biochemical evidence of cardiac injury is present [85]. This underscores that manipulating cardiac function should be triggered by a clinical problem (i.e., abnormal tissue perfusion) and not just by the presence of myocardial injury or even a low CO [77] as in those cases the side effects might outweigh any benefit. In this context, levosimendan has been associated with a lower likelihood of successful weaning from MV and a higher risk of supraventricular tachyarrhythmia [85]. In addition, levosimendan does not provide clear benefit over the use of dobutamine [86]. A theoretical advantage of levosimendan could be in patients admitted with sepsis in whom beta-blocker therapy contributes to the inadequate cardiac function.
Although experimental studies have shown possible benefit from the use of phosphodiesterase-3 inhibitors, clinical studies on outcomes in patients are scarce [83]. Although these might represent a typical example of an inodilator, the long half-life and the unpredictable effects on MAP limit its use in the already typically vasoplegic septic shock patients.
From the current studies available, it is clear that the use of vasopressors and inotropes in patients with septic shock is in need of more profound evidence of indication and benefit [87].
The concept of adrenergic modulation
A sustained hyperadrenergic response in septic shock and the use of adrenergic agents has been associated with adverse hemodynamic, microcirculatory, metabolic, and pro-inflammatory effects [88, 89]. Adrenergic modulation has a favorable impact on several physiological and clinical outcome parameters in septic shock. Dexmedetomidine (α2-agonist) attenuates the sympathetic response to stress, and decreases epinephrine levels without adverse consequences for tissue perfusion. Experimental studies have found anti-inflammatory effects and improvement in microcirculatory flow, and exogeneous lactate clearance [88]. Also, clinical studies have reported faster resolution of the hyperlactatemia [90]. Recent studies also showed that dexmedetomidine may reduce norepinephrine requirements; however, the impact on major outcomes has yet to be demonstrated [91, 92]. The supporting evidence for the use of β-blockers in sepsis is relatively weak. Experimental studies have shown favorable effects on heart rate, and some hemodynamic, inflammatory, metabolic variables, including a decrease in blood lactate levels [88]. The effect of esmolol on intrinsic myocardial function and vascular responsiveness is still controversial [93]. A clinical challenge in septic shock patients with persistent tachycardia is to distinguish a compensatory origin (low stroke volume) from maladaptive sympathetic overstimulation as in the former context beta blockers could be detrimental in the former. In the latter, beta blockers may decrease myocardial O2 consumption, increase diastolic filling and systemic flow and improve VAC [94]. Ongoing studies try to identify the best predictors of a favorable response to beta blockers. Till the results of these studies are available, the use of beta blocker in septic shock cannot be recommended [93].
Evolving concepts in refractory shock
Refractory septic shock is a life-threatening condition that is best defined by a state in which escalation of vasoactive therapy does not restore adequate tissue perfusion. At the bedside, this can be recognized by persistent hypotension and hypoperfusion in the absence of hypovolemia, while the patient is receiving more than 0.25 µg/kg min of norepinephrine [95]. Multiple factors may favor the onset of refractory shock (Table 3). Timely identification of refractory shock and the provoking factors may reduce the urge to increase vasopressors at the risk of severe side effects that may further worsen the clinical condition. It is important to recognize the difference between severely decreased vasopressor responsiveness and complications like for instance LV outflow tract obstruction due to high vasopressor support. Therefore, echocardiography should always accompany the diagnosis of refractory shock. In the absence of these complications, adjuvant therapies may benefit the patient. Various treatments have been studied and shown variable effects. Whilst the benefit of hydrocortisone in specific patients has been established the role of vitamins is less clear [96, 97]. In addition, non-adrenergic vasopressors (vasopressin, angiotensin 2) and drugs targeting endothelial factors (NO-scavenger/inhibitors, MB, endothelin), have shown clinical effects that might be beneficial but their impact on outcome remains to be determined [98]. Other studies have focused on individualizing treatment and identifying the response to vasoactive medication and corticosteroids that may vary in specific patients [99,100,101].
Extracorporeal blood purification techniques (ECBPT) offer a theoretical benefit of removing mediators and establishing immune homeostasis. Many studies have been performed (Table 4) [102,103,104,105,106,107,108,109]. However, as the immune response in sepsis is an individual process and, thus, cytokine profiles vary significantly between different sepsis phenotypes, the success of ECBPT is difficult to predict [110, 111]. In addition, adverse effects, including inadvertent removal of nutrients, trace elements and drugs have been reported with ECBPT [112] and the clinical benefit from these techniques remains controversial (Table 4) and requires further studies [113].
A ultimate rescue therapy for septic shock patients with refractory LV failure could be hemodynamic support with venous-arterial extracorporeal membrane oxygenation (VA-ECMO). A recent systematic review and meta-analysis indicated that especially septic shock patients with a very low LVEF might benefit [114].
Many treatments listed in this section lack sufficient clinical evidence and so general recommendations cannot be made. The use of these treatments should, thus, be individualized in which careful consideration should be given to the problem at hand in relation to the likelihood of benefit and harm of the intervention.
Conclusions
In this review, we have discussed current practice and evolving concepts in septic shock resuscitation. While the pillars of hemodynamic resuscitation have not changed in general, there is new information on how to optimize fluid administration and the use of vasoactive drugs. The lack of solid evidence in all fields is of growing concern, and this precludes to make strong recommendations for most interventions.
A broad research agenda focusing on individualization and phenotyping of septic shock patients using combined efforts of the different excellence groups is, therefore, imperative.
Abbreviations
- RCT:
-
Randomized controlled trials
- SSC:
-
Surviving Sepsis Campaign
- CO:
-
Cardiac output
- PLR:
-
Passive leg raising test
- SVV:
-
Stroke volume variation
- PPV:
-
Pulse pressure variation
- MAP:
-
Mean arterial pressure
- LV:
-
Left ventricular
- CRT:
-
Capillary refill time
- ICU:
-
Intensive care unit
- tRBCp:
-
Tissue red blood cell perfusion
- SOSD:
-
Salvage, optimization, stabilization, de-escalation
- DAP:
-
Diastolic arterial pressure
- ScvO2 :
-
Central venous oxygenation
- MV:
-
Mechanical ventilation
- VAC:
-
Ventriculo-arterial coupling
- Ea:
-
Arterial elastance
- Ees:
-
End-systolic elastance
- Eadyn :
-
Dynamic arterial elastance
- ATII:
-
Angiotensin II
- MB:
-
Methylene blue
- LVEF:
-
Left ventricular ejection fraction
- VTI:
-
Aortic velocity time integral
- ECBPT:
-
Extracorporeal blood purification techniques
- VA-ECMO:
-
Venous–arterial extracorporeal membrane oxygenation
References
Menk M, Estenssoro E, Sahetya SK, Neto AS, Sinha P, Slutsky AS, Summers C, Yoshida T, Bein T, Ferguson ND (2020) Current and evolving standards of care for patients with ARDS. Intensive Care Med 46:2157–2167
Vincent JL, Joosten A, Saugel B (2021) Hemodynamic monitoring and support. Crit Care Med 49:1638–1650
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, McIntyre L, Ostermann M, Prescott HC, Schorr C, Simpson S, Wiersinga WJ, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R, Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Coz Yataco A, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R, Gomersall C, Hodgson C, Moller MH, Iwashyna T, Jacob S, Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H, McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W, Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M (2021) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 47:1181–1247
Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, Jaeschke R, Mebazaa A, Pinsky MR, Teboul JL, Vincent JL, Rhodes A (2014) Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 40:1795–1815
Jansen TC, van Bommel J, Schoonderbeek FJ, Visser SJS, van der Klooster JM, Lima AP, Willemsen SP, Bakker J, Grp LS (2010) Early lactate-guided therapy in intensive care unit patients a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med 182:752–761
Hernandez G, Bellomo R, Bakker J (2019) The ten pitfalls of lactate clearance in sepsis. Intensive Care Med 45:82–85
Vincent JL (2018) How I treat septic shock. Intensive Care Med 44:2242–2244
Vincent J-L, Singer M, Einav S, Moreno R, Wendon J, Teboul JL, Bakker J, Hernandez G, Annane D, de Man A, Monnet X, Ranieri VM, Hamzaoui O, Takala J, Juffermans N, Chiche J-D, Myatra SN, De Backer D (2021) Equilibrating SSC guidelines with individualized care. Crit Care 25(1):397. https://doi.org/10.1186/s13054-021-03813-0
Berlin DA, Bakker J (2014) Understanding venous return. Intensive Care Med 40:1564–1566
Toscani L, Aya HD, Antonakaki D, Bastoni D, Watson X, Arulkumaran N, Rhodes A, Cecconi M (2017) What is the impact of the fluid challenge technique on diagnosis of fluid responsiveness? A systematic review and meta-analysis. Crit Care 21:207
Monnet X, Marik PE, Teboul JL (2016) Prediction of fluid responsiveness: an update. Ann Intensive Care 6:111
Guarracino F, Bertini P, Pinsky MR (2020) Heterogeneity of cardiovascular response to standardized sepsis resuscitation. Crit Care 24:99
Hernandez G, Ospina-Tascon GA, Damiani LP, Estenssoro E, Dubin A, Hurtado J, Friedman G, Castro R, Alegria L, Teboul JL, Cecconi M, Ferri G, Jibaja M, Pairumani R, Fernandez P, Barahona D, Granda-Luna V, Cavalcanti AB, Bakker J, Investigators A-S, the Latin America Intensive Care N (2019) Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: the ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA 321:654–664
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP (2017) Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 43:304–377
Vincent JL, Cecconi M, De Backer D (2020) The fluid challenge. Crit Care 24:703
Shi R, Monnet X, Teboul JL (2020) Parameters of fluid responsiveness. Curr Opin Crit Care 26:319–326
Vignon P, Repesse X, Begot E, Leger J, Jacob C, Bouferrache K, Slama M, Prat G, Vieillard-Baron A (2017) Comparison of echocardiographic indices used to predict fluid responsiveness in ventilated patients. Am J Respir Crit Care Med 195:1022–1032
Roger C, Zieleskiewicz L, Demattei C, Lakhal K, Piton G, Louart B, Constantin JM, Chabanne R, Faure JS, Mahjoub Y, Desmeulles I, Quintard H, Lefrant JY, Muller L, AzuRea G (2019) Time course of fluid responsiveness in sepsis: the fluid challenge revisiting (FCREV) study. Crit Care 23:179
Monnet X, Cipriani F, Camous L, Sentenac P, Dres M, Krastinova E, Anguel N, Richard C, Teboul JL (2016) The passive leg raising test to guide fluid removal in critically ill patients. Ann Intensive Care 6:46
Levy B, Fritz C, Tahon E, Jacquot A, Auchet T, Kimmoun A (2018) Vasoplegia treatments: the past, the present, and the future. Crit Care 22:52
Hernandez G, Teboul JL, Bakker J (2019) Norepinephrine in septic shock. Intensive Care Med 45:687–689
Feldheiser A, Gelman S, Chew M, Stopfkuchen-Evans M (2021) Vasopressor effects on venous return in septic patients: a review. Eur J Anaesthesiol 38:659–663
Innocenti F, Palmieri V, Tassinari I, Capretti E, De Paris A, Gianno A, Marchesini A, Montuori M, Pini R (2021) Change in myocardial contractility in response to treatment with norepinephrine in septic shock. Am J Respir Crit Care Med 204:365–368
Scheeren TWL, Bakker J, Kaufmann T, Annane D, Asfar P, Boerma EC, Cecconi M, Chew MS, Cholley B, Cronhjort M, De Backer D, Dubin A, Dunser MW, Duranteau J, Gordon AC, Hajjar LA, Hamzaoui O, Hernandez G, Kanoore Edul V, Koster G, Landoni G, Leone M, Levy B, Martin C, Mebazaa A, Monnet X, Morelli A, Payen D, Pearse RM, Pinsky MR, Radermacher P, Reuter DA, Sakr Y, Sander M, Saugel B, Singer M, Squara P, Vieillard-Baron A, Vignon P, Vincent JL, van der Horst ICC, Vistisen ST, Teboul JL (2021) Current use of inotropes in circulatory shock. Ann Intensive Care 11:21
Dubin A, Pozo MO, Casabella CA, Palizas F Jr, Murias G, Moseinco MC, Kanoore Edul VS, Palizas F, Estenssoro E, Ince C (2009) Increasing arterial blood pressure with norepinephrine does not improve microcirculatory blood flow: a prospective study. Crit Care 13:R92
Patel BM, Chittock DR, Russell JA, Walley KR (2002) Beneficial effects of short-term vasopressin infusion during severe septic shock. Anesthesiology 96:576–582
Russell JA, Walley KR, Singer J, Gordon AC, Hebert PC, Cooper DJ, Holmes CL, Mehta S, Granton JT, Storms MM, Cook DJ, Presneill JJ, Ayers D (2008) Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 358:877–887
Gordon AC, Mason AJ, Thirunavukkarasu N, Perkins GD, Cecconi M, Cepkova M, Pogson DG, Aya HD, Anjum A, Frazier GJ, Santhakumaran S, Ashby D, Brett SJ, Investigators V (2016) Effect of early vasopressin vs norepinephrine on kidney failure in patients with septic shock: the VANISH randomized clinical trial. JAMA 316:509–518
Nagendran M, Russell JA, Walley KR, Brett SJ, Perkins GD, Hajjar L, Mason AJ, Ashby D, Gordon AC (2019) Vasopressin in septic shock: an individual patient data meta-analysis of randomised controlled trials. Intensive Care Med 45:844–855
Vincent JL, Post EH (2016) Sepsis: vasopressin: a first-line agent for septic shock? Nat Rev Nephrol 12:718–719
Dubin A, Henriquez E, Hernandez G (2018) Monitoring peripheral perfusion and microcirculation. Curr Opin Crit Care 24:173–180
Joly HR, Weil MH (1969) Temperature of the great toe as an indication of the severity of shock. Circulation 39:131–138
van Genderen ME, Engels N, van der Valk RJ, Lima A, Klijn E, Bakker J, van Bommel J (2015) Early peripheral perfusion-guided fluid therapy in patients with septic shock. Am J Respir Crit Care Med 191:477–480
Zampieri FG, Damiani LP, Bakker J, Ospina-Tascon GA, Castro R, Cavalcanti AB, Hernandez G, Investigators A-S, the Latin America Intensive Care N (2020) Effects of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels among patients with septic shock: a Bayesian Reanalysis of the ANDROMEDA-SHOCK Trial. Am J Respir Crit Care Med 201:423–429
Zhou J, Song J, Gong S, Li L, Zhang H, Wang M (2017) Persistent hyperlactatemia-high central venous-arterial carbon dioxide to arterial-venous oxygen content ratio is associated with poor outcomes in early resuscitation of septic shock. Am J Emerg Med 35:1136–1141
Ospina-Tascon GA, Umana M, Bermudez WF, Bautista-Rincon DF, Valencia JD, Madrinan HJ, Hernandez G, Bruhn A, Arango-Davila C, De Backer D (2016) Can venous-to-arterial carbon dioxide differences reflect microcirculatory alterations in patients with septic shock? Intensive Care Med 42:211–221
Ince C, Boerma EC, Cecconi M, De Backer D, Shapiro NI, Duranteau J, Pinsky MR, Artigas A, Teboul JL, Reiss IKM, Aldecoa C, Hutchings SD, Donati A, Maggiorini M, Taccone FS, Hernandez G, Payen D, Tibboel D, Martin DS, Zarbock A, Monnet X, Dubin A, Bakker J, Vincent JL, Scheeren TWL, Cardiovascular Dynamics Section of the E (2018) Second consensus on the assessment of sublingual microcirculation in critically ill patients: results from a task force of the European Society of Intensive Care Medicine. Intensive Care Med 44:281–299
Berthelsen RE, Ostrowski SR, Bestle MH, Johansson PI (2019) Co-administration of iloprost and eptifibatide in septic shock (CO-ILEPSS)-a randomised, controlled, double-blind investigator-initiated trial investigating safety and efficacy. Crit Care 23:301
Legrand M, De Backer D, Depret F, Ait-Oufella H (2019) Recruiting the microcirculation in septic shock. Ann Intensive Care 9:102
Siam J, Kadan M, Flaishon R, Barnea O (2015) Blood flow versus hematocrit in optimization of oxygen transfer to tissue during fluid resuscitation. Cardiovasc Eng Technol 6:474–484
Perel A (2017) Iatrogenic hemodilution: a possible cause for avoidable blood transfusions? Crit Care 21:291
Hilty MP, Akin S, Boerma C, Donati A, Erdem O, Giaccaglia P, Guerci P, Milstein DM, Montomoli J, Toraman F, Uz Z, Veenstra G, Ince C (2020) Automated algorithm analysis of sublingual microcirculation in an international multicentral database identifies alterations associated with disease and mechanism of resuscitation. Crit Care Med 48:e864–e875
Hilty MP, Guerci P, Ince Y, Toraman F, Ince C (2019) MicroTools enables automated quantification of capillary density and red blood cell velocity in handheld vital microscopy. Commun Biol 2:217
Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, Stollings JL, Kumar AB, Hughes CG, Hernandez A, Guillamondegui OD, May AK, Weavind L, Casey JD, Siew ED, Shaw AD, Bernard GR, Rice TW, Investigators S, the Pragmatic Critical Care Research G (2018) Balanced crystalloids versus saline in critically ill adults. N Engl J Med 378:829–839
Jackson KE, Wang L, Casey JD, Bernard GR, Self WH, Rice TW, Semler MW, Investigators S, the Pragmatic Critical Care Research G (2021) Effect of early balanced crystalloids before ICU admission on sepsis outcomes. Chest 159:585–595
Jaynes MP, Murphy CV, Ali N, Krautwater A, Lehman A, Doepker BA (2018) Association between chloride content of intravenous fluids and acute kidney injury in critically ill medical patients with sepsis. J Crit Care 44:363–367
Zampieri FG, Machado FR, Biondi RS, Freitas FGR, Veiga VC, Figueiredo RC, Lovato WJ, Amendola CP, Serpa-Neto A, Paranhos JLR, Guedes MAV, Lucio EA, Oliveira-Junior LC, Lisboa TC, Lacerda FH, Maia IS, Grion CMC, Assuncao MSC, Manoel ALO, Silva-Junior JM, Duarte P, Soares RM, Miranda TA, de Lima LM, Gurgel RM, Paisani DM, Correa TD, Azevedo LCP, Kellum JA, Damiani LP, Brandao da Silva N, Cavalcanti AB, Si B, the Bm (2021) Effect of intravenous fluid treatment with a balanced solution vs 0.9% saline solution on mortality in critically ill patients: the BaSICS Randomized Clinical Trial. JAMA 326:1–12
Vincent JL, De Backer D (2013) Circulatory shock. N Engl J Med 369:1726–1734
Colon Hidalgo D, Patel J, Masic D, Park D, Rech MA (2020) Delayed vasopressor initiation is associated with increased mortality in patients with septic shock. J Crit Care 55:145–148
Ospina-Tascon GA, Hernandez G, Alvarez I, Calderon-Tapia LE, Manzano-Nunez R, Sanchez-Ortiz AI, Quinones E, Ruiz-Yucuma JE, Aldana JL, Teboul JL, Cavalcanti AB, De Backer D, Bakker J (2020) Effects of very early start of norepinephrine in patients with septic shock: a propensity score-based analysis. Crit Care 24:52
Permpikul C, Tongyoo S, Viarasilpa T, Trainarongsakul T, Chakorn T, Udompanturak S (2019) Early use of norepinephrine in septic shock resuscitation (CENSER): a randomized trial. Am J Respir Crit Care Med 199:1097–1105
Li Y, Li H, Zhang D (2020) Timing of norepinephrine initiation in patients with septic shock: a systematic review and meta-analysis. Crit Care 24:488
Silversides JA, Perner A, Malbrain M (2019) Liberal versus restrictive fluid therapy in critically ill patients. Intensive Care Med 45:1440–1442
Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, Van Regenmortel N (2014) Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther 46:361–380
Cordemans C, De Laet I, Van Regenmortel N, Schoonheydt K, Dits H, Martin G, Huber W, Malbrain ML (2012) Aiming for a negative fluid balance in patients with acute lung injury and increased intra-abdominal pressure: a pilot study looking at the effects of PAL-treatment. Ann Intensive Care 2(Suppl 1):S15
Chawla LS, Davison DL, Brasha-Mitchell E, Koyner JL, Arthur JM, Shaw AD, Tumlin JA, Trevino SA, Kimmel PL, Seneff MG (2013) Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care 17:R207
Hall A, Crichton S, Dixon A, Skorniakov I, Kellum JA, Ostermann M (2020) Fluid removal associates with better outcomes in critically ill patients receiving continuous renal replacement therapy: a cohort study. Crit Care 24:279
Wang L, Qiu C, Guan X, Chen M, Chen J, Si X, Du Z, Liu Y, Ouyang B (2018) Fluid removal with ultrasound guided protocol improves the efficacy and safety of dehydration in post-resuscitated critically ill patients: a quasi-experimental, before and after study. Shock 50:401–407
Ospina-Tascon GA, Teboul JL, Hernandez G, Alvarez I, Sanchez-Ortiz AI, Calderon-Tapia LE, Manzano-Nunez R, Quinones E, Madrinan-Navia HJ, Ruiz JE, Aldana JL, Bakker J (2020) Diastolic shock index and clinical outcomes in patients with septic shock. Ann Intensive Care 10:41
Sunagawa K, Maughan WL, Burkhoff D, Sagawa K (1983) Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol 245:H773-780
Monge Garcia MI, Jian Z, Settels JJ, Hunley C, Cecconi M, Hatib F, Pinsky MR (2018) Performance comparison of ventricular and arterial dP/dtmax for assessing left ventricular systolic function during different experimental loading and contractile conditions. Crit Care 22:325
Monge Garcia MI, Jian Z, Hatib F, Settels JJ, Cecconi M, Pinsky MR (2020) Dynamic arterial elastance as a ventriculo-arterial coupling index: an experimental animal study. Front Physiol 11:284
Garcia MI, Romero MG, Cano AG, Aya HD, Rhodes A, Grounds RM, Cecconi M (2014) Dynamic arterial elastance as a predictor of arterial pressure response to fluid administration: a validation study. Crit Care 18:626
Guinot PG, Bernard E, Levrard M, Dupont H, Lorne E (2015) Dynamic arterial elastance predicts mean arterial pressure decrease associated with decreasing norepinephrine dosage in septic shock. Crit Care 19:14
Persichini R, Silva S, Teboul JL, Jozwiak M, Chemla D, Richard C, Monnet X (2012) Effects of norepinephrine on mean systemic pressure and venous return in human septic shock. Crit Care Med 40:3146–3153
Fiorese Coimbra KT, de Freitas FGR, Bafi AT, Pinheiro TT, Nunes NF, de Azevedo LCP, Machado FR (2019) Effect of increasing blood pressure with noradrenaline on the microcirculation of patients with septic shock and previous arterial hypertension. Crit Care Med 47:1033–1040
Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, Mira JP, Dequin PF, Gergaud S, Weiss N, Legay F, Le Tulzo Y, Conrad M, Robert R, Gonzalez F, Guitton C, Tamion F, Tonnelier JM, Guezennec P, Van Der Linden T, Vieillard-Baron A, Mariotte E, Pradel G, Lesieur O, Ricard JD, Herve F, du Cheyron D, Guerin C, Mercat A, Teboul JL, Radermacher P, Investigators S (2014) High versus low blood-pressure target in patients with septic shock. N Engl J Med 370:1583–1593
Lamontagne F, Richards-Belle A, Thomas K, Harrison DA, Sadique MZ, Grieve RD, Camsooksai J, Darnell R, Gordon AC, Henry D, Hudson N, Mason AJ, Saull M, Whitman C, Young JD, Rowan KM, Mouncey PR, trial i (2020) Effect of reduced exposure to vasopressors on 90-day mortality in older critically ill patients with vasodilatory hypotension: a randomized clinical trial. JAMA 323:938–949
Liu ZM, Chen J, Kou Q, Lin Q, Huang X, Tang Z, Kang Y, Li K, Zhou L, Song Q, Sun T, Zhao L, Wang X, He X, Wang C, Wu B, Lin J, Yuan S, Gu Q, Qian K, Shi X, Feng Y, Lin A, He X, Study Group of i, Guan XD (2018) Terlipressin versus norepinephrine as infusion in patients with septic shock: a multicentre, randomised, double-blinded trial. Intensive Care Med 44:1816–1825
Russell JA, Vincent JL, Kjolbye AL, Olsson H, Blemings A, Spapen H, Carl P, Laterre PF, Grundemar L (2017) Selepressin, a novel selective vasopressin V1A agonist, is an effective substitute for norepinephrine in a phase IIa randomized, placebo-controlled trial in septic shock patients. Crit Care 21:213
Laterre PF, Berry SM, Blemings A, Carlsen JE, Francois B, Graves T, Jacobsen K, Lewis RJ, Opal SM, Perner A, Pickkers P, Russell JA, Windelov NA, Yealy DM, Asfar P, Bestle MH, Muller G, Bruel C, Brule N, Decruyenaere J, Dive AM, Dugernier T, Krell K, Lefrant JY, Megarbane B, Mercier E, Mira JP, Quenot JP, Rasmussen BS, Thorsen-Meyer HC, Vander Laenen M, Vang ML, Vignon P, Vinatier I, Wichmann S, Wittebole X, Kjolbye AL, Angus DC, Investigators S-A (2019) Effect of selepressin vs placebo on ventilator- and vasopressor-free days in patients with septic shock: the SEPSIS-ACT randomized clinical trial. JAMA 322:1476–1485
Khanna A, English SW, Wang XS, Ham K, Tumlin J, Szerlip H, Busse LW, Altaweel L, Albertson TE, Mackey C, McCurdy MT, Boldt DW, Chock S, Young PJ, Krell K, Wunderink RG, Ostermann M, Murugan R, Gong MN, Panwar R, Hastbacka J, Favory R, Venkatesh B, Thompson BT, Bellomo R, Jensen J, Kroll S, Chawla LS, Tidmarsh GF, Deane AM, Investigators A (2017) Angiotensin II for the treatment of vasodilatory shock. N Engl J Med 377:419–430
Bellomo R, Forni LG, Busse LW, McCurdy MT, Ham KR, Boldt DW, Hastbacka J, Khanna AK, Albertson TE, Tumlin J, Storey K, Handisides D, Tidmarsh GF, Chawla LS, Ostermann M (2020) Renin and survival in patients given angiotensin II for catecholamine-resistant vasodilatory shock. a clinical trial. Am J Respir Crit Care Med 202:1253–1261
Tumlin JA, Murugan R, Deane AM, Ostermann M, Busse LW, Ham KR, Kashani K, Szerlip HM, Prowle JR, Bihorac A, Finkel KW, Zarbock A, Forni LG, Lynch SJ, Jensen J, Kroll S, Chawla LS, Tidmarsh GF, Bellomo R, Angiotensin II for the Treatment of High-Output Shock 3 (ATHOS-3) Investigators (2018) Outcomes in patients with vasodilatory shock and renal replacement therapy treated with intravenous angiotensin II. Crit Care Med 46:949–957
Kirov MY, Evgenov OV, Evgenov NV, Egorina EM, Sovershaev MA, Sveinbjornsson B, Nedashkovsky EV, Bjertnaes LJ (2001) Infusion of methylene blue in human septic shock: a pilot, randomized, controlled study. Crit Care Med 29:1860–1867
Memis D, Karamanlioglu B, Yuksel M, Gemlik I, Pamukcu Z (2002) The influence of methylene blue infusion on cytokine levels during severe sepsis. Anaesth Intensive Care 30:755–762
Vieillard-Baron A (2011) Septic cardiomyopathy. Ann Intensive Care 1:6
Geri G, Vignon P, Aubry A, Fedou AL, Charron C, Silva S, Repesse X, Vieillard-Baron A (2019) Cardiovascular clusters in septic shock combining clinical and echocardiographic parameters: a post hoc analysis. Intensive Care Med 45:657–667
Vieillard-Baron A, Caille V, Charron C, Belliard G, Page B, Jardin F (2008) Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med 36:1701–1706
Belletti A, Benedetto U, Biondi-Zoccai G, Leggieri C, Silvani P, Angelini GD, Zangrillo A, Landoni G (2017) The effect of vasoactive drugs on mortality in patients with severe sepsis and septic shock. A network meta-analysis of randomized trials. J Crit Care 37:91–98
De Backer D, Creteur J, Dubois MJ, Sakr Y, Koch M, Verdant C, Vincent JL (2006) The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med 34:403–408
Vincent JL, Roman A, De Backer D, Kahn RJ (1990) Oxygen uptake/supply dependency. Effects of short-term dobutamine infusion. Am J Respir Crit Care Med 142:2–7
Ospina-Tascon GA, Calderon-Tapia LE (2020) Inodilators in septic shock: should these be used? Ann Transl Med 8:796
Potter EK, Hodgson L, Creagh-Brown B, Forni LG (2019) Manipulating the microcirculation in sepsis—the impact of vasoactive medications on microcirculatory blood flow: a systematic review. Shock 52:5–12
Gordon AC, Perkins GD, Singer M, McAuley DF, Orme RM, Santhakumaran S, Mason AJ, Cross M, Al-Beidh F, Best-Lane J, Brealey D, Nutt CL, McNamee JJ, Reschreiter H, Breen A, Liu KD, Ashby D (2016) Levosimendan for the prevention of acute organ dysfunction in sepsis. N Engl J Med 375:1638–1648
Bhattacharjee S, Soni KD, Maitra S, Baidya DK (2017) Levosimendan does not provide mortality benefit over dobutamine in adult patients with septic shock: a meta-analysis of randomized controlled trials. J Clin Anesth 39:67–72
Einav S, Helviz Y, Ippolito M, Cortegiani A (2021) Vasopressor and inotrope treatment for septic shock: an umbrella review of reviews. J Crit Care 65:65–71
Hernandez G, Tapia P, Alegria L, Soto D, Luengo C, Gomez J, Jarufe N, Achurra P, Rebolledo R, Bruhn A, Castro R, Kattan E, Ospina-Tascon G, Bakker J (2016) Effects of dexmedetomidine and esmolol on systemic hemodynamics and exogenous lactate clearance in early experimental septic shock. Crit Care 20:234
Stolk RF, van der Poll T, Angus DC, van der Hoeven JG, Pickkers P, Kox M (2016) Potentially inadvertent immunomodulation: norepinephrine use in sepsis. Am J Respir Crit Care Med 194:550–558
Miyamoto K, Nakashima T, Shima N, Kato S, Ueda K, Kawazoe Y, Ohta Y, Morimoto T, Yamamura H, on behalf of DTI (2018) Effect of dexmedetomidine on lactate clearance in patients with septic shock: a subanalysis of a multicenter randomized controlled trial. Shock 50:162–166
Morelli A, Sanfilippo F, Arnemann P, Hessler M, Kampmeier TG, D’Egidio A, Orecchioni A, Santonocito C, Frati G, Greco E, Westphal M, Rehberg SW, Ertmer C (2018) The effect of propofol and dexmedetomidine sedation on norepinephrine requirements in septic shock patients: a crossover trial. Crit Care Med 47:e89–e95
Cioccari L, Luethi N, Bailey M, Shehabi Y, Howe B, Messmer AS, Proimos HK, Peck L, Young H, Eastwood GM, Merz TM, Takala J, Jakob SM, Bellomo R, Group ACT, the SIIII (2020) The effect of dexmedetomidine on vasopressor requirements in patients with septic shock: a subgroup analysis of the Sedation Practice in Intensive Care Evaluation [SPICE III] Trial. Crit Care 24:441
Levy B, Fritz C, Piona C, Duarte K, Morelli A, Guerci P, Kimmoun A, Girerd N (2021) Hemodynamic and anti-inflammatory effects of early esmolol use in hyperkinetic septic shock: a pilot study. Crit Care 25:21
Morelli A, Romano SM, Sanfilippo F, Santonocito C, Frati G, Chiostri M, Agro FE, Ertmer C, Rehberg SW, Vieillard-Baron A (2020) Systolic-dicrotic notch pressure difference can identify tachycardic patients with septic shock at risk of cardiovascular decompensation following pharmacological heart rate reduction. Br J Anaesth 125:1018–1024
Annane D, Bellissant E, Cavaillon JM (2005) Septic shock. Lancet 365:63–78
Fujii T, Luethi N, Young PJ, Frei DR, Eastwood GM, French CJ, Deane AM, Shehabi Y, Hajjar LA, Oliveira G, Udy AA, Orford N, Edney SJ, Hunt AL, Judd HL, Bitker L, Cioccari L, Naorungroj T, Yanase F, Bates S, McGain F, Hudson EP, Al-Bassam W, Dwivedi DB, Peppin C, McCracken P, Orosz J, Bailey M, Bellomo R, Investigators VT (2020) Effect of Vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the VITAMINS randomized clinical trial. JAMA 323:423–431
Chang P, Liao Y, Guan J, Guo Y, Zhao M, Hu J, Zhou J, Wang H, Cen Z, Tang Y, Liu Z (2020) Combined treatment with hydrocortisone, Vitamin C, and thiamine for sepsis and septic shock: a randomized controlled trial. Chest 158:174–182
Annane D, Ouanes-Besbes L, de Backer D, Du B, Gordon AC, Hernandez G, Olsen KM, Osborn TM, Peake S, Russell JA, Cavazzoni SZ (2018) A global perspective on vasoactive agents in shock. Intensive Care Med 44:833–846
Wong HR, Cvijanovich NZ, Anas N, Allen GL, Thomas NJ, Bigham MT, Weiss SL, Fitzgerald JC, Checchia PA, Meyer K, Quasney M, Hall M, Gedeit R, Freishtat RJ, Nowak J, Lutfi R, Gertz S, Grunwell JR, Lindsell CJ (2018) Endotype transitions during the acute phase of pediatric septic shock reflect changing risk and treatment response. Crit Care Med 46:e242–e249
Anantasit N, Boyd JH, Walley KR, Russell JA (2014) Serious adverse events associated with vasopressin and norepinephrine infusion in septic shock. Crit Care Med 42:1812–1820
Ounissi M, Benkirane A, Dempsey E, Soares R, Jullien V, Pons G, Chhun S (2015) A review of potential pharmacogenetic effects on catecholamine responses. Drug Metab Rev 47:558–564
Dellinger RP, Bagshaw SM, Antonelli M, Foster DM, Klein DJ, Marshall JC, Palevsky PM, Weisberg LS, Schorr CA, Trzeciak S, Walker PM, Investigators ET (2018) Effect of targeted Polymyxin B hemoperfusion on 28-day mortality in patients with septic shock and elevated endotoxin level: the EUPHRATES randomized clinical trial. JAMA 320:1455–1463
Schadler D, Pausch C, Heise D, Meier-Hellmann A, Brederlau J, Weiler N, Marx G, Putensen C, Spies C, Jorres A, Quintel M, Engel C, Kellum JA, Kuhlmann MK (2017) The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: a randomized controlled trial. PLoS One 12:e0187015
Atan R, Peck L, Prowle J, Licari E, Eastwood GM, Storr M, Goehl H, Bellomo R (2018) A double-blind randomized controlled trial of high cutoff versus standard hemofiltration in critically ill patients with acute kidney injury. Crit Care Med 46:e988–e994
Joannes-Boyau O, Honore PM, Perez P, Bagshaw SM, Grand H, Canivet JL, Dewitte A, Flamens C, Pujol W, Grandoulier AS, Fleureau C, Jacobs R, Broux C, Floch H, Branchard O, Franck S, Roze H, Collin V, Boer W, Calderon J, Gauche B, Spapen HD, Janvier G, Ouattara A (2013) High-volume versus standard-volume haemofiltration for septic shock patients with acute kidney injury (IVOIRE study): a multicentre randomized controlled trial. Intensive Care Med 39:1535–1546
Lipcsey M, Tenhunen J, Pischke SE, Kuitunen A, Flaatten H, De Geer L, Sjolin J, Frithiof R, Chew MS, Bendel S, Kawati R, Larsson A, Mollnes TE, Tonnessen TI, Rubertsson S (2020) Endotoxin removal in septic shock with the alteco LPS adsorber was safe but showed no benefit compared to placebo in the double-blind randomized controlled trial-the asset study. Shock 54:224–231
Busund R, Koukline V, Utrobin U, Nedashkovsky E (2002) Plasmapheresis in severe sepsis and septic shock: a prospective, randomised, controlled trial. Intensive Care Med 28:1434–1439
Tumlin JA, Galphin CM, Tolwani AJ, Chan MR, Vijayan A, Finkel K, Szamosfalvi B, Dev D, DaSilva JR, Astor BC, Yevzlin AS, Humes HD (2015) A multi-center, randomized, controlled, pivotal study to assess the safety and efficacy of a selective cytopheretic device in patients with acute kidney injury. PLoS One 10:e0132482
Garbero E, Livigni S, Ferrari F, Finazzi S, Langer M, Malacarne P, Meca MCC, Mosca S, Olivieri C, Pozzato M, Rossi C, Tavola M, Terzitta M, Viaggi B, Bertolini G, GiViTi (2021) High dose coupled plasma filtration and adsorption in septic shock patients. Results of the COMPACT-2: a multicentre, adaptive, randomised clinical trial. Intensive Care Med 47:1303–1311
Seymour CW, Kennedy JN, Wang S, Chang CH, Elliott CF, Xu Z, Berry S, Clermont G, Cooper G, Gomez H, Huang DT, Kellum JA, Mi Q, Opal SM, Talisa V, van der Poll T, Visweswaran S, Vodovotz Y, Weiss JC, Yealy DM, Yende S, Angus DC (2019) Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA 321:2003–2017
Klein DJ, Foster D, Walker PM, Bagshaw SM, Mekonnen H, Antonelli M (2018) Polymyxin B hemoperfusion in endotoxemic septic shock patients without extreme endotoxemia: a post hoc analysis of the EUPHRATES trial. Intensive Care Med 44:2205–2212
Breilh D, Honore PM, De Bels D, Roberts JA, Gordien JB, Fleureau C, Dewitte A, Coquin J, Roze H, Perez P, Attou R, Redant S, Kugener L, Saux MC, Spapen HD, Ouattara A, Joannes-Boyau O, group Is (2019) Pharmacokinetics and pharmacodynamics of anti-infective agents during continuous veno-venous hemofiltration in critically ill patients: lessons learned from an ancillary study of the IVOIRE Trial. J Transl Int Med 7:155–169
Monard C, Rimmele T, Ronco C (2019) Extracorporeal blood purification therapies for sepsis. Blood Purif 47(Suppl 3):1–14
Ling RR, Ramanathan K, Poon WH, Tan CS, Brechot N, Brodie D, Combes A, MacLaren G (2021) Venoarterial extracorporeal membrane oxygenation as mechanical circulatory support in adult septic shock: a systematic review and meta-analysis with individual participant data meta-regression analysis. Crit Care 25:246
Funding
This is a non-funded article.
Author information
Authors and Affiliations
Contributions
GH, JB, EK and DDB conceived the manuscript; DA, JB, RC, MC, DDB, AD, LE, MNG, OH, GH, EK, CI, BL, XM, GOT, MO, MP, JR, BS, TWLS, JLT, AVB, JLV, FGZ drafted specific parts of the manuscript, critically revised and approved its final contents.
Corresponding author
Ethics declarations
Conflicts of interest
DA, JB, RC, DDB, AD, LE, MNG, OH, GH, EK, BL, XM, GOT, MO, MP, JR, TWLS, JLT, AVB, JLV, FGZ declare no conflicts of interest. BS is a consultant for and has received honoraria for giving lectures from Edwards Lifesciences Inc. (Irvine, CA, USA). BS is a consultant for and has received institutional restricted research grants and honoraria for giving lectures from Pulsion Medical Systems SE (Feldkirchen, Germany). BS has received institutional restricted research grants and honoraria for giving lectures from CNSystems Medizintechnik GmbH (Graz, Austria). BS is a consultant for and has received honoraria for giving lectures from Philips Medizin Systeme Böblingen GmbH (Böblingen, Germany). BS is a consultant for and has received honoraria for giving lectures from GE Healthcare (Chicago, IL, USA). BS is a consultant for Vygon (Aachen, Germany). BS is a consultant for and has received institutional restricted research grants from Retia Medical LLC. (Valhalla, NY, USA). BS was a consultant for and has received institutional restricted research grants from Tensys Medical Inc. (San Diego, CA, USA); FGZ has received grants for investigator-initiated trials from Bactiguard, Sweden, and Ionis Pharmaceuticals, USA, unrelated to the scope of this work. FGZ was the principal investigator of the BaSICS trial, which received logistics support from Baxter Hospitalar, Brazil; CI received educational grants from LaJolla Pharmaceuticals (LaJolla, Cal USA) and educational grants and speaker fees from Cytosorbents Europe (Berlin Germany). CI is Chief Scientific Officer and holds shares in Active Medical BV (Leiden, The Netherlands), a company that provides educational services, products and software related to clinical microcirculation using sublingual hand-held vital microscopes; AVB received a research grant from GSK. AVB received Fees from Air Liquide for being in an advisory board for conducting a clinical research; BL received grants from Novartis, Abiomed, Amomed, orion, Gettinge; MC performed consultancies for Edwards Lifesciences and Directed Systems;
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bakker, J., Kattan, E., Annane, D. et al. Current practice and evolving concepts in septic shock resuscitation. Intensive Care Med 48, 148–163 (2022). https://doi.org/10.1007/s00134-021-06595-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-021-06595-9