Abstract
Purpose
Nangibotide is a specific TREM-1 inhibitor that tempered deleterious host–pathogens interactions, restored vascular function, and improved survival, in animal septic shock models. This study evaluated the safety and pharmacokinetics of nangibotide and its effects on clinical and pharmacodynamic parameters in septic shock patients.
Methods
This was a multicenter randomized, double-blind, two-stage study. Patients received either continuous infusion of nangibotide (0.3, 1.0, or 3.0 mg/kg/h) or placebo. Treatment began < 24 h after shock onset and continued for up to 5 days. Safety primary outcomes were adverse events (AEs), whether serious or not, and death. Exploratory endpoints evaluated nangibotide effects on pharmacodynamics, organ function, and mortality, and were analyzed according to baseline sTREM-1 concentrations.
Results
Forty-nine patients were randomized. All treatment emergent AEs (TEAEs) were collected until Day 28. No significant differences were observed in TEAEs between treatment groups. No drug withdrawal linked to TEAE nor appearance of anti-drug antibodies were reported. Nangibotide pharmacokinetics appeared to be dose-proportional and clearance was dose-independent. Nangibotide did not significantly affect pharmacodynamic markers. Decrease in SOFA score LS mean change (± SE) from baseline to Day 5 in pooled nangibotide groups versus placebo was − 0.7 (± 0.85) in the randomized population and − 1.5 (± 1.12) in patients with high baseline plasma sTREM-1 concentrations (non-significant). This pattern was similar to organ support end points.
Conclusion
No significant increases in TEAEs were detected in nangibotide-treated patients versus placebo. These results encourage further evaluation of nangibotide and further exploration of plasma sTREM-1 concentrations as a predictive efficacy biomarker.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
By inhibiting a novel therapeutic target in septic shock patients, nangibotide modulates innate immune cell activation and restores vascular function, thus representing a potential therapeutic approach for septic shock. Further larger studies investigating the efficacy of nangibotide in the treatment of septic shock as well as the ability of sTREM-1 to predict nangibotide efficacy are needed. |
Introduction
Sepsis is a life-threatening condition caused by dysregulated host response to infection leading to organ failure. It accounts for an estimated 11 million deaths annually worldwide [1]. There are no approved septic shock-specific treatments, except angiotensin-II which has no true effect on sepsis outside vasopressor activity, and current interventions are focused on organ support and infection source control [2]. There is still a major unmet medical need for septic shock treatments that would help reduce the mortality of these patients.
The triggering receptor expressed on myeloid cells 1 (TREM-1) pathway is one of the most up-regulated pathways in critically ill patients [3, 4]. Initially described on myeloid cells [5], this transmembrane receptor is also expressed by other cell types including innate immune cells and activated endothelial cells [6,7,8,9]. Signaling mediated by TREM-1 synergizes with previously activated and specific Pattern Recognition Receptors (PRRs), including Toll-like receptors (TLRs) and/or NOD-like receptors, resulting in sustained amplification of the inflammatory response to infection independent of the pathogen, whether bacteria [4, 6, 7, 10,11,12,13,14], viruses [15, 16], or fungi [4, 17, 18]. This renders TREM-1 a universal innate immune amplifier. TREM-1 deletion by genetic modification as well as pharmacological inhibition blunts excessive inflammation while preserving the capacity for microbial control in various infectious models [19,20,21,22,23,24].
In septic shock, TREM-1 activation results in an overzealous and dysregulated host innate immune response to infection [4] associated with alterations in both endothelial cell integrity [9] and cardiac function [25].
TREM-1 also exists in a soluble form (sTREM-1) in the blood following cleavage of membrane-bound TREM-1 by metalloproteinases after TREM-1 receptor activation [26,27,28]. The presence of sTREM-1 in the circulation is an indicator of TREM-1 pathway activation and higher levels correlate with disease severity indicators, including Sequential/Sepsis-related Organ Failure Assessment (SOFA) score and 28-day mortality [29,30,31,32,33,34].
The TREM-1 investigational inhibitor nangibotide (LR12) is a 12-amino acid peptide which acts as a “ligand-trapping” molecule, selectively modulating the TREM-1-mediated inflammatory response amplification [25, 35]. In animal models of polymicrobial peritonitis septic shock, analogs of nangibotide restored a balanced inflammatory response, anti-microbial control, vascular function, and hemodynamic stability, which translated into organ protection and improved survival [9, 25, 35]. In the phase 1 study in healthy volunteers, nangibotide was well tolerated up to the highest dose tested (6 mg/kg/h) [36]. This phase 2a study assessed the safety, tolerability, pharmacokinetics, pharmacodynamics, and efficacy of nangibotide in patients with septic shock. Results were also analyzed according to sTREM-1 levels at baseline.
Methods
Study design
This was a multicenter, prospective, randomized, double-blind, two-stage, placebo-controlled phase 2a study conducted at 11 ICUs in four countries (Belgium, France, Spain, and The Netherlands) between July 2017 and June 2018 (clinicaltrials.gov: NCT03158948). Patients were randomized to receive either placebo or 0.3, 1, or 3 mg/kg/h of nangibotide (Online Resource, Fig. E1). Patients and clinical study site staff were blinded to study drug assignment. This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the appropriate Ethics Committees and competent authorities. Written informed consent was obtained from each participant, or authorized representative in case of incapacitated patients, prior to study enrollment.
Patients, randomization, and intervention
Eligible patients complied with the Sepsis-3 septic shock definition [37]. Eligibility criteria and prohibited treatments are provided in Table 1. Prohibited immunosuppressive drugs are listed in Table 2. A Clinical Coordinating Center confirmed patient eligibility (Online Resource, Section 1.2).
In Stage 1, nangibotide ascending doses (0.3, 1, and 3 mg/kg/h) were investigated in sequentially enrolled cohorts randomized 3:1 to nangibotide or placebo for a total of 12 patients. Escalation was monitored by a Data Safety Monitoring Board (DSMB) (Online Resource, Section 1.3). In Stage 2, patients were randomized 1:1:1:1 to placebo or nangibotide 0.3, 1, or 3 mg/kg/h using a parallel-group design with 12 patients per arm for a total of 48 patients. One additional patient was included in the 0.3 mg/kg/h arm before closing of the study. Full randomization methods are given in Online Resource, Section 1.4.
Nangibotide and placebo were administered as continuous intravenous infusions, as previously described [36]. Treatment was initiated within 24 h of septic shock onset. Patients received a 15-min 20 mg/kg/h loading dose of nangibotide or placebo followed by a maintenance dose of nangibotide or placebo. Treatment was administered until 12 (± 2) hours after vasopressor withdrawal with a maximum of 5 days. Day 0 corresponded to the start of study drug administration. All patients received standard of care according to the Surviving Sepsis Campaign recommendations, including fluid resuscitation and vasopressors, early antibiotics, and early infection source control [2]. Corticosteroid use for septic shock up to 300 mg/24 h of hydrocortisone was optional and left to investigator judgment.
Outcome assessments
Safety and tolerability were assessed by analyzing the frequency, intensity, and nature of treatment emergent adverse events (TEAEs) and the occurrence of death. TEAEs were defined as an event that first occurred or worsened in severity after baseline. TEAEs were collected until end of study (EoS; Day 28). The cause and relationship to the investigational product and whether the TEAE was reported as serious (SAE) was recorded. TEAEs considered as clinical events related to severe sepsis or sepsis complications were exempt from SAE reporting, unless the investigator deemed the event to be related to the study drug (Online Resource, Section 1.5, Table E1). The following parameters were measured as part of the safety assessment from Day 1 to Day 5 and at the EoS visit: vital signs, 12-lead ECG, hematology and biochemistry laboratory assessments, and International Normalized Ratio (INR). Immunogenicity was assessed by evaluation of anti-drug antibodies (ADA) on Days 0, 10 (French patients only), and 28.
Exploratory secondary endpoints included change from baseline in SOFA score at Days 1–5 and the number of vasopressor, invasive mechanical ventilation (IMV), and renal replacement therapy (RRT) free days alive from Day 0 to the EoS visit (Online Resource, Section 1.6). The proportion of patients free from organ support and alive at Day 28 and time to shock reversal (cessation of vasopressor support for 24 h) were also calculated. Other secondary clinical efficacy outcomes included all-cause and sepsis-related mortality at Days 5, 28, and 90. Relevant changes to patient functional and survival status were collected by phone at Day 90.
Additional exploratory secondary outcomes included pharmacokinetic and pharmacodynamic analyses. Plasma levels of nangibotide were assessed before, during (Days 0–5 or until End of Infusion [EoI]), and after EoI using a validated LS-HRMS assay [36]. Plasma sTREM-1 levels were measured retrospectively on Days 0–5 and 28 in a central laboratory using a commercially validated ELISA assay (Quantikine, RnD Systems, Minneapolis, USA). Circulating cytokines (TNFα, IL-6, IL-8, IL-10, CCL2, and IFNγ) and vascular endothelium activation markers (sCD62P, sCD62E, VEGFR-1, VCAM-1, Ang-1, and Ang-2) were analyzed on Days 0, 1, 3, 5, and 28 in a central laboratory using Luminex assays (Merck Millipore, Burlington, USA; Bio-Techne, Oxford, UK; RnD Systems, Minneapolis, USA). Analytical characteristics of methods used are described in the Online Resource (Section 1.7).
Clinical efficacy and pharmacodynamic endpoint analyses were further evaluated in two predefined subgroups: patients with low baseline plasma sTREM-1 concentrations (< median; referred to as “low sTREM-1”) and patients with high baseline plasma sTREM-1 concentrations (≥ median; referred to as “high sTREM-1”) after database lock.
Statistical analysis
A sample size of 48 was chosen in line with pilot study recommendations and according to the general rule of thumb to use 30 patients or more to estimate a parameter [38]. Forty-eight patients were considered enough to assess the most frequent TEAEs, and the study was not powered to assess efficacy endpoints. Fifty patients were randomized, one died before receiving the study drug, and an additional patient was included before the study was stopped, resulting in 13 patients in the 0.3 mg/kg/h dose arm. The study was unblinded at completion (Day 28), after database lock. Last observation carried forward (LOCF) was used for missing continuous pharmacodynamic and clinical efficacy endpoint values (Online Resource, Section 1.8 and Table E2) except for safety measures. Last available values were reported as Day 5/EoI. Statistical tests are described in the Online Resource (Section 1.9). Sensitivity analyses for organ support free days alive with death penalty of zero were conducted (Online Resource, Section 1.6). Pairwise comparisons versus placebo-based p values were not adjusted for multiplicity.
Pharmacokinetic parameters were estimated using non-compartmental analysis, as previously described [36]. Fold decrease from baseline in pharmacodynamic marker log2 values was calculated. The effect of nangibotide exposure on individual IL-6 concentrations measured from Days 1–5 was analyzed by nonlinear mixed-effects modeling (Online Resource, Section 1.10, Fig. E2). Analytical sets are described in the Online Resource, Section 1.11.
Results
Patients
Fifty patients were enrolled and randomized, and 49 patients were treated according to the protocol: 12 patients in Stage 1 and 37 patients in Stage 2. Each treatment group comprised 12 patients except the nangibotide 0.3 mg/kg/h dose group which included 13 patients. Eight (16%) patients died before Day 28. One additional patient died on Day 28 after the EoS visit was conducted on Day 27. Thirteen (26%) patients died before Day 90. All study discontinuations were due to death. A CONSORT diagram is presented in the Online Resource (Fig. E3).
Baseline parameters were well balanced between groups (Online Resource, Table E3). The overall median (IQR) baseline Acute Physiology and Chronic Health Evaluation (APACHE) II score was 24 (18.5 to 27.5), and the median (IQR) total SOFA score was 10 (8 to 12).
No significant differences were observed in treatment duration or duration of vasopressors before treatment administration (Online Resource, Tables E4&E5).
Primary endpoint
Safety and tolerability
No differences in the number of patients experiencing TEAEs or the number of TEAEs were observed between the study arms. No TEAEs led to treatment discontinuation. Overall, 234 TEAEs were observed in 46 (94%) patients. TEAE frequency (Table 3) and type (Online Resource, Table E6) were comparable between treatment groups. Atrial fibrillation occurred in one (8.3%) placebo-treated patient and five (38.5%), two (16.7%), and three (25.0%) patients in the 0.3, 1, and 3 mg/kg/h nangibotide groups, respectively.
The most frequent TEAEs (occurring in > 10% of patients) were anemia, atrial fibrillation, pleural effusion, and thrombocytopenia. Seven of the nine deaths before Day 28 were reported as TEAEs: two (17%) placebo-treated and five (14%) nangibotide-treated patients. Overall, 17 (35%) patients experienced 22 SAEs.
A table summarizing TEAEs reported as related to treatment before unblinding is presented in the Online Resource (Table E7). Two SAEs reported as related to treatment in the 1 mg/kg/h group were evaluated to be not formally linked to product administration by the DSMB.
There were no clinically relevant differences between treatment groups in vital signs, ECG or laboratory data. No patient had detectable ADAs. Mortality rates are reported in the Online Resource (Table E8).
Pharmacodynamics
Changes in median levels of sTREM-1, cytokines, and vascular endothelium activation markers from baseline to Day 1, Day 3, or Day 5/EoI were not significantly different between nangibotide-treated patients and placebo-treated patients (Fig. 1; Online Resource, Fig E4).
Change in sTREM-1 from baseline to Day 5/EoI. Due to the broad range of values obtained for pharmacodynamic markers, absolute values were log transformed and the difference versus baseline is expressed as log2 fold change, allowing for an increase in dynamic range presented in the graphs, e.g., halving is equal to a log2 fold change of − 1, a quartering is equal to a log2 fold change of − 2 and so on. Data are shown as median with interquartile range. p value by Mann–Whitney test
Pharmacokinetics and PK/PD modeling
Nangibotide pharmacokinetic parameters are reported in Table 4. Overall, nangibotide pharmacokinetics appeared to be dose proportional. Clearance was comparable in all nangibotide groups and thus was dose-independent. Similar patterns were observed in patients with or without RRT (Online Resource, Fig. E5).
An indirect response model [39] showed that nangibotide had a significant additional IL-6 inhibition effect compared to placebo when assessed according to the M3 methods but did not show any dose difference (Online Resource, Fig. E6) [40]. No other correlations were established with other cytokines or endothelial markers.
Exploratory secondary clinical efficacy endpoints
Overall population
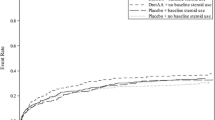
There were no statistically significant differences between nangibotide groups and placebo or significant dose effects for any of the secondary clinical efficacy endpoints in the overall population. Change in SOFA score at Day 5/EoI versus placebo (Fig. 2), vasopressor, IMV, and RRT free days alive as well as the proportion of patients alive and free of organ support at Day 28 are shown in Table 5. All deaths before Day 5 were septic shock-related, as adjudicated by an independent committee (Online Resource, Section 1.3). Results for change in SOFA score at other study time points and other exploratory clinical efficacy endpoints are shown in the Online Resource (Fig. E7; Table E9). No difference was seen in shock duration or patient functional and survival status at Day 90 between nangibotide dose groups and placebo (Online Resource, Table E10). Sensitivity analyses of organ support free days alive are reported in the Online Resource (Table E11).
Change in total SOFA score from baseline to Day 5/EoI. Data are shown as LS means and standard error for pooled nangibotide-treated arms (red) and placebo (blue), in all patients and in patients with high or low sTREM-1 levels at baseline (above or below the median value, respectively). Difference of nangibotide-treated patients versus placebo in SOFA change versus baseline value at Day 5/EoI is shown (ΔSOFA)
According to baseline plasma sTREM-1 concentrations
The overall baseline median (min, max) plasma sTREM-1 level was 433 (154, 1960) pg/mL. Twenty-four patients had low (< median) baseline plasma sTREM-1 levels (placebo, n = 7; nangibotide 0.3 mg/kg/h, n = 7; 1 mg/kg/h, n = 6; and 3 mg/kg/h, n = 4). Twenty-five patients had high (≥ median) baseline plasma sTREM-1 levels (placebo, n = 5; nangibotide 0.3 mg/kg/h, n = 6; 1 mg/kg/h, n = 6; and 3 mg/kg/h, n = 8). Baseline characteristics for patients with low and high sTREM-1 levels are shown in the Online Resource (Tables E12&E13). In nangibotide-treated patients, mean (± SD) baseline APACHE II score and total SOFA score were 26.5 (± 6.6) and 11.3 (± 2.9), respectively, in the high sTREM-1 subgroup and 20.5 (± 6) and 9.1 (± 2.7), respectively, in the low sTREM-1 subgroup. In the high sTREM-1 subgroup, mean (± SD) baseline total SOFA score was 12.4 (± 2.2) for placebo and 10.7 (± 3.8), 11.3 (± 3.5), and 11.1 (± 2.6) in the 0.3, 1, and 3 mg/kg/h nangibotide groups, respectively. Groups were well balanced for other patient characteristics. At Day 28, all-cause and septic shock-related overall trial mortality were 28 and 24%, respectively, in the high sTREM-1 subgroup and 8 and 4%, respectively, in the low sTREM-1 subgroup.
There were no statistically significant differences between nangibotide groups and placebo or significant dose effects for any of the secondary clinical efficacy endpoints in either high or low sTREM-1 patients (Figs. 1, 2; Table 6; Online Resource, Fig. E8&E9). At Day 5, the incidence of both all-cause and septic shock-related mortality was 24% (6/25) in the high sTREM-1 subgroup, whereas no patient had died in the low sTREM-1 subgroup. Mortality rates for low and high sTREM-1 patients are shown in the Online Resource (Table E8).
In the high sTREM-1 subgroup, 70% (14/20) and 40% (2/5) of patients were alive and free of medical support at Day 28 in the pooled nangibotide and placebo groups, respectively (Table 6).
Discussion
This is the first clinical study of a novel investigational drug, nangibotide, targeting TREM-1 in septic shock patients. No statistical differences were seen between nangibotide and placebo groups for safety and tolerability nor for studied exploratory biomarkers or clinical efficacy endpoints. Nangibotide pharmacokinetics appeared to be dose proportional, and its clearance appeared dose independent. No signs of immunogenicity were observed.
Some TEAEs (atrial fibrillation, arrhythmia, and thrombocytopenia) occurred more frequently in nangibotide-treated patients than placebo; however, these were not deemed to be treatment related, and none were reported as serious. These events are commonly linked to septic shock, and their etiology remains unclear. In particular, a decrease in thrombopoietic activity is associated with severe thrombocytopenia and mortality in sepsis [41]. These events were not observed in preclinical models [35, 42]. Nevertheless, specific attention should be paid to this type of event in future clinical studies.
In animal models, nangibotide decreases inflammatory cytokines and endothelial activation/injury markers [9, 25, 42]. In this study, there were no statistically significant differences in pharmacodynamic markers between placebo- and nangibotide-treated patients. The size of this study did not formally allow for detection of a pharmacodynamic signal. However, the results of an indirect response model showed a positive correlation between nangibotide concentration and a decrease in the IL-6 production rate (the higher the exposure to nangibotide, the greater the decrease in IL-6 production rate). In addition, the pattern of pharmacodynamic behavior in nangibotide-treated patients in terms of change versus baseline, in particular in high sTREM-1 patients for markers such as sTREM-1, IL-6, or Ang-2, constitutes an encouraging start to continue the exploration of these markers in larger clinical trials.
Pharmacokinetics of nangibotide-treated patients in this study were consistent with the nangibotide pharmacokinetics pattern previously observed in healthy volunteers, i.e., rapid, dose-independent clearance, and dose proportionality [36]. Half-life could not be calculated in this study due to limited sample size; however, a half-life of around 3 min has been previously described in humans [36] as well as in cynomolgous monkeys (unpublished data). Consistent with nangibotide pharmacokinetic behavior, patients who underwent hemofiltration displayed a very similar pharmacokinetics pattern to those without RRT. Further pharmacokinetic assessments are needed to better characterize the behavior of nangibotide in septic shock patients.
TREM-1 is an amplifier of TLR signaling known to directly promote deleterious host–pathogen interactions. By specifically inhibiting TREM-1 receptor activation, the mechanism of action of nangibotide is novel compared to previous septic shock therapeutic approaches [43,44,45]. Nangibotide has the potential to modulate endothelial dysregulation and regulate dysfunctional crosstalk between the immune system and the endothelium. Moreover, nangibotide may potentially prevent long-term sequelae, such as immunosuppression, by targeting initial immune dysregulation [46, 47]. Nangibotide has as a therapeutic target pathway that has not previously been addressed and that differs from cytokines. As nangibotide is an immune modulator, and not an immunosuppressor, it may better address the septic shock pathology as it does not interfere with appropriate immune responses mediated by TLRs, but modulates the loop of amplification maintained by TREM-1. In addition, TREM-1 is expressed by endothelial cells and nangibotide has been shown to modulate activation of the endothelium and to have vasoprotective effects in various models [9, 25]. We also believe that a precision medicine approach and continuing nangibotide development exploring sTREM-1 as potential predictive efficacy biomarker will enhance the chances of efficacy signal detection in line with recent observations [48, 49].
The optimal dose and length of treatment could not be deduced from this small study. An apparent inverse dose effect may be concluded from changes in SOFA score. However, differences in patient characteristics between the treatment arms could have accounted for these differences. In terms of treatment length, future clinical trials should explore TREM-1 pathway activation during septic shock to identify the optimal duration of treatment. sTREM-1 should also be explored as a monitoring marker for nangibotide use. We have used the median sTREM-1 concentration as a threshold to distinguish between patients belonging to high versus low sTREM-1 groups. However, this cutoff needs to be refined. The best fit yielding the greatest size effects for a relevant endpoint will be chosen for future clinical trials evaluating nangibotide efficacy. The effect of nangibotide in patients below this cutoff should be examined further before confirmatory trials prospectively selecting biomarker positive patients take place.
This study has several limitations. Exclusion of septic shock-related AEs from SAE reporting may have led to insufficient identification of true drug-related AEs. The study was not powered to draw conclusions on efficacy and pharmacodynamic endpoints. The exclusion of patients with APACHE II > 34, in which expected mortality is very high, may have introduced bias. Full pharmacokinetic characterization was not possible as the short half-life of nangibotide requires preanalytical complex processing of multiple samples in a short time period; this was not feasible in the clinical setting. Clinical and pharmacodynamic parameters were missing for patients interrupting treatment before Day 5, and missing data were replaced by LOCF (63% at Day 3 and 79% at Day 5). It must be mentioned that the PK/PD modeling with the M3 method had a warning concerning the covariance step which could not be solved, limiting the robustness of the results for the IL-6 model. We cannot exclude that the results observed could be confounded a posteriori with the difference in baseline measurement of some prognostic variables such as sTREM-1 or severity scores. The correlation of sTREM-1 levels at baseline with other measures of severity should be assessed in larger trials.
Conclusion
No safety concerns about nangibotide were raised in this small trial in septic shock patients. Larger studies are needed to investigate the effect of nangibotide and the potential role of sTREM-1 plasma concentrations as a predictive biomarker for patient selection.
Data availability
Sharing of de-identified patient data does not comply with the General Data Protection Regulation (GDPR) in the EU. Therefore, individual, de-identified participant data will currently not be shared. This clinical trial was designed and started before the regulation came into effect. The informed consent form that the participants signed for this study did not address the sharing of individual patient data. Therefore, the authors are currently not allowed to share such data.
References
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, Naghavi M (2020) Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. The Lancet 395(10219):200–211. https://doi.org/10.1016/S0140-6736(19)32989-7
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche J-D, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent J-L, Wiersinga WJ, Zimmerman JL, Dellinger RPJICM (2017) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43(3):304–377. https://doi.org/10.1007/s00134-017-4683-6
Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, López MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG (2011) A genomic storm in critically injured humans. J Exp Med 208(13):2581–2590. https://doi.org/10.1084/jem.20111354
Bouchon A, Facchetti F, Weigand MA, Colonna M (2001) TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 410(6832):1103–1107. https://doi.org/10.1038/35074114
Colonna M, Facchetti F (2003) TREM-1 (triggering receptor expressed on myeloid cells): a new player in acute inflammatory responses. J Infect Dis 187(Suppl 2):S397–S401. https://doi.org/10.1086/374754
Bouchon A, Dietrich J, Colonna M (2000) Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol 164(10):4991–4995. https://doi.org/10.4049/jimmunol.164.10.4991
Derive M, Massin F, Gibot S (2010) Triggering receptor expressed on myeloid cells-1 as a new therapeutic target during inflammatory diseases. Self Nonself 1(3):225–230. https://doi.org/10.4161/self.1.3.12891
Jolly L, Lemarie J, Carrasco K, Popovic B, Derive M, Boufenzer A, Gibot S (2017) Triggering receptor expressed on myeloid cells-1: a new player in platelet aggregation. Thromb Haemost 117(9):1772–1781. https://doi.org/10.1160/th17-03-0156
Jolly L, Carrasco K, Derive M, Lemarie J, Boufenzer A, Gibot S (2018) Targeted endothelial gene deletion of triggering receptor expressed on myeloid cells-1 protects mice during septic shock. Cardiovasc Res 114(6):907–918. https://doi.org/10.1093/cvr/cvy018
Gibot S, Massin F, Le Renard P, Bene MC, Faure GC, Bollaert PE, Levy B (2005) Surface and soluble triggering receptor expressed on myeloid cells-1: expression patterns in murine sepsis. Crit Care Med 33(8):1787–1793. https://doi.org/10.1097/01.ccm.0000172614.36571.75
Knapp S, Gibot S, de Vos A, Versteeg HH, Colonna M, van der Poll T (2004) Cutting edge: expression patterns of surface and soluble triggering receptor expressed on myeloid cells-1 in human endotoxemia. J Immunol 173(12):7131–7134. https://doi.org/10.4049/jimmunol.173.12.7131
Bleharski JR, Kiessler V, Buonsanti C, Sieling PA, Stenger S, Colonna M, Modlin RL (2003) A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J Immunol 170(7):3812–3818. https://doi.org/10.4049/jimmunol.170.7.3812
Netea MG, Azam T, Ferwerda G, Girardin SE, Kim SH, Dinarello CA (2006) Triggering receptor expressed on myeloid cells-1 (TREM-1) amplifies the signals induced by the NACHT-LRR (NLR) pattern recognition receptors. J Leukoc Biol 80(6):1454–1461. https://doi.org/10.1189/jlb.1205758
Arts RJ, Joosten LA, van der Meer JW, Netea MG (2013) TREM-1: intracellular signaling pathways and interaction with pattern recognition receptors. J Leukoc Biol 93(2):209–215. https://doi.org/10.1189/jlb.0312145
Roe K, Gibot S, Verma S (2014) Triggering receptor expressed on myeloid cells-1 (TREM-1): a new player in antiviral immunity? Front Microbiol 5:627. https://doi.org/10.3389/fmicb.2014.00627
Ivan FX, Rajapakse JC, Welsch RE, Rozen SG, Narasaraju T, Xiong GM, Engelward BP, Chow VT (2012) Differential pulmonary transcriptomic profiles in murine lungs infected with low and highly virulent influenza H3N2 viruses reveal dysregulation of TREM1 signaling, cytokines, and chemokines. Funct Integr Genomics 12(1):105–117. https://doi.org/10.1007/s10142-011-0247-y
Smith NL, Denning DW (2011) Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur Respir J 37(4):865–872. https://doi.org/10.1183/09031936.00054810
Huang W, Ling S, Jia X, Lin B, Huang X, Zhong J, Li W, Lin X, Sun Y, Yuan J (2014) Tacrolimus (FK506) suppresses TREM-1 expression at an early but not at a late stage in a murine model of fungal keratitis. PLoS ONE 9(12):e114386. https://doi.org/10.1371/journal.pone.0114386
Mohamadzadeh M, Coberley SS, Olinger GG, Kalina WV, Ruthel G, Fuller CL, Swenson DL, Pratt WD, Kuhns DB, Schmaljohn AL (2006) Activation of triggering receptor expressed on myeloid cells-1 on human neutrophils by marburg and ebola viruses. J Virol 80(14):7235–7244. https://doi.org/10.1128/jvi.00543-06
Ruiz-Pacheco JA, Vivanco-Cid H, Izaguirre-Hernandez IY, Estrada-Garcia I, Arriaga-Pizano L, Chacon-Salinas R, Fonseca-Coronado S, Vaughan G, Tovar KR, Rivera-Osorio MP, Escobar-Gutierrez A (2014) TREM-1 modulation during early stages of dengue virus infection. Immunol Lett 158(1–2):183–188. https://doi.org/10.1016/j.imlet.2014.01.003
Hyun J, McMahon RS, Lang AL, Edwards JS, Badilla AD, Greene ME, Stone GW, Pallikkuth S, Stevenson M, Dykxhoorn DM, Kottilil S, Pahwa S, Thomas E (2019) HIV and HCV augments inflammatory responses through increased TREM-1 expression and signaling in Kupffer and Myeloid cells. PLoS Pathog 15(7):e1007883. https://doi.org/10.1371/journal.ppat.1007883
Kozik J-H, Trautmann T, Carambia A, Preti M, Lütgehetmann M, Krech T, Wiegard C, Heeren J, Herkel J (2016) Attenuated viral hepatitis in Trem1 −/− mice is associated with reduced inflammatory activity of neutrophils. Sci Rep 6(1):28556. https://doi.org/10.1038/srep28556
Kumar M, Belcaid M, Nerurkar VR (2016) Identification of host genes leading to West Nile virus encephalitis in mice brain using RNA-seq analysis. Sci Rep 6:26350. https://doi.org/10.1038/srep26350
Weber B, Schuster S, Zysset D, Rihs S, Dickgreber N, Schurch C, Riether C, Siegrist M, Schneider C, Pawelski H, Gurzeler U, Ziltener P, Genitsch V, Tacchini-Cottier F, Ochsenbein A, Hofstetter W, Kopf M, Kaufmann T, Oxenius A, Reith W, Saurer L, Mueller C (2014) TREM-1 deficiency can attenuate disease severity without affecting pathogen clearance. PLoS Pathog 10(1):e1003900. https://doi.org/10.1371/journal.ppat.1003900
Derive M, Boufenzer A, Bouazza Y, Groubatch F, Alauzet C, Barraud D, Lozniewski A, Leroy P, Tran N, Gibot S (2013) Effects of a TREM-like transcript 1-derived peptide during hypodynamic septic shock in pigs. Shock 39(2):176–182. https://doi.org/10.1097/SHK.0b013e31827bcdfb
Gomez-Pina V, Soares-Schanoski A, Rodriguez-Rojas A, Del Fresno C, Garcia F, Vallejo-Cremades MT, Fernandez-Ruiz I, Arnalich F, Fuentes-Prior P, Lopez-Collazo E (2007) Metalloproteinases shed TREM-1 ectodomain from lipopolysaccharide-stimulated human monocytes. J Immunol 179(6):4065–4073. https://doi.org/10.4049/jimmunol.179.6.4065
Carrasco K, Boufenzer A, Jolly L, Le Cordier H, Wang G, Heck AJR, Cerwenka A, Vinolo E, Nazabal A, Kriznik A, Launay P, Gibot S, Derive M (2018) TREM-1 multimerization is essential for its activation on monocytes and neutrophils. Cell Mol Immunol 16(5):460–472. https://doi.org/10.1038/s41423-018-0003-5
Gibot S, Kolopp-Sarda MN, Bene MC, Bollaert PE, Lozniewski A, Mory F, Levy B, Faure GC (2004) A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J Exp Med 200(11):1419–1426. https://doi.org/10.1084/jem.20040708
Su L, Liu D, Chai W, Liu D, Long Y (2016) Role of sTREM-1 in predicting mortality of infection: a systematic review and meta-analysis. BMJ Open 6(5):e010314. https://doi.org/10.1136/bmjopen-2015-010314
Charles PE, Noel R, Massin F, Guy J, Bollaert PE, Quenot JP, Gibot S (2016) Significance of soluble triggering receptor expressed on myeloid cells-1 elevation in patients admitted to the intensive care unit with sepsis. BMC Infect Dis 16(1):559. https://doi.org/10.1186/s12879-016-1893-4
Gibot S, Cravoisy A, Levy B, Bene M-C, Faure G, Bollaert P-E (2004) Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med 350(5):451–458. https://doi.org/10.1056/NEJMoa031544
Rios-Toro JJ, Marquez-Coello M, Garcia-Alvarez JM, Martin-Aspas A, Rivera-Fernandez R, Saez de Benito A, Giron-Gonzalez JA (2017) Soluble membrane receptors, interleukin 6, procalcitonin and C reactive protein as prognostic markers in patients with severe sepsis and septic shock. PLoS ONE 12(4):e0175254. https://doi.org/10.1371/journal.pone.0175254
Zhang J, She D, Feng D, Jia Y, Xie L (2011) Dynamic changes of serum soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) reflect sepsis severity and can predict prognosis: a prospective study. BMC Infect Dis 11:53. https://doi.org/10.1186/1471-2334-11-53
Jedynak M, Siemiatkowski A, Mroczko B, Groblewska M, Milewski R, Szmitkowski M (2018) Soluble TREM-1 serum level can early predict mortality of patients with sepsis, severe sepsis and septic shock. Arch Immunol Ther Exp (Warsz) 66(4):299–306. https://doi.org/10.1007/s00005-017-0499-x
Derive M, Bouazza Y, Sennoun N, Marchionni S, Quigley L, Washington V, Massin F, Max JP, Ford J, Alauzet C, Levy B, McVicar DW, Gibot S (2012) Soluble TREM-like transcript-1 regulates leukocyte activation and controls microbial sepsis. J Immunol 188(11):5585–5592. https://doi.org/10.4049/jimmunol.1102674
Cuvier V, Lorch U, Witte S, Olivier A, Gibot S, Delor I, Garaud JJ, Derive M, Salcedo-Magguilli M (2018) A first-in-man safety and pharmacokinetics study of nangibotide, a new modulator of innate immune response through TREM-1 receptor inhibition. Br J Clin Pharmacol 84(10):2270–2279. https://doi.org/10.1111/bcp.13668
Singer M, Deutschman CS, Seymour C et al (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315(8):801–810. https://doi.org/10.1001/jama.2016.0287
Lancaster GA, Dodd S, Williamson PR (2004) Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 10(2):307–312. https://doi.org/10.1111/j.2002.384.doc.x
Dayneka NL, Garg V, Jusko WJ (1993) Comparison of four basic models of indirect pharmacodynamic responses. J Pharmacokinet Biopharm 21(4):457–478. https://doi.org/10.1007/bf01061691
Bergstrand M, Karlsson MO (2009) Handling data below the limit of quantification in mixed effect models. AAPS J 11(2):371–380. https://doi.org/10.1208/s12248-009-9112-5
Koyama K, Katayama S, Muronoi T, Tonai K, Goto Y, Koinuma T, Shima J, Nunomiya S (2018) Time course of immature platelet count and its relation to thrombocytopenia and mortality in patients with sepsis. PLoS ONE 13(1):e0192064. https://doi.org/10.1371/journal.pone.0192064
Derive M, Boufenzer A, Gibot S (2014) Attenuation of responses to endotoxin by the triggering receptor expressed on myeloid cells-1 inhibitor LR12 in nonhuman primate. Anesthesiology 120(4):935–942. https://doi.org/10.1097/aln.0000000000000078
Opal SM, Laterre PF, Francois B, LaRosa SP, Angus DC, Mira JP, Wittebole X, Dugernier T, Perrotin D, Tidswell M, Jauregui L, Krell K, Pachl J, Takahashi T, Peckelsen C, Cordasco E, Chang CS, Oeyen S, Aikawa N, Maruyama T, Schein R, Kalil AC, Van Nuffelen M, Lynn M, Rossignol DP, Gogate J, Roberts MB, Wheeler JL, Vincent JL (2013) Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA 309(11):1154–1162. https://doi.org/10.1001/jama.2013.2194
Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, Gardlund B, Marshall JC, Rhodes A, Artigas A, Payen D, Tenhunen J, Al-Khalidi HR, Thompson V, Janes J, Macias WL, Vangerow B, Williams MD (2012) Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med 366(22):2055–2064. https://doi.org/10.1056/NEJMoa1202290
Vincent JL, Francois B, Zabolotskikh I, Daga MK, Lascarrou JB, Kirov MY, Pettila V, Wittebole X, Meziani F, Mercier E, Lobo SM, Barie PS, Crowther M, Esmon CT, Fareed J, Gando S, Gorelick KJ, Levi M, Mira JP, Opal SM, Parrillo J, Russell JA, Saito H, Tsuruta K, Sakai T, Fineberg D (2019) Effect of a recombinant human soluble thrombomodulin on mortality in patients with sepsis-associated coagulopathy: the SCARLET randomized clinical trial. JAMA 321(20):1993–2002. https://doi.org/10.1001/jama.2019.5358
Francois B, Jeannet R, Daix T, Walton AH, Shotwell MS, Unsinger J, Monneret G, Rimmele T, Blood T, Morre M, Gregoire A, Mayo GA, Blood J, Durum SK, Sherwood ER, Hotchkiss RS (2018) Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial. JCI Insight 3(5):98960. https://doi.org/10.1172/jci.insight.98960
Patil NK, Bohannon JK, Sherwood ER (2016) Immunotherapy: A promising approach to reverse sepsis-induced immunosuppression. Pharmacol Res 111:688–702. https://doi.org/10.1016/j.phrs.2016.07.019
Antcliffe DB, Burnham KL, Al-Beidh F, Santhakumaran S, Brett SJ, Hinds CJ, Ashby D, Knight JC, Gordon AC (2019) Transcriptomic signatures in sepsis and a differential response to steroids from the VANISH randomized trial. Am J Respir Crit Care Med 199(8):980–986. https://doi.org/10.1164/rccm.201807-1419oc
Seymour CW, Kennedy JN, Wang S, Chang C-CH, Elliott CF, Xu Z, Berry S, Clermont G, Cooper G, Gomez H, Huang DT, Kellum JA, Mi Q, Opal SM, Talisa V, van der Poll T, Visweswaran S, Vodovotz Y, Weiss JC, Yealy DM, Yende S, Angus DC (2019) Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA 321(20):2003–2017. https://doi.org/10.1001/jama.2019.5791
Acknowledgements
We thank Simon Lambden and Jean-Marie Grouin for critical review of this manuscript as well as the data monitoring and data adjudication committee members, Professors Michel Wolff and Jean Chastre. Support of PK NCA calculation and modeling was performed by Calvagone and MnS. Support for third-party writing assistance for this article, furnished by Megan Christian, was provided by Prism Ideas and funded by Inotrem SA.
Funding
This study, and editorial support for the preparation of this manuscript, was funded by Inotrem SA.
Author information
Authors and Affiliations
Contributions
JJG, SW, MSM, BF, and PFL conceived and designed the study. BF was the Principal investigator. BF, XW, RF, JPM, TD, SG, PP, MS, and PFL collected data. MSM and FV developed analysis tools. MSM, FV, BF, PFL, JJG, SG, MD, VC, and AO analyzed the data. BF, SG, MSM, and PFL wrote the manuscript. All authors reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
BF reports personal fees from Inotrem during the conduct of the study, and personal fees from Biomérieux, Aridis, Ashai-Kasai, Polyphor, AM-Pharma, and Ferring outside the submitted work. XW reports fees from Inotrem during the conduct of the study, and fees from AKPA and Ferring outside the submitted work. RF reports personal fees from MSD, Pfizer, Shionogi, Grifols, Toray, and BD outside the submitted work. PFL reports personal fees from Inotrem outside the submitted work. FRV reports personal fees from Inotrem during the conduct of the study. PP reports personal fees from Inotrem during the conduct of the study, and fees from AM-Pharma, EBI, and Ferring outside the submitted work. SW has acted as a consultant to Inotrem. MD and SG hold patent EP2011055519, licensed to Inotrem. MD, AO, VC, JJG, and MSM are employees of Inotrem. JPM, TD, and MS have nothing to disclose.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval of the study protocol and all amendments was granted by the following Ethics Committees: France: Comité de Protection des Personnes Ile de France (initial approval 13th September 2017; 2017-04-02 MS1 RIPH 1°). The Netherlands: Radboud University Medical Centre, Commissie Mensgebonden Onderzoek Regio Arnhem-Nijmegen (initial approval 19th July 2017; 2017-3326), Belgium: Cliniques Universitaires Saint Luc, Comité d’Éthique Hospitalo-Facultaire (initial approval 27th April 2017; 2017/15MAR138) Spain: Hospital Clinico San Carlos, Comité de Ética de la Investigación con Medicamentos (initial approval 31st May 2017; 17/162-R_M).
Informed consent
Written informed consent was obtained from each participant, or authorized representative in case of incapacitated patients, prior to study enrollment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
François, B., Wittebole, X., Ferrer, R. et al. Nangibotide in patients with septic shock: a Phase 2a randomized controlled clinical trial. Intensive Care Med 46, 1425–1437 (2020). https://doi.org/10.1007/s00134-020-06109-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-020-06109-z