Abstract
Purpose
Weaning failure from mechanical ventilation may be due to lung de-recruitment or weaning-induced pulmonary oedema (WIPO). Both can be diagnosed by lung ultrasound (LUS) and transthoracic echocardiography (TTE), respectively. We conducted a prospective observational study, combining TTE and LUS, to determine if LUS alone may identify elderly patients at high risk of weaning or extubation failure.
Methods
Before and at the end of spontaneous breathing trials (SBT) in 40 elderly patients, we prospectively performed LUS and TTE. Extubation was decided by an independent operator. LUS included global and anterolateral LUS score. TTE included measurement of E/A and E/Ea ratios to determine LV filling pressures. SBT LUS scores for prediction of weaning outcome and for the diagnosis of WIPO were studied.
Results
Weaning or extubation failure was observed in 45% (95% CI 28–61) of patients. ROC analysis for ability of global SBT LUS to predict weaning/extubation failure and extubation failure found AUC of 0.80 and 0.81, respectively. AUC for anterolateral SBT LUS to predict weaning/extubation failure and extubation failure was 0.79 and 0.81, respectively. Increased LV filling pressure during SBT was observed without increase of anterolateral LUS score. Inversely, increase of anterolateral LUS was observed without increased filling pressure and was associated with extubation failure. Global and anterolateral SBT LUS were not correlated to E/Ea.
Conclusion
In elderly patients, global and anterolateral LUS scores were associated with weaning and extubation failures while echocardiographic indices of filling pressures were not.
Clinical trial number and registry URL
ClinicalTrials.gov No. NCT03261440.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In elderly patients with high risk of weaning or extubation failure, lung ultrasound at end of SBT can aid in predicting weaning outcome. |
Introduction
The weaning process represents 40–50% of total duration of mechanical ventilation. Whatever its primary cause, the switch from mechanical ventilation (MV) to a spontaneous breath trial (SBT) induces significant changes in lung aeration [1]. Lung ultrasound (LUS) is a reliable tool to detect lung aeration and can be used to reliably predict extubation failure [2]. Since failure in weaning process is associated with poor outcome [3], a reliable prediction of extubation failure and the identification of its underlying mechanism may be of importance on prognosis. For example, diastolic dysfunction and the subsequent weaning-induced pulmonary oedema (WIPO) could be effectively treated by fluid removal or vasodilators [4]. Pulmonary oedema results in increased extra-vascular lung water (EVLW) that extends to lung periphery and is detected by LUS as vertical ultrasound artefacts called B-lines. The B-line number is correlated to pulmonary wedge pressure [5]. Variation of this number is correlated to variation of EVLW during haemodialysis [6] or stress test [7]. Thus B-line assessment to evaluate pulmonary oedema during heart failure is now recommended [8].
During a spontaneous breathing trial (SBT), a prevailing A-line pattern changing to a diffuse B-line pattern is suggestive of cardiogenic oedema as the cause of weaning failure [9]. Recently, a number of B-lines ≥ 6 on four anterior points during SBT has been proposed as diagnostic test for WIPO [10]. Although the diffuse bilateral B-lines indicate interstitial syndrome and increased lung density [1], it is not specific for cardiogenic pulmonary oedema, given that loss of aeration or de-recruitment can also create this LUS finding.
Transthoracic echocardiography (TTE) can be used to estimate left ventricular filling pressure [11]: if performed before and during a spontaneous breathing trial (SBT), an increase in pre-load pressure is identified by increase in E/A and in E/E′ [12].
We hypothesized that the addition of echocardiography to LUS in selected elderly critically ill patients with isolated diastolic left ventricle (LV) dysfunction could help identify the risk of weaning failure as well as understand the cause. So, we conducted a prospective observational study to estimate the value of LUS alone, TTE alone, and LUS and TTE in combination to 1/ to identify elderly patients at high risk of weaning failure from MV, and 2/ to determine if LUS alone could identify patients at risk of weaning failure.
Methods
This prospective, observational study was conducted in four teaching hospital ICUs (CHU Dijon, France, St. Joseph Hospital, Paris, France, Fondazione IRCCS Policlinico S. Matteo, Pavia, Italy and King Abdulaziz Medical City, National Guard Health Affairs, Riyadh, Saudi Arabia). This study was approved by local Institutional Ethics Committees and registered with ClinicalTrials.gov No. NCT03261440. Ultrasound lung and cardiac examination were a part of a routine diagnostic strategy aimed at optimizing respiratory and haemodynamic status of the patients. So, this observational study was classified as a study of standard care and, therefore, the need for informed consent was waived. Oral information was provided to the patient or relatives. No patient or family member expressed concern about participation.
Inclusion criteria: patients aged > 65 years, LV ejection fraction > 50% at enrolment on the apical four-chamber view, mechanically ventilated for more than 48 h and eligible for their first SBT according to the attending physician’s judgement that the underlying disease which led to intubation had sufficiently resolved.
Exclusion criteria: tracheostomy, known neuromuscular pathologies, ejection fraction < 50% at enrolment, significant COPD history, previous SBT failure.
Ultrasound examination
All ultrasound examinations were conducted using a CX-50 Philips or Vivid-iTM GE Healthcare and included both a lung and cardiac examination.
Lung ultrasound: A comprehensive scan was taken in six regions for each lung (superior and inferior areas in the anterior, lateral, and posterior fields using anterior and posterior axillary lines as landmarks). Four ultrasound aeration patterns were defined in each region [13]: (1) normal aeration (N—score 0): presence of lung sliding with A lines or fewer than two isolated B lines; (2) moderate loss of lung aeration: multiple, well-defined B lines (B1 lines—score 1); (3) severe loss of lung aeration: multiple coalescent B lines (B2 lines—score 3); and (4) lung consolidation (C—score 3), presence of a tissue-like pattern. For a given region of interest, points were allocated according to the worst ultrasound pattern observed. Global, anterior and antero-lateral LUS scores were calculated as the sum of all, anterior and antero-lateral regions ranging, therefore, 0–36, 0–12 and 0–24, respectively.
Cardiac ultrasound: All the examinations were made on the apical 4-chamber view. Presence of a left ventricular (LV) systolic dysfunction was assessed by eye-ball evaluation (ejection fraction ≤ 50% with LV dilatation). The transmitral flow was recorded by pulsed Doppler with the sample volume placed at the mitral valve tips. The mitral inflow was analysed for peak velocity of early (E) and late (A) filling. Velocities of the mitral annulus were recorded using tissue Doppler imaging (TDI) program with a 5-mm sample volume placed at the lateral corner of the mitral annulus [11]. The early diastolic (Ea) velocity of mitral annular displacement was measured from the TDI recording.
Definitions
Filling pressures: LV filling pressure can be evaluated using Doppler study of mitral flow and Doppler tissue imaging [11].
Low filling pressures or impaired relaxation patterns were defined by the presence of E/A ≤ 1 and E/Ea ≤ 13 [11]. High filling pressures or pseudonormal patterns were defined by E/A > 1 and E/Ea > 13 [11]. High filling pressures with restrictive patterns were defined by a E/A > 2.
Weaning-induced pulmonary oedema was defined by. an antero-lateral LUS score ≥ 5 with an increase in the LV filling pressure during a SBT. Derecruitment was defined by an increase in LUS scores without an increase in LV filling pressures during the SBT.
Weaning outcome: Weaning failure was defined by the failure of SBT [14]. Extubation failure was defined by a need for reintubation, non-invasive ventilation or death within 48 h after extubation.
Protocol
The SBT was performed through a T-tube as previously described or under mechanical ventilation with PEEP ≤ 5 cmH2O and pressure support of ≤ 7 cmH2O. The type of SBT performed was chosen by the physician in charge of the patient independently of the protocol. LUS examination and mitral Doppler study were performed prior to SBT under mechanical ventilation and at the end of SBT. The decision to stop SBT or to extubate was made by the physician in charge independently of the investigators.
Statistical analysis
In this interim analysis study, the estimated sample size to distinguish failing and non-failing patients on the basis of lung ultrasound score [2] [17 ± 3 vs. 13 ± 3], expecting a 30% failure rate and a 10% dropout, with alpha error 0.05 and power 0.95, was 38. So, we included 40 patients. Results are expressed as median (interquartile range) for quantitative variables and number and percentage for categorical ones. Two comparisons were performed: (1) patients successfully extubated (weaning success) vs. patients with weaning failure or extubation failure and (2) patients with weaning success vs. patients with extubation failure. Unpaired Wilcoxon/Mann–Whitney U-test for numerical data and the Fisher’s exact test for categorical ones were used. ROC curves analysis was performed to study ability of LUS score, anterolateral LUS score, E/A at end of SBT to predict weaning failure, and/or extubation failure. Sensitivity specificity, positive/negative predictive values, and positive/negative likelihood were also calculated. Cut off points for multivariate analysis were obtained by Youden index. To evaluate the association between endpoint variable (development of extubation/weaning failure) and ultrasound features, odds ratios (ORs) and the corresponding 95% confidence interval (CI) were calculated; a logistic regression model was carried out to identify the mutually adjusted effect among endpoints and the independent variables chosen on the basis of the statistical significance (univariate analysis, p ≤ 0.05) and of the clinical judgment; age and sex were used as adjusting variables. P-value ≤ 0.05 was considered significant (two-sided). All the analyses were conducted with STATA/SE for Macintosh, version 14.2.
Results
Patients
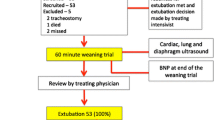
Flowchart of the study is given in Fig. 1. 40 patients were included (consecutively in each unit) in the study: eight patients were determined to have weaning failure and therefore remained intubated and 32 patients were extubated. Among them, ten patients presented an extubation failure and the remaining 22 were successfully weaned. Clinical characteristics are given in Tables 1 and 2. Patients’ median age was 72 [67–78] years, mechanically ventilated for a median duration of 8.5 [6.0–15.5] days before the first weaning attempt, and with significant cardiac comorbidity (20.0% of patients were admitted after cardiac surgery or for cardiogenic pulmonary oedema). Consequently, a high incidence of 45% (IC 95%: 28–61) of both weaning + extubation failure was observed. No significant differences were found between groups for clinical characteristics.
LUS for predicting weaning or extubation failure
No significant difference was found between groups in LUS score prior to SBT (Tables 3 and 4). Significant differences in all LUS scores were found between failure groups vs. success group at the end of SBT (Tables 3 and 4). ROC analysis for ability of SBT LUS scores to predict weaning/extubation failure and extubation failure are given in Fig. 2a, b, respectively.
Cardiovascular abnormalities before and during spontaneous breathing trial
No significant difference was found between groups in echocardiographic parameters either during or prior to SBT. ROC analysis for E/A and E/Ea to predict weaning/extubation failure and extubation failure are given in Fig. 3a, b, respectively. Mitral flow pattern during MV and at end of SBT (i.e. degree of diastolic dysfunction: impaired relaxation, pseudonormal and restrictive patterns) were not significantly different between groups (Tables 3 and 4). A mitral pattern variation ≥ 1 step was observed overall in 15.0% of patients but was not associated with failure (Tables 3 and 4).
LUS and mitral flow during weaning process and WIPO
At the end of the SBT, an antero-lateral LUS score ≥ 5 was found in 30 patients and in 21 this was associated with high filling pressure. An anterolateral LUS score ≥ 5 was associated with high prevalence of weaning or extubation failure (56.7%), independently from LV filling pressure: in patients with anterolateral LUS scores ≥ 5 and high filling pressure, extubation or weaning failure had a similar prevalence compared to patients with antero-lateral LUS ≥5 and low LV filling pressure (57.1% vs. 55.6%; p = 1.0000). Among patients with antero-lateral LUS scores ≥ 5 and high LV filling pressure, only three could be classified as WIPO, i.e. an increase of LV pressure was induced by the switch from MV to SBT (Fig. 4).
Moreover, there was no correlation between LUS scores and diastolic pattern: some patients with increased LV pre-load, as measured by E/Ea > 13, had low LUS scores, while other patients with normal LV-preload had high LUS scores (Fig. 5a, b).
In multivariate regression analysis, only global and anterolateral LUS scores at the end of the SBT were independent risk factors for weaning and / or extubation failure (Table 5).
Discussion
This study found that in elderly patients, only increased LUS (global or anterolateral) score was an independent risk factor for weaning or extubation failures, while increased LV filling pressure measured by echocardiography during SBT was not associated with weaning or extubation failures. Our results show that increases in LV filling pressure may occur without inducing pulmonary oedema. Conversely, during the weaning process, antero-lateral LUS score may occur without an increase in LV filling pressure. LUS identifies a lung loss of aeration induced by both WIPO and other mechanisms, such as derecruitment; therefore, an increase in LUS scores was not specific for WIPO but instead accurately identified the patients at risk of weaning/extubation failure.
SBT is associated with changes in aeration. If significant, these changes in lung aeration can be detected using LUS [15, 16]. The best way to semi-quantify loss of aeration at the bedside is to use a LUS rating system [2, 13, 17, 18]. In the context of weaning, the LUS score can help distinguish patients at high and low risk of post-extubation distress. In patients passing a 1-hour SBT, the LUS score is predictive of extubation success or failure: in patients successfully extubated, overall lung aeration does not significantly change during the SBT, whereas in patients with extubation failure, lung aeration decreases during the SBT. After a successful SBT, a score > 17 was highly predictive of extubation failure, while a score < 13 was highly predictive of weaning success in an unselected ICU population [2]. We obtained very similar results in elderly patients at the first attempt to wean from mechanical ventilation.
LUS is simple and easy to learn [19]. The change from dominant A-lines to diffuse B-lines during an SBT is easy to diagnose with LUS for the majority of ICU physicians and corresponds to an increase in lung tissue density in the explored region. LUS score predicts extubation failure better than other ultrasound techniques presumably because LUS detects any increase in tissue density, independently from its aetiology. The decrease in pulmonary aeration measured by LUS score is the final common pathway of different mechanisms inducing weaning or extubation failure, namely cardiogenic pulmonary oedema and derecruitment. Hence, LUS could be a key tool in the ICU during weaning process.
Switching a patient from a controlled ventilation mode to a SBT is a stress test for the ICU patient and can induce cardiogenic pulmonary oedema in cases of volume overload as well as LV systolic or diastolic dysfunction. This can be diagnosed through advanced cardiac echography skills and Doppler study of mitral flow and Doppler tissue of mitral annulus [11, 20]. Moreover, cardiac echography may also be used also to diagnose which cardiogenic cause is responsible for this failure [9]. As expected, in our study including elderly patients, when the pre-test probability of cardiac weaning failure is high, cardiac echography has limited utility [20]. When the pre-test probability is low or intermediate, as in an unselected population of critically ill patients, cardiac ultrasound might be more useful in predicting weaning failure [21,22,23].
LUS may help in detecting WIPO. LUS pattern of cardiogenic pulmonary oedema is characterized by the presence of multiple B-lines in all examined regions [24] since alveolar flooding is homogeneously distributed. Simple sum of B-lines yields a score correlated to the extent of extravascular lung water (EVLW) and was correlated to surrogate markers of pulmonary oedema as EVLW measured by transpulmonary thermodilution or pulmonary wedge pressure [5, 7]. Variation of EVLW during hemodialysis [6] or stress test [7] also correlates with changes in the number of B lines, and their use in the assessment of pulmonary oedema during heart failure is now recommended [8]. Recently, a number of B-lines ≥ 6 on 4 anterior points during SBT has been proposed as a diagnostic test for WIPO [10].
But, as above, B-lines alone are not enough to affirm WIPO. In fact, loss of lung tissue aeration associated with B-lines is not specific of cardiogenic pulmonary oedema, but it can be alternatively explained by lung derecruitment during SBT. Moreover, our findings suggest that the combination of B-lines and high filling pressure are also not specific for WIPO. During an increase in left atrial pressure or in hypervolemia, there is an increase in pulmonary venous pressure and a more homogeneous redistribution of perfusion to the apices by distension and recruitment of capillaries, and an increase of the lymphatic flow in parallel. All these mechanisms allow limiting the increase in capillary pressure and oedema formation [25, 26]. This was observed in heart failure patients where haemodynamic congestion (i.e. an increase of filling pressure) is not always associated with clinical congestion [8] even in cases of severe haemodynamic congestion [27, 28]. We observed similar findings in a population of elderly patients undergoing their first SBT: high filling pressures were not synonymous with cardiogenic pulmonary edema in this setting.
The combination of lung ultrasound with other ultrasound techniques in the assessment of the patients to be weaned has been suggested, not only to identify the frailest patients early, but also to understand the underlying mechanism, since the main causes of weaning failure can be evidenced by ultrasound (i.e. unresolved lung disease, diaphragm dysfunction and cardiac failure) [9, 29]. A combined cardiac, lung and diaphragm ultrasound approach may offer a more comprehensive understanding of patient’s status [20] and can potentially fill the gap of single-organ assessment.
Our findings suggest a 2-step ultrasound-aided strategy to improve the weaning process: (1) in all patients, LUS assessment at the end of SBT is required to identify those patients at high risk of extubation failure, and (2) in the weaning failure group, cardiac and diaphragm ultrasound could be used to identify the mechanism and guide treatments to promote extubation success.
Our study has some limitations. First, this was a multi-centre study, with only 40 patients included. Second, the diagnosis of weaning-induced pulmonary oedema was not based on pulmonary artery catheter or on a review by experts. However, echocardiogragraphy and Doppler studies are validated tools to diagnose increased LV pre-load. Third, we tested mainly patients with SBT performed with T-tubes, but for ten patients low pressure support was used which is not as challenging for the heart [30] as spontaneous breathing with T-tube.
In conclusion, our study shows that LUS, but not echocardiography, can predict extubation outcome. New studies are needed to test the hypothesis that LUS, by allowing for a more comprehensive assessment of the patients at risk for extubation failure, can also improve the weaning outcome.
Change history
10 February 2020
The original version of this article unfortunately contained a mistake. Two of the authors forgot to mention recent collaborations in their COI. The correct COI would have been: Dr Silvia Mongodi received feed for lectures from General Electrics; and Professor Francesco Mojoli received feed for lectures from General Electrics, Hamilton Medical and SEDA SpA. The Authors apologise for the missing information.
References
Chiumello D, Mongodi S, Algieri I, Vergani GL, Orlando A, Via G, Crimella F, Cressoni M, Mojoli F (2018) Assessment of lung aeration and recruitment by CT scan and ultrasound in acute respiratory distress syndrome patients. Crit Care Med 46:1761–1768
Soummer A, Perbet S, Brisson H, Arbelot C, Constantin JM, Lu Q, Rouby JJ (2012) Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress*. Crit Care Med 40:2064–2072
Beduneau G, Pham T, Schortgen F, Piquilloud L, Zogheib E, Jonas M, Grelon F, Runge I, Nicolas T, Grange S, Barberet G, Guitard PG, Frat JP, Constan A, Chretien JM, Mancebo J, Mercat A, Richard JM, Brochard L (2017) Epidemiology of weaning outcome according to a new definition The WIND study. Am J Respir Crit Care Med 195:772–783
Liu J, Shen F, Teboul JL, Anguel N, Beurton A, Bezaz N, Richard C, Monnet X (2016) Cardiac dysfunction induced by weaning from mechanical ventilation: incidence, risk factors, and effects of fluid removal. Crit Care 20:369
Lichtenstein DA, Meziere GA, Lagoueyte JF, Biderman P, Goldstein I, Gepner A (2009) A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest 136:1014–1020
Noble VE, Murray AF, Capp R, Sylvia-Reardon MH, Steele DJR, Liteplo A (2009) Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis. Time course for resolution. Chest 135:1433–1439
Agricola E, Picano E, Oppizzi M, Pisani M, Meris A, Fragasso G, Margonato A (2006) Assessment of stress-induced pulmonary interstitial edema by chest ultrasound during exercise echocardiography and its correlation with left ventricular function. J Am Soc Echocardiogr 19:457–463
Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJ, Filippatos G, European Society of C, European Society of Intensive Care M (2010) Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail 12:423–433
Mongodi S, Via G, Bouhemad B, Storti E, Mojoli F, Braschi A, Group AS (2013) Usefulness of combined bedside lung ultrasound and echocardiography to assess weaning failure from mechanical ventilation: a suggestive case*. Crit Care Med 41:e182–185
Ferre A, Guillot M, Lichtenstein D, Meziere G, Richard C, Teboul JL, Monnet X (2019) Lung ultrasound allows the diagnosis of weaning-induced pulmonary oedema. Intensive Care Med 45:601–608
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29:277–314
Lamia B, Maizel J, Ochagavia A, Chemla D, Osman D, Richard C, Teboul JL (2009) Echocardiographic diagnosis of pulmonary artery occlusion pressure elevation during weaning from mechanical ventilation. Crit Care Med 37:1696–1701
Bouhemad B, Brisson H, Guen M, Arbelot C, Lu Q, Rouby JJ (2011) Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am J Respir Crit Care Med 183:341–347
Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, Pearl R, Silverman H, Stanchina M, -Baron A, Welte T (2007) Weaning from mechanical ventilation. Eur Respir J 29:1033–1056
Bouhemad B, Mongodi S, Via G, Rouquette I (2015) Ultrasound for "lung monitoring" of ventilated patients. Anesthesiology 122:437–447
Mojoli F, Bouhemad B, Mongodi S, Lichtenstein D (2018) Lung ultrasound for critically ill patients. Am J Respir Crit Care Med 199:701–714
Bouhemad B, Liu ZH, Arbelot C, Zhang M, Ferarri F, Guen M, Girard M, Lu Q, Rouby JJ (2010) Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. Crit Care Med 38:84–92
Mongodi S, Bouhemad B, Orlando A, Stella A, Tavazzi G, Via G, Iotti GA, Braschi A, Mojoli F (2017) Modified lung ultrasound score for assessing and monitoring pulmonary aeration. Ultraschall Med 38:530–537
Rouby JJ, Arbelot C, Gao Y, Zhang M, Lv J, An Y, Wang C, Bin D, Barbas CSV, Neto FL, Caltabeloti F, Lima E, Cebey A, Perbet S, Constantin JM (2018) Training for lung ultrasound score measurement in critically ill patients. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.201802-0227LE
Mayo P, Volpicelli G, Lerolle N, Schreiber A, Doelken P, -Baron A (2016) Ultrasonography evaluation during the weaning process: the heart, the diaphragm, the pleura and the lung. Intensive Care Med 42:1107–1117
Moschietto S, Doyen D, Grech L, Dellamonica J, Hyvernat H, Bernardin G (2012) Transthoracic echocardiography with doppler tissue imaging predicts weaning failure from mechanical ventilation: evolution of the left ventricle relaxation rate during a spontaneous breathing trial is the key factor in weaning outcome. Crit Care 16:R81
Gerbaud E, Erickson M, Grenouillet-Delacre M, Beauvieux MC, Coste P, Durrieu-Jais C, Hilbert G, Castaing Y, Vargas F (2012) Echocardiographic evaluation and N-terminal pro-brain natriuretic peptide measurement of patients hospitalized for heart failure during weaning from mechanical ventilation. Minerva Anestesiol 78:415–425
Caille V, Amiel JB, Charron C, Belliard G, -Baron A, Vignon P (2010) Echocardiography: a help in the weaning process. Crit Care 14:R120
Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T, International Liaison Committee on Lung Ultrasound for International Consensus Conference on Lung U (2012) International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 38:577–591
Guyton AC (1965) Interstitial fluid presure. II. Pressure-volume curves of interstitial space. Circ Res 16:452–460
Staub NC, Nagano H, Pearce ML (1967) Pulmonary edema in dogs, especially the sequence of fluid accumulation in lungs. J Appl Physiol 22:227–240
Mahdyoon H, Klein R, Eyler W, Lakier JB, Chakko SC, Gheorghiade M (1989) Radiographic pulmonary congestion in end-stage congestive heart failure. Am J Cardiol 63:625–627
Stevenson LW, Perloff JK (1989) The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA 261:884–888
Silva S, Aissa D, Cocquet P, Hoarau L, Ruiz J, Ferre F, Rousset D, Mora M, Mari A, Fourcade O, Riu B, Jaber S, Bataille B (2017) Combined thoracic ultrasound assessment during a successful weaning trial predicts postextubation distress. Anesthesiology 127:666–674
Cabello B, Thille AW, Roche-Campo F, Brochard L, Gomez FJ, Mancebo J (2010) Physiological comparison of three spontaneous breathing trials in difficult-to-wean patients. Intensive Care Med 36:1171–1179
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bouhemad, B., Mojoli, F., Nowobilski, N. et al. Use of combined cardiac and lung ultrasound to predict weaning failure in elderly, high-risk cardiac patients: a pilot study. Intensive Care Med 46, 475–484 (2020). https://doi.org/10.1007/s00134-019-05902-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-019-05902-9