Abstract
Purpose
This study aimed to elucidate the impact of protocolized family support intervention on length of stay (LOS) in the intensive care unit (ICU) through a systematic review and meta-analysis.
Methods
Medline, EMBASE, the Cochrane Central Register of Controlled Trials, and other web-based databases were referenced since inception until November 26, 2018. We included randomized-controlled trials wherein protocolized family support interventions were conducted for enhanced communication and shared medical decision-making. LOS (in days) and mortality were evaluated using a random-effects model, and adjusted LOS was estimated using a mixed-effects model.
Results
We included seven randomized-controlled trials with 3477 patients. Protocolized family support interventions were found to significantly reduce the ICU LOS {mean difference = − 0.89 [95% confidence interval (CI) = − 1.50 to − 0.27]} and hospital LOS [mean difference = − 3.78 (95% CI = − 5.26 to − 2.29)]; the results of the mixed-effect model showed that they significantly reduced ICU LOS after adjusting for the therapeutic goal [mean difference = − 1.30 (95% CI = − 2.35 to − 0.26)], methods of measurement [mean difference = − 0.89 (95% CI = − 1.55 to − 0.22)], and timing of intervention [mean difference = − 1.05 (95% CI = − 2.05 to − 0.05)]. Similar results were found after adjusting for patients’ disease severity [mean difference = − 1.21 (95% CI = − 2.03 to − 0.39)] and the trim-and-fill method [mean difference = − 0.86 (95% CI = − 1.44 to − 0.28)]. There was no difference in mortality rate in ICU and hospital between the protocolized intervention and control groups.
Conclusions
Protocolized family support intervention for enhanced communication and shared decision-making with the family reduced ICU LOS in critically ill patients without impacting mortality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Our meta-analysis found that protocolized family support intervention for enhanced communication and shared decision-making reduced ICU LOS without affecting mortality, even after adjusting for disease severity. The benefits of protocolized family support intervention were more evident in sensitivity analyses with comfort care settings, post-intervention LOS, and proactive intervention. |
Introduction
Various types of family support interventions for critically ill patients and their surrogates have been studied to reduce the psychological problems [1,2,3] stemming chiefly from inadequate communication [4,5,6]. Although the details of each type of intervention differed across each study, these interventions commonly focused on facilitating effective communication between family and medical staff. For effective communication with family members, major professional guidelines have emphasized the concept of “family-centered care” in an intensive care unit (ICU) and advocated a shared decision-making approach [7, 8].
ICU length of stay (LOS) is a representative ICU index and has been shown to be a responsive measure for communication interventions in the ICU [9,10,11]. The previous studies have shown that patients with lengthy ICU admissions were associated with high caregiver burdens [12,13,14]. Moreover, the capacity strained by unnecessarily prolonged ICU LOS can be perceived as a strong barrier to patient- and family-centered care that disrupts routine opportunities for clinicians to communicate with family members [15, 16]. A lack of quality communication in the ICU often leads to confusion among the family members, which is associated with unrealistic expectations and unnecessarily prolonged ICU stays [17]. On the other hand, effective communication through well-organized protocol-based family meetings enables the caregivers to understand which intervention is being implemented, overcome the fear arising from lack of information, and cooperate with clinicians to make shared decisions [18,19,20].
However, the effect of protocolized family support intervention on ICU LOS has not been estimated in the previous systematic reviews (SRs) and meta-analyses. Only a few clinical trials and systematic reviews have been found to evaluate ICU LOS as the primary outcome [21, 22]. Our study focused more explicitly on protocolized interventions and includes recent research [23,24,25,26]. We hypothesized that family support intervention with well-established protocols can lead to reduced ICU LOS by promoting effective communication between family members and medical staff, and helping them reach an agreement on good, timely decisions that are in the best interests of the ICU patients.
Methods
Protocol and registration
The present study complied with the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [27]. The review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO, CRD42018117506).
Eligibility criteria
Studies that met the following inclusion criteria were considered eligible: (1) one or more family members, including surrogates and relatives, of a critically ill adult were included, (2) the critically ill patient was at a high risk of mortality, extended ventilation, or prolonged hospitalization owing to medical complications in the ICU; this criterion excluded the patients who were simply admitted to ICU for close observation after surgery, (3) the intervention was meant to help the patient’s family and medical staff engage in shared decision-making, (4) family support intervention was performed based on the pre-established protocol, (5) an ICU LOS or hospital LOS was clearly reported, and (6) the study design was a parallel-group randomized-controlled trial (RCT). Studies based on families of pediatric patients and those in which the compliance with the intervention was too low to be evaluated were not included. Family was broadly defined as individuals whom the patients wanted involved in their care, regardless of biological or legal relations [28].
Information sources and search strategy
Medline, EMBASE, and the Cochrane Central Register of Controlled Trials were referenced for potentially eligible published or unpublished clinical studies (Search date: November 26, 2018). The Peer Review of Electronic Search Strategies checklist was used to design the search strategy [29]. Additional data sources included the United States National Library of Medicine (www.clinicaltrials.gov), the European Union Clinical Trials Register (https://www.clinicaltrialsregister.eu), and conference abstracts from the international congress of the American Thoracic Society, British Thoracic Society, and European Respiratory Society. Our search strategy was complemented by manual searches for references cited in recent articles, SRs, and meta-analyses. No restrictions were applied on study period, ethnicity, or language. The detailed search strategy is described in Supplementary appendix 1.
Study selection
We selected pertinent studies based on the PRISMA flow diagram [30]. After removing duplicate studies, two independent reviewers (HWL and YKP) undertook a calibration exercise with a sample of 200 randomly selected studies and achieved over 95% agreement. All potentially eligible studies were individually screened by the reviewers for conducting a full-text review to assess whether they met the eligibility criteria. Any conflicts or disagreements regarding eligibility were resolved by referring to the original articles and discussing them with a third reviewer (YJL).
Data extraction and quality assessment
A standardized format was prepared [31]. HWL and YKP extracted data on study characteristics (first author, published year, and study design), baseline features of the patients and their families (number of participants, age, sex, ethnicity, and relationship), the patient’s therapeutic goal (comfort care or curative care), types of family support interventions (providing medical information or emotional support), the timing of intervention (a proactive intervention beginning since ≤ 72 h of ICU admission or randomization), and the range of medical staff involved. Two continuous variables (ICU LOS and hospital LOS) and two dichotomous variables (number of deceased patients in ICU and hospital) were used as clinical outcomes. Data on ICU LOS were collected using two different methods of measurement: post-intervention LOS versus LOS at baseline. We extracted unadjusted and adjusted LOS data in all studies, if they were available.
The risk of bias (ROB) of eligible studies was assessed using the Cochrane Collaboration’s tool for assessing ROB for RCTs [32]. However, the results of the ROB assessment were not used to exclude any individual study from our analysis.
Outcomes
The primary outcome was ICU LOS in the intervention and control groups. Secondary outcomes included hospital LOS and the all-cause mortality rate in the ICU and hospital.
Data synthesis and analysis
Primary and secondary outcomes were analyzed using a random-effects model, because heterogeneity regarding study protocol and participants was detected in all the included studies. A fixed-effects model was only used as a sensitivity analysis to check if similar results were yielded. LOS was presented as mean difference with 95% confidence interval (CI) and mortality was presented as odds ratio (OR) with 95% CI, in terms of summary statistics. If the mean and standard deviation (SD) were not given, other values including the median and range, the median and interquartile range (IQR) [33], and the mean and p value (Chapter 7.3.3) [31] were converted to mean and SD to obtain mean difference. Heterogeneity was statistically evaluated with I2 statistics and Cochran’s Q test.
Subgroup analyses of ICU LOS were conducted to determine how effect size and heterogeneity changed according to the pre-defined variables (therapeutic goal, method of measuring LOS, and timing of intervention) using a random-effects model. Considering the influence of these pre-defined variables on ICU LOS, a meta-regression analysis was conducted using a mixed-effect model. We also adjusted ICU LOS and hospital LOS by predicted probability of mortality estimated by disease severity with a mixed-effect model. Publication bias was qualitatively assessed by funnel plot asymmetry and quantitatively analyzed by Egger’s and Begg’s test (Chapter 10.4.3.1) [31]. Funnel plot asymmetry was analyzed: if asymmetry was detected in the funnel plot, the trim-and-fill method was used to calculate a corrected OR by estimating the number of missing studies [34].
Effect size estimates with two-tailed significance of P < 0.05 were regarded statistically significant. All the analyses were performed using Review Manager version 5.3 (Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014) and R version 3.4.0 statistical computing software (R Foundation for Statistical Computing, Vienna, Austria) with the metafor package [35].
Results
Study selection
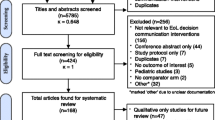
A total of 6122 studies were identified after removal of duplicates, and 106 potentially relevant articles were retrieved for full-text review (Fig. 1). The final 7 RCTs selected met the eligibility criteria and included 3477 patients [18, 21, 23,24,25,26, 36]. The reasons for excluding the other 99 articles are summarized in Supplementary appendix 2. ICU LOS and hospital LOS were reported in seven and four RCTs, respectively. Inter-observer reliability for study selection was high (κ = 0.92), which was assessed with a sample of 300 randomly selected studies.
Characteristics of the included studies and participants
The characteristics are summarized in Table 1. Medical information was provided to the patients’ family members in seven RCTs, while emotional support was provided in five. Protocolized family support interventions were implemented with clinicians in five, nurses in six, and other facilitators in four RCTs. These interventions were conducted in several countries: five in the United States and two in France. Although eligibility criteria in each study were heterogeneous, the included patients can be summarized into five categories: deceased patients (one RCT), patients at a high risk of mortality (four RCTs), patients with prolonged mechanical ventilation (one RCT), patients at a high risk of mechanical ventilation (one RCT), and patients at a high risk of prolonged hospitalization (one RCT).
The characteristics of the included patients are described in Table 1. Mean age was about 66.2 years and about 53.7% of them were male. In about 69.4% of cases, women made medical decisions as a surrogate for families. Among family members, spouses had the largest share (40.2%) in terms of decision-making, followed by children (32%), parents (9.4%), and siblings (11.4%). The disease severity was reported in five studies. Mean in-hospital mortality was 62.4%.
Risk-of-bias assessment within studies
The quality of eligible studies is described in Supplementary appendix 3. All seven RCTs had low ROB in random sequence generation. Specific methodology for allocation concealment was described in four RCTs. One RCT blinded medical personnel. Although description about blinding of outcome assessment was not found in any RCT, our primary and secondary outcomes were not likely to be influenced by lack of blinding, because these outcomes were objective findings. Low risk of attrition bias and reporting bias was found in six RCTs. No additional sources of bias were found in any of the RCTs.
Intensive care unit length of stay
For 3,477 patients in seven RCTs, the estimated mean difference of ICU LOS was − 0.89 days (95% CI − 1.50 to − 0.27) determined in a meta-analysis using a random-effects model (Fig. 2). A fixed-effects model yielded the same results. Significant heterogeneity was not detected by either model (I2 = 0%, P = 0.62).
In a subgroup analysis by therapeutic goal, the benefit of protocolized family support intervention was only evident in comfort care settings [estimated mean difference = − 1.26 (95% CI − 2.21 to − 0.31), P = 0.009] (Supplementary appendix 4). The pooled effect of protocolized family support intervention was differently estimated according to method of measurement; only post-intervention LOS showed significant results [estimated mean difference = − 0.89 (95% CI − 1.55 to − 0.22), P = 0.009] (Supplementary appendix 5). Results of a subgroup analysis by timing of intervention showed that only proactive (≤ 72 h) intervention had a significant effect on reducing LOS [estimated mean difference = − 1.07 (95% CI − 2.12 to − 0.02), P = 0.05] (Supplementary appendix 6). Significant heterogeneity was not found in these subgroups.
In a sensitivity analysis adjusted by pre-defined variables using a mixed-effects model, protocolized family support intervention significantly decreased ICU LOS after adjustment by therapeutic goal [comfort care, estimated mean difference = − 1.30 (95% CI − 2.35 to − 0.26), P = 0.01], method of measurement of LOS [post-intervention, estimated mean difference = − 0.89 (95% CI − 1.55 to − 0.22), P = 0.01], and the timing of intervention [proactive intervention, estimated mean difference = − 1.05 (95% CI − 2.05 to − 0.05), P = 0.04]. After adjusting for disease severity (predicted probability of mortality), a significantly reduced ICU LOS was found in the group with protocolized intervention [adjusted mean difference = − 1.21 (95% CI − 2.03 to − 0.39), P = 0.004].
Adjusted LOS could be extracted only in one study [23]. In the sensitivity analysis including adjusted LOS instead of unadjusted LOS, similar results were obtained [estimated mean difference = − 0.79 (95% CI − 1.23 to − 0.36), P < 0.001].
Hospital length of stay
For 1,562 patients in four RCTs, the estimated mean difference of hospital LOS was − 3.78 days (95% CI − 5.26 to − 2.29) in a meta-analysis using a random-effects model (Fig. 3). A fixed-effects model yielded similar results [estimated mean difference = − 3.88 (95% CI − 5.84 to − 1.91), P = < 0.001]. Small heterogeneity was detected in both models (I2 = 31%, P = 0.23). After adjusting for the predicted probability of mortality, a significantly reduced hospital LOS was found in the group with protocolized intervention [adjusted mean difference = − 5.24 (95% CI − 9.79 to − 0.70), P = 0.02].
Adjusted LOS was found only in one study [23]. In the sensitivity analysis including adjusted LOS instead of unadjusted LOS, results were also significant [estimated mean difference = − 3.54 (95% CI − 5.36 to − 1.72), P < 0.001].
Mortality in ICU and hospital
In two RCTs, the observed mortality rate in ICU was not significantly different between the intervention group and the control group [estimated OR = 0.76 (95% CI 0.45–1.29)] (Supplementary appendix 7). In four RCTs, the observed mortality rate in hospital also was not significantly different between the two groups [estimated OR = 1.13 (95% CI 0.85–1.49)] (Supplementary appendix 8). Significant heterogeneity was not detected in both meta-analyses.
Publication bias
The results of funnel plots supported by Egger’s and Begg’s tests indicate no publication bias for ICU LOS and hospital LOS (Fig. 4). The adjusted mean difference for ICU LOS by the trim-and-fill method showed similar results [estimated mean difference = − 0.86 (95% CI − 1.44 to − 0.28), P = 0.004]. No adjustment for publication bias was needed for the analysis of hospital LOS.
Discussion
Our SR and meta-analysis focused on the effect of protocolized family support interventions on reducing ICU LOS and hospital LOS in critically ill patients. We found that such interventions significantly decreased ICU LOS by a mean of about 1 day and hospital LOS by that of about 4 days. Subgroup and sensitivity analyses showed that these interventions were beneficial in comfort care settings, post-intervention LOS, and proactive intervention. Even after adjusting for patients’ disease severity and the trim-and-fill method, the effect was similar. There was no significant difference in mortality rate between the intervention and control groups. Protocolized family support interventions may decrease ICU LOS by reducing the potentially inappropriate life-sustaining treatments for patients requiring comfort care, while not increasing mortality by hindering the discontinuance of lifesaving treatments for patients who could survive.
One of the most reasonable and efficient goals of ICU care is decreasing ICU LOS [17], when medically appropriate, as well as to improve the quality of care, reduce medical cost [37], and efficiently use limited ICU resources [38]. However, in most previous RCTs about the effect of family support intervention, the primary measured outcome was the family members’ psychological symptoms (post-traumatic stress disorder, depression, and anxiety) [18, 23,24,25,26] or satisfaction with ICU care [36]. In one SR, LOS was qualitatively evaluated in individual-level studies, and a pooled effect of family support intervention was not estimated [1]. Another meta-analysis similarly showed that family-centered care intervention may decrease ICU LOS by about one day [2]. The previous SRs reported descriptive information about LOS reductions by palliative ICU care in several studies [3, 39]. Our study, on the other hand, focused on pre-established protocol-based family support interventions using rigorous inclusion criteria to include high-quality RCTs. In addition, the sensitivity analysis of the present study elucidates specific medical settings in which a more obvious effect of protocolized family support intervention is derived.
Among the various types of family support interventions [1,2,3, 39], there are reasons to analyze interventions for well-organized protocol-based interventions in our study. To achieve efficient communication between family members and medical staff and make appropriate timely decisions, there should be well-organized protocols containing detailed key components as follows: establishing a trusting partnership with empathetic attitudes and emotional support, assessing patient’s or family’s situation and preference, reviewing prognosis and treatment options, being realistic and empathetic, and frequently asking family members if they have any questions about the current status, medical decisions, and prognosis [7, 18, 40]. Based on pre-established protocols with these items, medical staff can prepare family meetings without inadvertently missing out on important information points or key questions and maintain an appropriate and consistent quality of interventions. In fact, some RCTs showed that protocolized family support intervention was associated with lower psychological symptoms in family members [18, 24] and higher family ratings of quality of communication and patient-centeredness of care [23]. On the other hand, interventions that merely involve sharing educational materials or leaflets without any direct communication or conducting family meetings without a unified protocol are likely to be ineffective, and shared decision-making may be unsatisfactory.
In our study, protocolized family support interventions were found to significantly reduce ICU LOS especially in comfort care settings. The mean difference of LOS can be an indicator for withdrawal of futile life support through effective shared decision-making [41]. Family members of ICU patients with impaired consciousness often find themselves in distress while making important decisions, because these decisions include complicated and potentially distressing issues, such as the dilemma between their duty to preserve the patient’s life and the patient’s right to a dignified death [42]. Effective and high-quality communication between family members and medical staff could help family members to make timely appropriate decisions related to this issue [43], and earlier withdrawal of potentially inappropriate life support is associated with reduced length of stay and increased family member satisfaction in ICU [23, 44]. Therefore, our study suggests setting up treatment goals for care in a timely manner through well-organized family meetings, especially in the setting of comfort care, which could prevent inappropriate use of potentially futile treatments [1] and improve patient- and family-centered outcomes.
There have been conflicting results about the benefit of proactive communication with family members in the ICU. The previous studies reported that the early integration of family communication was associated with a higher quality of death as rated by family members [45] and reduced ICU LOS [11, 46]. On the other hand, Garrouste-Orgeas’s study showed that LOS did not reduce after the proactive participation of a nurse in family conferences [26]. In that study, a nurse facilitated mutual understanding between medical staff and family members, but played a limited role in family conferences. In our study, sensitivity analysis by timing of intervention showed that reduction in ICU LOS was only found in proactive intervention, which supports the American College of Critical Care Medicine guideline that recommends holding a family meeting with a multi-professional ICU team within 24–48 h of ICU admission [7]. Proactive intervention with empathetic attitudes would help clinicians develop an intimate relationship with the families earlier, leading to an early consensus to avoid delayed decisions of essential treatment and potentially futile treatments in a timely manner.
Methodological variabilities were found in each protocol of trials. The first is the frequency of family interventions. Additional family meetings could be held according to the patient’s request or medical need without prescribed rules. The number of family meetings was not fully reported in all included RCTs. It was unclear whether there was a difference in the actual number of meetings for each protocol or how such a difference would affect the results. The second is the purpose of the communication with family. While there were studies in which protocols were written to focus on emotional support [23, 25], other studies focused on providing medical information to family members [26, 36]. However, providing emotional support and medical information cannot be performed separately, and in most cases, the two would be conducted together. The third is the degree of medical staff’s involvement. Clinicians usually play a major role in family conferences to make shared decisions. However, two studies were centered on the role of nurse or facilitator [24, 26]. Considering that the nurse and facilitator could help the family conference proceed smoothly and effectively, most medical staff needs to be involved in efforts for better communication with patient’s family.
This study has its limitations. First, although rigorous eligibility criteria were applied in our systematic review and meta-analysis, the characteristics of protocolized family support interventions were varied in detailed implementations. Some studies seemed to have different objectives for family support intervention. For example, Schneiderman et al.’s study [21] involved a trial of ethical consultation and Curtis et al.’s [36] involved a trial of quality improvement of communication. Despite this major concern about heterogeneity, the reason for pooling these studies was to determine if using pre-established protocols can be an important factor for successful family support intervention. In fact, all the included studies were under a common umbrella in that family support interventions were conducted according to pre-established protocol. Second, we did not control for all the variables that affect LOS reduction in ICU or hospital. However, it is unlikely that other variables had a significant impact on LOS when considering the effective randomization in individual RCTs. After adjusting for predicted probability of mortality, we still obtained significant reduction in ICU LOS or hospital LOS. Third, unblinded medical staff might affect the discharge decision. Owing to the nature of intervention, performance bias is likely. Only Carson et al. [25] described about blinding of study staff to group assignment.
In conclusion, ICU LOS significantly reduced without impacting mortality after implementation of protocolized family support intervention for shared decision-making. The benefits of the intervention were more evident when conducted proactively or in the context of comfort care or high risk of death.
Availability of data and material
The data sets used and/or analyzed can be obtained from the corresponding author on reasonable request.
Change history
08 October 2019
The original version of this article unfortunately contained a mistake. One of the affiliations was incorrect: Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Veterans Health Service Medical Center, Portland, USA.
08 October 2019
The original version of this article unfortunately contained a mistake. One of the affiliations was incorrect: Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Veterans Health Service Medical Center, Portland, USA.
References
Kynoch K, Chang A, Coyer F, McArdle A (2016) The effectiveness of interventions to meet family needs of critically ill patients in an adult intensive care unit: a systematic review update. JBI Database Syst Rev Implement Rep 14:181–234
Goldfarb MJ, Bibas L, Bartlett V, Jones H, Khan N (2017) Outcomes of patient- and family-centered care interventions in the ICU: a systematic review and meta-analysis. Crit Care Med 45:1751–1761
Kyeremanteng K, Gagnon LP, Thavorn K, Heyland D, D’Egidio G (2018) The impact of palliative care consultation in the ICU on length of stay: a systematic review and cost evaluation. J Intensive Care Med 33:346–353
Wendler D, Rid A (2011) Systematic review: the effect on surrogates of making treatment decisions for others. Ann Intern Med 154:336–346
Azoulay E, Pochard F, Kentish-Barnes N, Chevret S, Aboab J, Adrie C, Annane D, Bleichner G, Bollaert PE, Darmon M, Fassier T, Galliot R, Garrouste-Orgeas M, Goulenok C, Goldgran-Toledano D, Hayon J, Jourdain M, Kaidomar M, Laplace C, Larche J, Liotier J, Papazian L, Poisson C, Reignier J, Saidi F, Schlemmer B (2005) Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med 171:987–994
Gries CJ, Engelberg RA, Kross EK, Zatzick D, Nielsen EL, Downey L, Curtis JR (2010) Predictors of symptoms of posttraumatic stress and depression in family members after patient death in the ICU. Chest 137:280–287
Kon AA, Davidson JE, Morrison W, Danis M, White DB (2016) Shared decision making in ICUs: an American College of critical care medicine and american thoracic society policy statement. Crit Care Med 44:188–201
Davidson JE, Aslakson RA, Long AC, Puntillo KA, Kross EK, Hart J, Cox CE, Wunsch H, Wickline MA, Nunnally ME, Netzer G, Kentish-Barnes N, Sprung CL, Hartog CS, Coombs M, Gerritsen RT, Hopkins RO, Franck LS, Skrobik Y, Kon AA, Scruth EA, Harvey MA, Lewis-Newby M, White DB, Swoboda SM, Cooke CR, Levy MM, Azoulay E, Curtis JR (2017) Guidelines for family-centered care in the neonatal, pediatric, and adult ICU. Crit Care Med 45:103–128
Adanir T, Erdogan I, Hunerli G, Unveren G, Dasci H, Cetin HY, Ozsan I, Aydin U (2014) The effect of psychological support for the relatives of intensive care unit patients on cadaveric organ donation rate. Transpl Proc 46:3249–3252
Campbell ML, Guzman JA (2003) Impact of a proactive approach to improve end-of-life care in a medical ICU. Chest 123:266–271
Lilly CM, De Meo DL, Sonna LA, Haley KJ, Massaro AF, Wallace RF, Cody S (2000) An intensive communication intervention for the critically ill. Am J Med 109:469–475
Wong DT, Gomez M, McGuire GP, Kavanagh B (1999) Utilization of intensive care unit days in a Canadian medical-surgical intensive care unit. Crit Care Med 27:1319–1324
Stricker K, Rothen HU, Takala J (2003) Resource use in the ICU: short- vs. long-term patients. Acta Anaesthesiol Scand 47:508–515
Zimmerman JE, Kramer AA, McNair DS, Malila FM, Shaffer VL (2006) Intensive care unit length of stay: benchmarking based on Acute Physiology and Chronic Health Evaluation (APACHE) IV. Crit Care Med 34:2517–2529
Bagshaw SM, Opgenorth D, Potestio M, Hastings SE, Hepp SL, Gilfoyle E, McKinlay D, Boucher P, Meier M, Parsons-Leigh J, Gibney RT, Zygun DA, Stelfox HT (2017) Healthcare provider perceptions of causes and consequences of ICU capacity strain in a large publicly funded integrated health region: a qualitative study. Crit Care Med 45:e347–e356
Clay AM, Parsh B (2016) Patient- and family-centered care: it’s not just for pediatrics anymore. AMA J Ethics 18:40–44
Gruenberg DA, Shelton W, Rose SL, Rutter AE, Socaris S, McGee G (2006) Factors influencing length of stay in the intensive care unit. Am J Critical Care 15:502–509
Lautrette A, Darmon M, Megarbane B, Joly LM, Chevret S, Adrie C, Barnoud D, Bleichner G, Bruel C, Choukroun G, Curtis JR, Fieux F, Galliot R, Garrouste-Orgeas M, Georges H, Goldgran-Toledano D, Jourdain M, Loubert G, Reignier J, Saidi F, Souweine B, Vincent F, Barnes NK, Pochard F, Schlemmer B, Azoulay E (2007) A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med 356:469–478
Hutchison PJ, McLaughlin K, Corbridge T, Michelson KN, Emanuel L, Sporn PH, Crowley-Matoka M (2016) Dimensions and role-specific mediators of surrogate trust in the ICU. Crit Care Med 44:2208–2214
Mosenthal AC, Murphy PA, Barker LK, Lavery R, Retano A, Livingston DH (2008) Changing the culture around end-of-life care in the trauma intensive care unit. J Trauma 64:1587–1593
Schneiderman LJ, Gilmer T, Teetzel HD, Dugan DO, Blustein J, Cranford R, Briggs KB, Komatsu GI, Goodman-Crews P, Cohn F, Young EW (2003) Effect of ethics consultations on nonbeneficial life-sustaining treatments in the intensive care setting: a randomized controlled trial. JAMA 290:1166–1172
Khandelwal N, Kross EK, Engelberg RA, Coe NB, Long AC, Curtis JR (2015) Estimating the effect of palliative care interventions and advance care planning on ICU utilization: a systematic review. Crit Care Med 43:1102–1111
White DB, Angus DC, Shields AM, Buddadhumaruk P, Pidro C, Paner C, Chaitin E, Chang CH, Pike F, Weissfeld L, Kahn JM, Darby JM, Kowinsky A, Martin S, Arnold RM (2018) A randomized trial of a family-support intervention in intensive care units. N Engl J Med 378:2365–2375
Curtis JR, Treece PD, Nielsen EL, Gold J, Ciechanowski PS, Shannon SE, Khandelwal N, Young JP, Engelberg RA (2016) Randomized trial of communication facilitators to reduce family distress and intensity of end-of-life care. Am J Respir Crit Care Med 193:154–162
Carson SS, Cox CE, Wallenstein S, Hanson LC, Danis M, Tulsky JA, Chai E, Nelson JE (2016) Effect of palliative care-led meetings for families of patients with chronic critical illness: a randomized clinical trial. JAMA 316:51–62
Garrouste-Orgeas M, Max A, Lerin T, Gregoire C, Ruckly S, Kloeckner M, Brochon S, Pichot E, Simons C, El-Mhadri M, Bruel C, Philippart F, Fournier J, Tiercelet K, Timsit JF, Misset B (2016) Impact of proactive nurse participation in ICU family conferences: a mixed-method study. Crit Care Med 44:1116–1128
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6:e1000100
Brown SM, Rozenblum R, Aboumatar H, Fagan MB, Milic M, Lee BS, Turner K, Frosch DL (2015) Defining patient and family engagement in the intensive care unit. Am J Respir Crit Care Med 191:358–360
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C (2016) PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 75:40–46
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Higgins JP, Green S (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The cochrane collaboration. Available at https://handbook-5-1.cochrane.org/. Accessed 30 June 2019
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed) 343:d5928
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14:135
Duval S, Tweedie R (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56:455–463
Viechtbauer WJJoss (2010) Conducting meta-analyses in R with the metafor package. Available at https://www.jstatsoft.org/article/view/v036i03. Accessed 30 June 2019
Curtis JR, Nielsen EL, Treece PD, Downey L, Dotolo D, Shannon SE, Back AL, Rubenfeld GD, Engelberg RA (2011) Effect of a quality-improvement intervention on end-of-life care in the intensive care unit: a randomized trial. Am J Respir Crit Care Med 183:348–355
Oostenbrink JB, Buijs-Van der Woude T, van Agthoven M, Koopmanschap MA, Rutten FF (2003) Unit costs of inpatient hospital days. PharmacoEconomics 21:263–271
Hughes M, MacKirdy FN, Norrie J, Grant IS (2001) Outcome of long-stay intensive care patients. Intensive Care Med 27:779–782
Aslakson R, Cheng J, Vollenweider D, Galusca D, Smith TJ, Pronovost PJ (2014) Evidence-based palliative care in the intensive care unit: a systematic review of interventions. J Palliat Med 17:219–235
Nelson JE, Walker AS, Luhrs CA, Cortez TB, Pronovost PJ (2009) Family meetings made simpler: a toolkit for the intensive care unit. J Crit Care 24:626.e627-614
Martins B, Oliveira RA, Cataneo AJM (2017) Palliative care for terminally ill patients in the intensive care unit: systematic review and metaanalysis. Palliat Support Care 15:376–383
Long AC, Kross EK, Engelberg RA, Downey L, Nielsen EL, Back AL, Curtis JR (2014) Quality of dying in the ICU: is it worse for patients admitted from the hospital ward compared to those admitted from the emergency department? Intensive Care Med 40:1688–1697
Gries CJ, Curtis JR, Wall RJ, Engelberg RA (2008) Family member satisfaction with end-of-life decision making in the ICU. Chest 133:704–712
Gerstel E, Engelberg RA, Koepsell T, Curtis JR (2008) Duration of withdrawal of life support in the intensive care unit and association with family satisfaction. Am J Respir Crit Care Med 178:798–804
Glavan BJ, Engelberg RA, Downey L, Curtis JR (2008) Using the medical record to evaluate the quality of end-of-life care in the intensive care unit. Crit Care Med 36:1138–1146
Lamba S, Murphy P, McVicker S, Harris Smith J, Mosenthal AC (2012) Changing end-of-life care practice for liver transplant service patients: structured palliative care intervention in the surgical intensive care unit. J Pain Symptom Manag 44:508–519
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding
The authors declare that they have taken no support from any organization for the submitted work. The authors also have no conflicts of interest to declare.
Author information
Authors and Affiliations
Contributions
Study concept and design: HWL and YJL. Data acquisition: HWL and YKP. Data analysis and interpretation: HWL and EJJ. Drafting of the manuscript: HWL. Critical revision of the manuscript and important intellectual content: HWL and YJL. Study supervision: YJL.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
134_2019_5681_MOESM4_ESM.tiff
Supplementary appendix 4. Forest plot of subgroup analysis for the intensive care unit length of stay by therapeutic goal (TIFF 1154 kb)
134_2019_5681_MOESM5_ESM.tiff
Supplementary appendix 5. Forest plot of subgroup analysis for the intensive care unit length of stay by method of measurement (TIFF 1160 kb)
134_2019_5681_MOESM6_ESM.tiff
Supplementary appendix 6. Forest plot of subgroup analysis for the intensive care unit length of stay by timing of intervention (TIFF 1205 kb)
Rights and permissions
About this article
Cite this article
Lee, H.W., Park, Y., Jang, E.J. et al. Intensive care unit length of stay is reduced by protocolized family support intervention: a systematic review and meta-analysis. Intensive Care Med 45, 1072–1081 (2019). https://doi.org/10.1007/s00134-019-05681-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-019-05681-3