Abstract
Purpose
Changes of lactate concentration over time were reported to be associated with survival in septic patients. We aimed to evaluate delta-lactate (ΔLac) 24 h after admission (Δ24Lac) to an intensive care unit (ICU) in critically ill patients for short- and long-term prognostic relevance.
Methods
In total, 26,285 lactate measurements of 2191 patients admitted to a German ICU were analyzed. Inclusion criterion was a lactate concentration at admission above 2.0 mmol/L. Maximum lactate concentrations of day 1 and day 2 were used to calculate Δ24Lac. Follow-up of patients was performed retrospectively. Association of Δ24Lac and both in-hospital and long-term mortality were investigated. An optimal cut-off was calculated by means of the Youden index.
Results
Patients with lower Δ24Lac were of similar age, but clinically sicker. As continuous variable, higher Δ24Lac was associated with decreased in-hospital mortality (per 1% Δ24Lac; HR 0.987 95%CI 0.985–0.990; p < 0.001) and an optimal Δ24Lac cut-off was calculated at 19%. Δ24Lac ≤ 19% was associated with both increased in-hospital (15% vs 43%; OR 4.11; 95%CI 3.23–5.21; p < 0.001) and long-term mortality (HR 1.54 95%CI 1.28–1.87; p < 0.001), even after correction for APACHE II, need for catecholamines and intubation. We matched 256 patients with Δ24Lac ≤ 19% to case–controls > 19% corrected for APACHE II scores, baseline lactate level and sex: Δ24Lac ≤ 19% remained associated with lower in-hospital and long-term survival.
Conclusions
Lower Δ24Lac was robustly associated with adverse outcome in critically ill patients, even after correction for confounders. Δ24Lac might constitute an independent, easily available and important parameter for risk stratification in the critically ill.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increased blood lactate levels have been described as marker of impaired organ perfusion in critically ill patients [1, 2]. To understand the pathophysiology of hyperlactatemia, it is first important to consider the physiology of lactate metabolism. Briefly, at the cellular level, under stable conditions glucose is converted to pyruvate by glycolysis delivering adenosine triphosphate (ATP). Under stress conditions, increased glycolysis leads to an increased pyruvate production causing conversion of pyruvate to lactate [3]. Most commonly, hyperlactatemia in the context of circulatory failure, is considered a result of anaerobic glycolysis, and the relationship between tissue hypoxia and lactate generation has been confirmed in animal and clinical studies [3,4,5,6]. However, hyperlactatemia does not solely reflect anaerobic glycolysis. Generally, hyperlactatemia occurs when lactate production exceeds lactate consumption [7], which makes clinical interpretation a challenging task [8]. Several conditions have been described to influence blood lactate levels. For instance, in septic patients, further metabolic alterations such as increased catecholamine-stimulated Na+/K+-ATPase activity or aerobic glycolysis, contribute to increased lactate production [9,10,11]. Further, drugs impairing oxidative phosphorylation, such as propofol, metformin or antiretroviral agents, can augment lactic acid production [7]. On the other hand, decreased lactate elimination can lead to hyperlactatemia. Thus, as the liver accounts for up to 70% of lactate clearance, fulminant liver dysfunction is another possible mechanism [7, 12]. Furthermore, the half-life of blood lactate in its proper sense differs from the actual half-life in different pathological conditions. Whereas Huckabee and colleagues have shown in an experimental design a half-life of blood lactate of approximately 20 min [13], real-world studies in patients with grand-mal seizures revealed considerably longer time frames until blood lactate levels halved [14, 15].
Independently on the origin of hyperlactatemia, numerous clinical studies established blood lactate levels as reliable outcome predictor in different subgroups of critically ill patients, such as patients with cardiogenic shock, sepsis or trauma [16,17,18,19,20]. Different cut-offs of serum lactate concentration have been reported to predict adverse outcomes. Thus, a threshold of 4 mmol/L has been reported to be associated with adverse outcome in a multicentric analysis of the Surviving Sepsis Campaign database including 28,150 patients, recommending normalization of lactate to guide initial resuscitation in septic patients [21, 22].
Recently, kinetics of lactate levels as a prognostic predictor have shifted into focus of clinical studies [23, 24]. In the present study, we aimed to investigate prognostic impact of lactate concentration changes in a large, real-world cohort of unselected critically ill patients admitted to an intensive care unit (ICU).
Methods
Study subjects
We included in the present study 2191 patients admitted to the medical ICU of the Jena University Hospital between January 2004 and December 2009 with an arterial lactate concentration above 2.0 mmol/L on admission to the ICU. The primary endpoint of the study was all-cause ICU mortality. Secondary endpoint was long-term mortality. Patients were collected prospectively, and their medical history, clinical data and standard laboratory parameters were documented. Follow-up of patients was performed retrospectively. Mortality data were collected by review of medical records in the in-hospital patient data management system (COPRA System GmbH, Berlin, Germany) and/or patient contact.
The study has been approved by the local ethics committee of the Jena University Hospital. A sub-group of this cohort was analyzed in other contexts before [25, 26].
Lactate measurements
There was no prespecified time-based protocol regarding lactate measurements. Lactate concentrations were obtained at a regular basis (at least two times during each 8-h shift, adding up to at least six times per day) and additionally in each patient, at the discretion of treating physicians and nurses in case of clinical alterations such as increases in catecholamine doses, decreasing blood pressure or other signs of patients’ impairment. This reflects a real-world scenario with regards to timing and frequency of lactate concentration determination. In order to further characterize lactate changes over time delta-lactate (ΔLac) was calculated. Since the main focus of this study is on lactate concentration changes over the first 24 h of admission, Δ24Lac was calculated. To obtain Δ24Lac we set the maximum lactate concentration of the day of admission in relation to the maximum lactate concentration on day 2. This allows to evaluate a clinically meaningful and practical parameter since the mathematical use of all measured lactate parameters and derived cut-offs seems not practical in the clinical setting.
Calculation of SAPS II and APACHE II scores
Initial Simplified Acute Physiology Score II (SAPS II) and Acute Physiology And Chronic Health Evaluation II (APACHE II) scores were calculated within 24 h after admission as reported before [27, 28].
Statistical analysis
The association between ICU mortality and the Δ24Lac was analyzed by both univariable and multivariable logistic regression and odds ratios (OR) were calculated. First, Δ24Lac was analyzed as a continuous variable in univariable logistic regression (change per %). Then, for detection of the optimal cut-off value of the Δ24Lac for prediction of intra-ICU mortality we performed ROC-analysis, calculated the area under the curve (AUC) and an optimal cut-off by means of the Youden-Index. Patients with Δ24Lac values below and above the determined optimal cut-off of Δ24Lac (19%) were compared: Patients were retrospectively subdivided into two groups, representing subjects with low (≤ 19%) versus high ΔLac (> 19%) in the first 24 h after admission. Differences between groups were assessed using ANOVA. Metric data was summarized as mean ± standard error of the mean (SEM). Categorical data are expressed as numbers (percentage). Chi-square test was applied to assess differences between groups. Univariable and multivariable Cox regression analyses were used to analyze associations of Δ24Lac with long-term mortality, to adjust for confounding factors and calculate hazard ratios (HR). For the multivariable Cox regression analysis, a backward elimination has been performed. A p value of < 0.05 was considered statistically significant. For case-control matching we matched patients by APACHE II, baseline lactate level and sex. To compare in-hospital mortality between these paired groups we used McNemar’s test.
SPSS version 22.0 (IBM, Chicago, IL, USA) and MedCalc version 17.4.4 (MedCalc Software, Ostend, Belgium) were used for statistical analyses.
Results
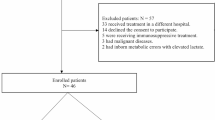
Our database consists of 6449 patients admitted to an ICU in whom lactate concentration on admission day were reported. Of these, 3740 patients evidenced an initial lactate concentration below 2.0 mmol/L and were excluded from further calculations. Intra-ICU mortality in this excluded cohort was 6%. Baseline characteristics of these patients are shown in Supplemental Table 1. Of 2709 patients evidencing a lactate concentration above 2.0 mmol/L on admission day, in 2191 patients, at least one lactate concentration was reported per day, allowing the calculation of Δ24Lac. These patients were included in the present analysis. In total in these patients, 26,285 lactate measurements, in median 17 per patient, were screened. Follow-up data allowing analysis of long-term outcome were available for 1484 patients. Baseline characteristics of the patient cohort are presented in the Table 1.
As continuous variable, higher was associated with decreased ICU mortality (per 1% Δ24Lac; OR 0.987 95%CI 0.985–0.990; p < 0.001). ROC-analysis was performed for prediction of ICU mortality by Δ24Lac (AUC 0.70; 95%CI 0.68–0.73) and an optimal Δ24Lac cut-off was calculated to be 19% (18.56; 95%CI 15.25–32.56) by means of the Youden-Index. Patients in the Δ24Lac ≤ 19% group were of similar age (p = 0.36), but with higher severity of disease than patients in the Δ24Lac > 19% group as reflected by both higher SAPS II (p < 0.001) and APACHE II (p < 0.001) scores. In patients with lower Δ24Lac, laboratory signs of multi-organ failure such as liver parameters (ASAT: p = 0.01; ALAT: p = 0.02) were more pronounced. There were no differences in baseline lactate, hemoglobin and procalcitonin levels, but patients with low Δ24Lac needed catecholamines more often (Table 1).
We analyzed short-term and long-term survival depending on the Δ24Lac. Δ24Lac ≤ 19% was associated with increased ICU mortality (15% vs 43%; OR 4.11; 95%CI 3.23–5.21; p < 0.001), even after correction for SAPS II (OR 2.17; 95%CI 1.60–2.94; p < 0.001) and APACHE II (OR 2.27; 95%CI 1.68–3.06; p < 0.001) scores, as well as need for catecholamines and intubation (OR 2.30; 95%CI 1.69–3.12; p < 0.001).
Δ24Lac ≤ 19% was further associated with increased long-term mortality (HR 2.22; 95%CI 1.90–2.61; p < 0.001; Fig. 1) regardless of admission diagnosis (Supplementary Table 1). This association remained after correction for APACHE II scores (HR 1.53; 95%CI 1.27–1.85; p < 0.001), SAPS II scores (HR 1.46; 95%CI 1.21–1.76; p < 0.001) and even APACHE II, need for catecholamines and intubation (HR 1.55 95%CI 1.28–1.87; p < 0.001) (Table 2).
We matched 256 patients with Δ24Lac ≤ 19% to case-controls evidencing Δ24Lac > 19% corrected for APACHE II scores, baseline lactate level and sex. Δ24Lac ≤ 19% not only remained associated with lower in-hospital survival, with the difference being 13.28% (95%CI 6.20%–20.36%; p = 0.004).
Discussion
In our study cohort of critically ill medical patients, the decrease in maximum lactate levels during the first 24 h was associated with in-hospital as well as long-term mortality. This association remained highly significant even after correction for severity of disease.
In clinical practice, serum lactate concentration is a well-established prognostic marker in critically ill patients [22, 29, 30]. In current literature, optimal cut-offs of single static arterial lactate measurements in terms of prediction of outcome vary considerably. In this context, volatility of blood lactate levels has attracted broad interest of clinicians and researchers in recent years. A number of groups hypothesized that early changes of lactate concentration could be a useful tool for risk evaluation [29,30,31]. Thus, Nguyen and colleagues have shown in a study collective of 111 patients with severe sepsis or septic shock that a higher early lactate clearance (LC), as the kinetics of lactate are defined in several studies, obtained by comparison of blood lactate concentrations at and 6 h after admission to emergency department, was significantly associated with decreased mortality rate [29]. In a work published in 2007, Donnino and coworkers have shown in a collective of 79 patients with cardiac arrest the significance of LC as independent predictor of 24 h survival [30]. In these observational studies, the authors demonstrated that higher LC is associated with higher survival rates [29, 30], whereas, vice versa, prolonged duration until restoration of normal blood lactate levels is associated with increased mortality [31]. However, the stated studies investigated specified patient cohorts and subject numbers were low. In 2011, Nichol et al. [32] provided a multi-center analysis investigating dynamic lactate parameters with over 5000 patients. They could demonstrate that time-weighted average lactate and the change in lactate over the first 24 h after admission independently predicted in-hospital mortality. Recently, Haas and colleagues conducted a retrospective analysis of a remarkable number of 14,040 ICU patients [33]. However, contrary to our study, the aim of this study was specifically to evaluate the etiology and association with in-hospital mortality of severe lactatemia and LC including patients with blood lactate concentrations > 10 mmol/L. It could be demonstrated that severe hyperlactatemia is associated with very high ICU mortality, especially when LC within 12 h was low [33]. By contrast, we provide here an extensive analysis of a large cohort of unselected ICU patients with mid- and high-range lactate elevations, starting with lactate concentrations > 2.0 mmol/L, thus, including very heterogeneous collective of patients, reflecting real-world ICU practice. In this regard, we think that the focus on maximum lactate concentrations is a strength of our analysis, as it might better reflect real-world lactate measurements compared to time-based protocols: In each patient, lactate was measured at least six times a day per protocol (two times during an 8-h shift), and additionally at the treating physician’s discretion in case of patient’s health deterioration, such as increased catecholamine doses. In our opinion, in this way, the maximum lactate concentration of each patient was measured relatively reliably, although there was no prespecified protocol for lactate determinations.
In addition to demonstrating an association of lower Δ24Lac with increased short-term mortality, we show here for the first time the impact not only on in-hospital, but also long-term mortality, analyzing follow-up data for up to 9 years. We aimed to provide a useful tool for daily clinical practice and calculated a Δ24Lac cut-off being 19% and confirmed its reliability in predicting in-hospital and long-term mortality in multivariable and matched-controlled analyses. This cut-off differs from the cut-off of 32.8% reported in the publication of Haas et al. [33]; however, it must be taken into account that this higher cut-off has been calculated for lactate changes within 12 h for patients with severe lactatemia.
Besides being a potentially potent risk parameter, lactate clearance might as well constitute a valuable treatment goal in ICU patients and might help guiding fluid resuscitation therapy [34]. Analogous to goal-directed therapy concept guided by central venous oxygen saturation (ScvO2), some groups have investigated therapy regimes guided by changes of serum lactate concentrations. For instance, Jones and colleagues reported in a randomized multi-center study no differences in outcomes between septic patients receiving ScvO2-guided or therapy additionally guided by lactate clearance [35]. By contrast, Jansen et al. [36] could demonstrate a reduced in-hospital mortality in patients with a treatment guided by the combination of ScvO2 and lactate levels with the objective to decrease lactate concentration by 20% or more per 2 h in the initial 8 h. However, the production, metabolism and elimination of lactate is a highly complex process, requiring a thorough consideration of possible underlying causes of hyperlactatemia to establish an appropriate therapy regime [8]. A very recent ongoing multicenter randomized controlled trial, ANDROMEDA-SHOCK study, deals with this problem by comparing early goal directed therapy targeting peripheral perfusion versus lactate levels in septic shock patients [37]. In the present study, patients did not underlie a pre-specified treatment algorithm and were treated at the responsible physicians’ discretion.
The long follow-up period allowing analysis of long-term mortality, as well as the high number and heterogeneity of the patients are the strengths of our study. Main limitations are its retrospective and single-center design. One of the main restrictions is thus the lack of documented lactate measurements at short intervals as well as some clinical parameters. In a work published 2016, Vincent and colleagues conclude after an extensive review of studies on prognostic value of serial lactate measurements that for optimal prediction of prognosis lactate changes should be recorded every 1–2 h [24]. On the other hand, a study by Hernandez et al. [38] in septic shock patients has demonstrated a slower normalization of lactate levels as compared to other parameters such as ScvO2 or capillary refill time. Thus, after 24 h, only 52% of in-hospital survivors have re-achieved normal lactate levels. In the present study, we were not able to provide cut-offs for such short intervals, but only for changes 24 h after hospital admission. However, we suggest here a cut-off for ΔLac at 24 h with a significant correlation to both in-hospital and long-term mortality, making it an easily applicable and valuable risk stratification tool. Another limitation linked to the retrospective design is the lack of a pre-specified treatment algorithm specifically targeting ΔLac, making the theory on lactate kinetics as therapy goal purely speculative in the context of our study. However, we suggest here a possible benchmark for future studies evaluating fluid management guided by a pre-specified mixture of ΔLac and clinical parameters.
Conclusion
ΔLac was robustly associated with short- and long-term mortality in our study cohort of critically ill patients, constituting a quick and easy risk stratification parameter. For future prospective studies with treatment algorithms specifically including ΔLac as target, we provide real-world data and suggest a handy Δ24Lac cut-off of approximately one fifth.
References
Broder G, Weil MH (1964) Excess lactate: an index of reversibility of schock in human patients. Science 143(3613):1457–1459
Weil MH, Afifi AA (1970) Experimental and clinical studies on lactate and pyruvate as indicators of the severity of acute circulatory failure (shock). Circulation 41(6):989–1001
Bakker J, Nijsten MW, Jansen TC (2013) Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care 3(1):12
Zhang H, Vincent JL (1993) Oxygen extraction is altered by endotoxin during tamponade-induced stagnant hypoxia in the dog. Circ Shock 40(3):168–176
Ronco JJ, Fenwick JC, Tweeddale MG, Wiggs BR, Phang PT, Cooper DJ, Cunningham KF, Russell JA, Walley KR (1993) Identification of the critical oxygen delivery for anaerobic metabolism in critically ill septic and nonseptic humans. JAMA 270(14):1724–1730
Friedman G, de Backer D, Shahla M, Vincent JL (1998) Oxygen supply dependency can characterize septic shock. Intensive Care Med 24(2):118–123
Kraut JA, Madias NE (2014) Lactic acidosis. N Engl J Med 371(24):2309–2319
Hernandez G, Bellomo R, Bakker J (2018) The ten pitfalls of lactate clearance in sepsis. Intensive Care Med. https://doi.org/10.1007/s00134-018-5213-x
James JH, Luchette FA, McCarter FD, Fischer JE (1999) Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet 354(9177):505–508
McCarter FD, Nierman SR, James JH, Wang L, King J-K, Friend LA, Fischer JE (2002) Role of skeletal muscle Na+–K+ ATPase activity in increased lactate production in sub-acute sepsis. Life Sci 70(16):1875–1888
Barth E, Albuszies G, Baumgart K, Matejovic M, Wachter U, Vogt J, Radermacher P, Calzia E (2007) Glucose metabolism and catecholamines. Crit Care Med 35(9 Suppl):S508–S518
Jeppesen JB, Mortensen C, Bendtsen F, Møller S (2013) Lactate metabolism in chronic liver disease. Scand J Clin Lab Invest 73(4):293–299
Huckabee WE (1958) Relationships of pyruvate and lactate during anaerobic metabolism. I. Effects of infusion of pyruvate or glucose and of hyperventilation. J Clin Invest 37(2):244–254
Orringer CE, Eustace JC, Wunsch CD, Gardner LB (1977) Natural history of lactic acidosis after grand-mal seizures. A model for the study of an anion-gap acidosis not associated with hyperkalemia. N Engl J Med 297(15):796–799
Vincent JL, Dufaye P, Berré J, Leeman M, Degaute JP, Kahn RJ (1983) Serial lactate determinations during circulatory shock. Crit Care Med 11(6):449–451
Jansen TC, van Bommel J, Bakker J (2009) Blood lactate monitoring in critically ill patients: a systematic health technology assessment. Crit Care Med 37(10):2827–2839
Cecconi M, de Backer D, Antonelli M, Beale R, Bakker J, Hofer C, Jaeschke R, Mebazaa A, Pinsky MR, Teboul JL, Vincent JL, Rhodes A (2014) Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 40(12):1795–1815
Jung C, Fuernau G, de Waha S, Eitel I, Desch S, Schuler G, Figulla HR, Thiele H (2015) Intraaortic balloon counterpulsation and microcirculation in cardiogenic shock complicating myocardial infarction: an IABP-SHOCK II substudy. Clin Res Cardiol 104(8):679–687
Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, Bellamy SL, Christie JD (2009) Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med 37(5):1670–1677
Manikis P, Jankowski S, Zhang H, Kahn RJ, Vincent JL (1995) Correlation of serial blood lactate levels to organ failure and mortality after trauma. Am J Emerg Med 13(6):619–622
Casserly B, Phillips GS, Schorr C, Dellinger RP, Townsend SR, Osborn TM, Reinhart K, Selvakumar N, Levy MM (2015) Lactate measurements in sepsis-induced tissue hypoperfusion: results from the surviving sepsis campaign database. Crit Care Med 43(3):567–573
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche J-D, Coopersmith C, de Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, van der Poll T, Vincent J-L, Wiersinga WJ, Zimmerman JL, Dellinger RP (2017) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 45(3):486–552
Levraut J, Ichai C, Petit I, Ciebiera J-P, Perus O, Grimaud D (2003) Low exogenous lactate clearance as an early predictor of mortality in normolactatemic critically ill septic patients. Crit Care Med 31(3):705–710
Vincent J-L, Quintairos E, Silva A, Couto L, Taccone FS (2016) The value of blood lactate kinetics in critically ill patients: a systematic review. Crit Care 20(1):257
Lichtenauer M, Wernly B, Ohnewein B, Franz M, Kabisch B, Muessig J, Masyuk M, Lauten A, Schulze PC, Hoppe UC, Kelm M, Jung C (2017) The lactate/albumin ratio: a valuable tool for risk stratification in septic patients admitted to ICU. Int J Mol Sci 18(9):1893
Wernly B, Lichtenauer M, Franz M, Kabisch B, Muessig J, Masyuk M, Hoppe UC, Kelm M, Jung C (2017) Model for end-stage liver disease excluding INR (MELD-XI) score in critically ill patients: easily available and of prognostic relevance. PLoS One 12(2):e0170987
Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE (1981) APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med 9(8):591–597
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270(24):2957–2963
Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, Tomlanovich MC (2004) Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med 32(8):1637–1642
Donnino MW, Miller J, Goyal N, Loomba M, Sankey SS, Dolcourt B, Sherwin R, Otero R, Wira C (2007) Effective lactate clearance is associated with improved outcome in post-cardiac arrest patients. Resuscitation 75(2):229–234
McNelis J, Marini CP, Jurkiewicz A, Szomstein S, Simms HH, Ritter G, Nathan IM (2001) Prolonged lactate clearance is associated with increased mortality in the surgical intensive care unit. Am J Surg 182(5):481–485
Nichol A, Bailey M, Egi M, Pettila V, French C, Stachowski E, Reade MC, Cooper DJ, Bellomo R (2011) Dynamic lactate indices as predictors of outcome in critically ill patients. Crit Care 15(5):R242
Haas SA, Lange T, Saugel B, Petzoldt M, Fuhrmann V, Metschke M, Kluge S (2016) Severe hyperlactatemia, lactate clearance and mortality in unselected critically ill patients. Intensive Care Med 42(2):202–210
Ghneim MH, Regner JL, Jupiter DC, Kang F, Bonner GL, Bready MS, Frazee R, Ciceri D, Davis ML (2013) Goal directed fluid resuscitation decreases time for lactate clearance and facilitates early fascial closure in damage control surgery. Am J Surg 206(6):995–999 (discussion 999–1000)
Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA (2010) Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA 303(8):739–746
Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, Willemsen SP, Bakker J (2010) Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med 182(6):752–761
Hernández G, Cavalcanti AB, Ospina-Tascón G, Zampieri FG, Dubin A, Hurtado FJ, Friedman G, Castro R, Alegría L, Cecconi M, Teboul J-L, Bakker J (2018) Early goal-directed therapy using a physiological holistic view: the ANDROMEDA-SHOCK-a randomized controlled trial. Ann Intensive Care 8(1):52
Hernandez G, Luengo C, Bruhn A, Kattan E, Friedman G, Ospina-Tascon GA, Fuentealba A, Castro R, Regueira T, Romero C, Ince C, Bakker J (2014) When to stop septic shock resuscitation: clues from a dynamic perfusion monitoring. Ann Intensive Care 4:30
Acknowledgements
We would like to thank Katharina Bannier and Julian Gonschorrek for their support in collecting the patients’ follow-up. MM was supported by a GEROK scholarship by the Collaborative Research Center 1116 (DFG).
Author information
Authors and Affiliations
Contributions
MM and BW analyzed and interpreted the data, drafted the submitted manuscript and approved the final version to be published. MF, BK, CJ and PCS substantially contributed to acquisition and interpretation of data, revised the manuscript critically and approved the final version to be published. ML, JMM, AL, UCH and MK substantially contributed to interpretation of data, revised the manuscript critically for important intellectual content and approved the final version to be published. GZ gave statistical advice. JB and CJ substantially contributed to conception and design and to the interpretation of data, revised the manuscript critically for important intellectual content and approved the final version to be published.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study has been approved by the local ethics committee of the Jena University Hospital.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Masyuk, M., Wernly, B., Lichtenauer, M. et al. Prognostic relevance of serum lactate kinetics in critically ill patients. Intensive Care Med 45, 55–61 (2019). https://doi.org/10.1007/s00134-018-5475-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-018-5475-3