Abstract
Purpose
The clinical significance of septic myocardial dysfunction is controversial, a fact that may be explained by the influence of loading conditions. Many indices may be useful to characterize cardiac function during septic shock, but their feasibility and physiological coherence in the clinical setting are unknown.
Methods
Hemodynamic and echocardiographic data with tissue Doppler and speckle tracking were prospectively recorded on the first 3 days of human septic shock. Hypokinesia, normokinesia, and hyperkinesia were defined as a left ventricular ejection fraction (LVEF) of <45, 45–60, and >60%, respectively. Twelve hemodynamic indices exploring contractility and loading conditions were assessed and analyzed.
Results
Two hundred and ninety-seven echocardiographies were performed in 132 patients. During the first 24 h (H1–24), 48 (36.4%) patients were hyperkinetic, 55 (41.7%) were normokinetic, and 29 (22.0%) patients were hypokinetic. Thirteen patients had a secondary hypokinesia absent at H1–24 but present at H25–48 or H49–72, for an overall incidence of 42 (31.8%) during the first 3 days. Despite a limited feasibility (<50%), global LV longitudinal peak systolic strain was impaired in a majority (>70%) of the patients assessed, including all those with depressed LVEF, and declined early in patients whose LVEF secondarily deteriorated. Most contractility indices were inversely correlated with afterload indices. Hyperkinetic patients exhibited the worst reduction in afterload indices. Hospital mortality was significantly higher in patients with LV hyperkinesia than in their counterparts: 30 (62.5%) vs. 35 (41.7%), p = 0.02.
Conclusions
Speckle tracking-derived strain was reduced in the majority of patients with septic shock, revealing covert septic myocardial dysfunction, but had poor feasibility. We found an inverse correlation between most of the contractility and afterload indices. Precise evaluation of afterload is crucial for adequate interpretation of LV systolic function in this setting.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Septic myocardial dysfunction (SMD) was described during septic shock by Parker et al. in 1984 [1], but its prevalence, clinical significance, and prognosis are still being debated [2]. The prevalence of left ventricle (LV) systolic dysfunction varies widely (18–65%) during human septic shock, depending on studies [2]. This variability may be explained by timing of evaluation, the accuracy of the routine indices used to assess LV systolic function, and most importantly their dependency on loading conditions [3]. Some authors have hypothesized that in the more severe patients, vasoplegia and reduced afterload may favor preserved or high LV ejection fraction (LVEF) [4], whereas the preservation or restoration of afterload could unmask poor intrinsic LV contractility [2]. However, a comprehensive demonstration of these assumptions in the clinical setting is lacking.
In recent years, new echocardiographic tools have been developed to assess LV function, such as speckle tracking, which evaluates LV deformation over time. Because this technique is angle-independent and less pressure-dependent and more sensitive than LVEF [5, 6], it might allow a more in-depth analysis of the prevalence of LV systolic dysfunction during septic shock and its early detection. Other parameters that can help to evaluate cardiac contractility include tissue Doppler imaging, LV end-systolic maximal elastance, and ventricular–arterial coupling. However, the feasibility, clinical significance, and physiological coherence of these different indices have not been assessed during human septic shock.
The aims of the present study were twofold: (i) to assess the feasibility and physiological coherence of the various indices of cardiac function proposed to assess hemodynamic during septic shock; (ii) to test the role of loading conditions on evaluation of cardiac contractility in septic shock.
Patients and methods
Patients
Patients who met septic shock criteria (as defined according to the American College of Chest Physicians (ACCP)/Society of Critical Care Medicine (SCCM) Consensus Conference [7]) were prospectively included at the medical ICU of Henri Mondor University Hospital (Creteil, France) between November 2010 and March 2013. Patients were included at the onset of septic shock, as defined by start of vasopressor infusion. Non-inclusion criteria were chronic heart failure, defined as a baseline (before ICU admission) LVEF below 45% or a severe valve heart disease. The study was approved by the institutional ethics committee of the French Intensive Care Society as a component of standard care and patient consent was waived. Written and oral information about the study was given to the families. Patient severity was evaluated by the McCabe and Jackson score for underlying diseases, the SAPS II score for acute illness at ICU admission, and the SOFA score for organ dysfunction during septic shock. Norepinephrine was the first-choice vasopressor therapy (used to target a mean arterial pressure of 65 mmHg or more); dobutamine was added in the presence of decreased LVEF (<45%) with ongoing signs of hypoperfusion despite adequate mean arterial pressure (epinephrine could be considered if the latter condition was not met). Follow-up for the study was until hospital discharge.
Echocardiography
To evaluate cardiac function, we performed transthoracic (TTE) or multiplane transesophageal echocardiographies (TEE, when TTE did not allow accurate measurements because of poor acoustic windows) each day during the first 72 h of septic shock (or until vasopressor weaning or death, if they occurred before the 72nd hour). These echocardiographies were performed by trained operators (competence in advanced critical care echocardiography) using an iE33 system (Philips Ultrasound, Bothell, WA, USA) with a standard procedure, to assess LV and right ventricle (RV) size, filling, function, and output, as detailed in the Electronic Supplementary Material (ESM). All measures performed during each examination were averaged over a minimum of three cardiac cycles (five to ten in case of non-sinus rhythm). Because calendar day 1 only represented a few hours in some cases, we separated echocardiographic data based on time elapsed since inclusion, dividing them into three periods: from the first to the 24th hour (H1–24), from the 25th to the 48th hour (H25–48), and from the 49th to the 72nd hour (H49–72). On each of these assessments, LVEF was defined as depressed (<45% or when an inotrope infusion was needed to achieve a value ≥45%), normal (between 45 and 60%), or increased (>60%) [8]. Hypokinesia was defined as the occurrence of depressed LVEF at H1–24 (primary hypokinesia) or after (secondary hypokinesia); the remaining patients were classified as hyperkinetic (LVEF was never depressed and increased at least once) or normokinetic (LVEF was never depressed and never increased).
Speckle tracking imaging
Apical long-axis (four- and two-chamber) clips obtained with a frame rate ≥50 Hz underwent off-line speckle tracking analyses using the semi-automated Philips’ Qlab 8.1 CMQ package (Philips Ultrasound, Bothell, WA, USA) by two trained operators (see ESM). In the Lagrangian strain calculation of strain = displacement/relaxed length, displacement was measured as a weighted average of the myocardial deformation across the myocardium with the weighting greatest at the endocardium. The relaxed length was measured at the endocardial boundary. The cutoff used to assess depressed contractility by speckle tracking was an absolute value of global LV longitudinal peak systolic strain below 16.5% [9].
Assessment of contractility and loading conditions
Preload was assessed using estimates of LV filling pressures (E/A and E/e′ ratios from pulsed-wave Doppler early (E) and late (A) and tissue Doppler early (e′) diastolic wave velocity at the lateral mitral valve annulus) and respiratory variations of vena cava (as surrogates of fluid responsiveness). Afterload was assessed using diastolic arterial pressure (invasive measurement), systemic vascular resistance, end-systolic arterial elastance, and LV end-systolic wall stress (see ESM). LV systolic function was assessed using indices obtained by two-dimensional echocardiography (LVEF), tissue Doppler imaging (tissue Doppler peak systolic wave at the lateral mitral valve annulus) [10], speckle tracking imaging (global longitudinal peak systolic strain and strain rate of the LV), LV end-systolic maximal elastance, and ventricular–arterial coupling, which is the ratio of LV end-systolic maximal elastance and end-systolic arterial elastance (see ESM for formulas).
Statistical analysis
The data were analyzed using the IBM SPSS Statistics for Windows (Version 24.0, IBM Corp Armonk, NY, USA) and R 3.1.2 (The R Foundation for Statistical Computing, Vienna, Austria). Continuous data were expressed as medians [25–75th centiles] unless otherwise specified, and were compared using the Kruskal–Wallis test followed by pairwise Mann–Whitney test with Benjamini–Hochberg correction to control the false discovery rate at the 0.05 level. Categorical variables, expressed as percentages, were evaluated using the Chi-square test or Fisher exact test. The two aims of our study were achieved as follows. First, we tested the feasibility of various indices of cardiac function, and assessed their physiological coherence using hierarchical clustering; this method builds homogeneous clusters based on dissimilarities or distances between cases and proceeds iteratively to join the most similar cases (see ESM). Because longitudinal strain has been suggested as a particularly sensitive method to assess contractility, we tested its usefulness to predict secondary hypokinesia. Second, we assessed the role of loading conditions on cardiac contractility by using bivariate correlations that were further summarized in a correlation matrix (corrplot package within the R environment). Correlations were tested using the Spearman method with Benjamini–Hochberg correction to control the false discovery rate at the 0.05 level. Two-tailed p values less than 0.05 were considered significant.
Results
Patient characteristics and feasibility of echocardiographic parameters
The study flow chart is provided in Fig. S1. The study comprises 132 patients (89 men and 43 women), with a median age of 63.8 [50.6–74.7] years, including 106 (80%) under mechanical ventilation. All patients required a vasopressor infusion to maintain blood pressure and 92 (70%) had an arterial lactate concentration above 2 mmol L−1 [11]; 56 (42%) patients died in ICU. A total of 279 echocardiographies (132 at H1–24, 74 at H25–48, and 73 at H49–72) were performed in these patients during the first 3 days of septic shock. The feasibility of echocardiographic parameters varied widely (Fig. 1). Global LV longitudinal strain rate had the worst feasibility (42%).
LV kinetics
At H1–24, 29 (22.0%) patients were hypokinetic (LVEF < 45%), 55 (41.7%) were normokinetic (LVEF between 45 and 60%), and 48 (36.4%) were hyperkinetic (LVEF > 60%). Overall, during the first 3 days of septic shock, LV hypokinesia was diagnosed in a total of 42 (31.8%) patients [including 29 (22.0%) with primary hypokinesia (present at H1–24) and 13 (9.8%) with secondary hypokinesia (absent at H1–24 but present at H25–48 or H49–72)] (Fig. 2). All patients’ clinical characteristics and comorbidities were similar between groups at initial assessment (H1–24), except for a higher prevalence of cirrhosis in patients with primary hyperkinesia as compared to their counterparts (Table 1). As expected, patients with primary hypokinesia had reduced LV contractility indices and received more inotropes (Table 1). Global LV longitudinal peak systolic strain was impaired in a majority of the patients that could be assessed for this parameter: at H1–24, 57/78 (73.1%) and 63/78 (80.3%) patients had an absolute value below 16.5 and 18.5%, respectively, including all those with an LVEF < 45%. As expected, LVEF and absolute values of global LV longitudinal peak systolic strain were lower at H1–24 in patients with primary hypokinesia as compared to their counterparts; in addition, the latter parameter was already lower at H1–24 in patients with normal LVEF but who exhibited a secondary hypokinesia (Fig. 3).
Left ventricle ejection fraction (EF) and absolute values of global left ventricle longitudinal peak systolic strain (AS) during the first 24 h of septic shock (H1–24), according to the occurrence and timing of hypokinesia: no hypokinesia (green boxes), primary hypokinesia (present at H1–24, red boxes), or secondary hypokinesia (absent at H1–24 but present at H25–48 or H49-72, blue boxes). The box-and-whisker plots represent median (thick horizontal bar), 25th and 75th percentiles (bottom/top of the boxes), 5th and 95th percentiles (thin horizontal bars). *p < 0.05 (corrected Mann–Whitney test after Kruskal–Wallis test) as compared to no hypokinesia; # p < 0.05 (corrected Mann–Whitney test after Kruskal–Wallis test) as compared to primary hypokinesia
Role of loading conditions
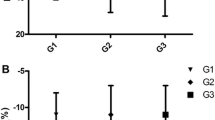
LV end-diastolic volumes and preload indices were similar between LV kinetics groups at H1–24; in contrast, patients with primary hyperkinesia exhibited the most severe reduction in afterload indices (Table 1). An unsupervised computer-generated hierarchical clustering of echocardiographic parameters at H1–24 identified three coherent clusters involving the following physiological pathways: contractility, afterload, and preload (Fig. 4a). In the correlation matrix (Fig. 4b), most contractility indices were not associated with preload indices, but were inversely correlated with afterload indices. The correlations of LVEF with LV longitudinal peak systolic strain and LV end-systolic wall stress at H1–24 are shown in Fig. 5a and b as an illustration. In-ICU and in-hospital mortality were significantly higher in patients with LV hyperkinesia as compared to their counterparts: 26 (54.2%) vs. 30 (35.7%), p = 0.04 and 30 (62.5%) vs. 35 (41.7%), p = 0.02, respectively.
Hierarchical clustering (a) and matrix correlation (b) of contractility and loading conditions indices recorded during the first 24 h of septic shock. In a, the parameters were reordered using computerized hierarchical clustering with the corrplot package of R statistical environment. Hierarchical clustering is a statistical method for finding comparatively homogeneous clusters of cases based on measured characteristics. The analysis starts with each case as a separate cluster (i.e., there are as many clusters as cases), and then combines the clusters sequentially, reducing the number of clusters at each step. The clustering method uses the dissimilarities between objects. The algorithm uses a set of dissimilarities or distances between cases when constructing the clusters and proceeds iteratively to join the most similar cases. Distances between clusters were recomputed by the Lance–Williams dissimilarity update formula according to the complete linkage method. In b, the three big squares drawn in the chart are based on the results of hierarchical clustering and contain each cluster’s members (contractility cluster in the upper left, afterload cluster in the middle, and preload cluster in the lower right). Numbers and the blue-white-red color spectrum denote Spearman correlation coefficients (with Benjamini–Hochberg correction to control the false discovery rate at the 0.05 level); positive correlations are blue, negative correlations are red; the areas of color pixels and their intensity show the absolute value of corresponding correlation coefficients; non-significant coefficients are left blank. There was a strong correlation between most indices within the contractility cluster (blue pixels in the upper-left cluster) and within the afterload cluster (blue pixels in the middle cluster). In addition, most contractility indices were inversely correlated with afterload indices (red pixels above and on the left of the middle cluster), but not with preload indices. All available echocardiographic parameters were recorded during first 24 h of septic shock (H1–24). List of abbreviations: respiratory variation of inferior vena cava in % (IVC); respiratory variation of superior vena cava in % (SVC); ratio of early to late diastolic wave velocities at the mitral valve (EA); ratio of early pulsed-wave Doppler to early tissue Doppler diastolic wave velocity at the lateral mitral valve annulus (Ee); LV ejection fraction in % (EF); absolute values of global LV longitudinal peak systolic strain in % (AS); global LV longitudinal peak systolic strain rate in s−1 (SR); tissue Doppler peak systolic wave at mitral lateral annulus in cm s−1 (s); ventricular-arterial coupling (VAC); LV end-systolic maximal elastance in mmHg mL−1 (ME); end-systolic arterial elastance in mmHg mL−1 (AE); systemic vascular resistance in mmHg L−1 min (SVR); LV end-systolic wall stress in mmHg mL (WS); diastolic arterial pressure in mmHg (DAP)
Discussion
We herein report a reduction of LV contractility in one-third (as assessed by LVEF) and more than two-thirds (as assessed by speckle tracking-derived LV longitudinal peak systolic strain) of patients during septic shock. The latter index was reduced early in patients whose LVEF secondarily deteriorated. There was an inverse correlation between contractility and afterload indices, with hyperkinetic patients exhibiting the most severe reduction in afterload indices.
Prevalence of hypokinesia and feasibility of echocardiographic indices
The 32% prevalence of hypokinesia evaluated by LVEF in our study is consistent with previous large-size studies [12, 13]. This prevalence varied widely in smaller cohorts and these discrepancies could be explained in part by differences in selection criteria (e.g., septic shock vs. severe sepsis), times of assessment and/or thresholds used to define reduced LVEF (e.g., 45 vs. 50%) [14–16]. When assessed by speckle tracking, the prevalence of SMD in our patients was much higher (>70%) and all patients with reduced LVEF had impaired LV longitudinal peak systolic strain. In addition, LV longitudinal peak systolic strain at H1–24 was lower in patients with secondary hypokinesia as compared to those with preserved LVEF on successive examinations. These findings suggest that speckle tracking may prove useful in predicting secondary overt SMD and may help reveal mild SMD not apparent with conventional echocardiography [6, 17]. Our data also corroborate animal studies suggesting an almost ubiquitous depression of intrinsic LV contractility during sepsis when assessed using speckle tracking or the gold standard technique of pressure–volume loops, which is independent from loading conditions [18, 19]. Unfortunately, this technique is not routinely applicable at the bedside. We found the feasibility of echocardiographic indices to be highly variable in our study. Although strain measurement is angle-independent, less subjective than other measurements (computer-generated), and very sensitive to detect altered contractility, its feasibility during septic shock (less than 50% in our study) seems limited by the need for high frame rate and adequate image quality [20].
Role of loading conditions
Variability in LVEF (and the resulting prevalence of human SMD) may mainly reflect the influence of loading conditions. LVEF and other systolic indices reflect the coupling between LV contractility and LV afterload [21], the latter being particularly reduced during septic shock. In other words, normal LVEF may be observed when afterload is severely impaired, despite seriously decreased intrinsic LV contractility; conversely, arterial tone preservation or restoration may unmask depressed LVEF [4]. Afterload was scrutinized using various parameters in our study, all of which were significantly reduced in the group of patients with hyperkinesia as compared with other patients. Among contractility parameters, only tissue Doppler peak systolic wave at the lateral mitral valve annulus did not significantly correlate with afterload. These findings are in accordance with previous studies suggesting its relative independence from loading conditions [10].
A recent study [22] documented that dynamic LV intraventricular obstruction triggered by hypovolemia in hyperdynamic patients at the early stage of septic shock (within the first 6 h following admission to the ICU) was associated with a worse prognosis. We did not specifically explore LV intraventricular obstruction in our study, but the absence of significant correlation between contractility parameters and preload indices is consistent with substantial fluid resuscitation at the time of our echocardiographic examinations. We found comparable diastolic LV volumes in patients with hypokinesia as compared to others, in keeping with previous studies suggesting the lack of preload adaptation during septic shock [23]. On the contrary, systolic LV volumes were significantly reduced in hyperkinetic patients, suggesting that hyperkinesia was due to a reduction in afterload (with increased stroke volume) rather than to hypovolemia. Cor pulmonale may theoretically alter LV filling and promote LV hyperkinesia [2]; although RV dilatation was common in this series of patients with septic shock, cor pulmonale, which is usually associated with severe ARDS [24], was rare (<10%) and evenly distributed in groups with hyperkinesia, normokinesia, and hypokinesia.
Outcome of SMD
In our study, the reduction of LV contractility was not associated with increased mortality, regardless of the parameter used. On the contrary, there was an association between hyperkinesia, reduced afterload, and increased mortality. These results are in accordance with previous reports suggesting reduced survival in septic and non-septic patients with increased contractility indices using conventional [23, 25] or tissue Doppler echocardiography [26]. Whether vasoplegia drives the excess mortality in septic hyperkinetic patients is a question warranting future research. The role of baseline cardiovascular alterations in cirrhotic patients with septic shock should also be scrutinized.
Implications, strengths and limitations
The strengths of our study include its prospective design and size, the severity of the patients selected, the comprehensive evaluation of myocardial contractility and loading conditions with control for false discovery rate, and the use of tissue Doppler and strain imaging. Our study has the following implications in the clinical and research settings: (i) systolic strain is a sensitive tool to depict covert SMD; (ii) knowledge of afterload is crucial to adequately interpret LV systolic function during human septic shock; and (iii) LV hypokinesia is not associated with excess mortality during septic shock. This point has significant clinical implications, as the use of inotropes during septic shock is generally driven by the assessment of systolic function in general, and of LVEF in particular. As pointed out in a recent review on the topic [3], most of the recent clinical literature has focused on cardiac performance to characterize septic cardiomyopathy, without considering the impact of systemic arterial circulation and heart–vessel interaction, so that readers may underestimate the crucial role of the latter. Very few clinical studies, of limited power (<30 patients), have attempted to investigate this interaction [4, 27], doing so by exploring a single parameter of LV afterload and using conventional echocardiographic indices. Our study is the first to comprehensively assess this interaction, using multiple parameters including new echocardiographic tools such as speckle tracking, in a large population of severe patients with septic shock.
Our study also has limitations. First, the design was monocentric and the number of patients with evolution over time was too low to scrutinize this point. Second, the study was not fully blinded and results of conventional echocardiographic measurements influenced patient treatment. Patients with decreased LVEF received more inotropes, a strategy which might have altered their hemodynamics and outcome. Dobutamine was shown to improve macrocirculation [28] and microcirculation [29] during septic shock, and future trials are needed to assess whether it alters the outcome of septic shock patients with LV hypokinesia at echocardiography. Third, we did not perform a reproducibility study, did not assess all potentially interesting parameters (e.g., mitral annular plane systolic excursion), and the inconsistent feasibility of the assessed parameters generated missing data.
Conclusion
LV longitudinal strain was more than twice as often depressed than LVEF during septic shock, revealing covert SMD; early strain reduction was found in patients with secondary hypokinesia, but this technique had poor feasibility. LV hypokinesia did not alter the prognosis of septic shock, given the common use of inotropes in this subgroup. Contractility indices were inversely correlated with afterload, with the exception of tissue Doppler peak systolic wave at the lateral mitral valve annulus. Our data suggest a widespread reduction of LV contractility during human septic shock and highlight the fact that precise evaluation of afterload is crucial to adequately interpret LV systolic function in this setting.
References
Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, Damske BA, Parrillo JE (1984) Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med 100:483–490
Aneman A, Vieillard-Baron A (2016) Cardiac dysfunction in sepsis. Intensive Care Med 42:2073–2076
Zaky A, Deem S, Bendjelid K, Treggiari MM (2014) Characterization of cardiac dysfunction in sepsis: an ongoing challenge. Shock 41:12–24
Jardin F, Brun-Ney D, Auvert B, Beauchet A, Bourdarias JP (1990) Sepsis-related cardiogenic shock. Crit Care Med 18:1055–1060
Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula J, Sengupta PP (2010) Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr 23:351–369 (quiz 453–355)
Orde SR, Pulido JN, Masaki M, Gillespie S, Spoon JN, Kane GC, Oh JK (2014) Outcome prediction in sepsis: speckle tracking echocardiography based assessment of myocardial function. Crit Care 18:R149
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med 31:1250–1256
Vieillard-Baron A, Caille V, Charron C, Belliard G, Page B, Jardin F (2008) Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med 36:1701–1706
Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH (2013) Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr 26:185–191
Aissaoui N, Guerot E, Combes A, Delouche A, Chastre J, Leprince P, Leger P, Diehl JL, Fagon JY, Diebold B (2012) Two-dimensional strain rate and Doppler tissue myocardial velocities: analysis by echocardiography of hemodynamic and functional changes of the failed left ventricle during different degrees of extracorporeal life support. J Am Soc Echocardiogr 25:632–640
Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD, Singer M, Sepsis Definitions Task Force (2016) Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315:775–787
Pulido JN, Afessa B, Masaki M, Yuasa T, Gillespie S, Herasevich V, Brown DR, Oh JK (2012) Clinical spectrum, frequency, and significance of myocardial dysfunction in severe sepsis and septic shock. Mayo Clin Proc 87:620–628
Landesberg G, Gilon D, Meroz Y, Georgieva M, Levin PD, Goodman S, Avidan A, Beeri R, Weissman C, Jaffe AS, Sprung CL (2012) Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur Heart J 33:895–903
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R (2012) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:580–637
Etchecopar-Chevreuil C, Francois B, Clavel M, Pichon N, Gastinne H, Vignon P (2008) Cardiac morphological and functional changes during early septic shock: a transesophageal echocardiographic study. Intensive Care Med 34:250–256
De Geer L, Engvall J, Oscarsson A (2015) Strain echocardiography in septic shock—a comparison with systolic and diastolic function parameters, cardiac biomarkers and outcome. Crit Care 19:122
Chang WT, Lee WH, Lee WT, Chen PS, Su YR, Liu PY, Liu YW, Tsai WC (2015) Left ventricular global longitudinal strain is independently associated with mortality in septic shock patients. Intensive Care Med 41:1791–1799
Barraud D, Faivre V, Damy T, Welschbillig S, Gayat E, Heymes C, Payen D, Shah AM, Mebazaa A (2007) Levosimendan restores both systolic and diastolic cardiac performance in lipopolysaccharide-treated rabbits: comparison with dobutamine and milrinone. Crit Care Med 35:1376–1382
Natanson C, Danner RL, Elin RJ, Hosseini JM, Peart KW, Banks SM, MacVittie TJ, Walker RI, Parrillo JE (1989) Role of endotoxemia in cardiovascular dysfunction and mortality. Escherichia coli and Staphylococcus aureus challenges in a canine model of human septic shock. J Clin Investig 83:243–251
Vignon P, Huang SJ (2015) Global longitudinal strain in septic cardiomyopathy: the hidden part of the iceberg? Intensive Care Med 41:1851–1853
Robotham JL, Takata M, Berman M, Harasawa Y (1991) Ejection fraction revisited. Anesthesiology 74:172–183
Chauvet JL, El-Dash S, Delastre O, Bouffandeau B, Jusserand D, Michot JB, Bauer F, Maizel J, Slama M (2015) Early dynamic left intraventricular obstruction is associated with hypovolemia and high mortality in septic shock patients. Crit Care 19:262
Vieillard Baron A, Schmitt JM, Beauchet A, Augarde R, Prin S, Page B, Jardin F (2001) Early preload adaptation in septic shock? A transesophageal echocardiographic study. Anesthesiology 94:400–406
Mekontso Dessap A, Boissier F, Charron C, Begot E, Repesse X, Legras A, Brun-Buisson C, Vignon P, Vieillard-Baron A (2016) Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med 42:862–870
Paonessa JR, Brennan T, Pimentel M, Steinhaus D, Feng M, Celi LA (2015) Hyperdynamic left ventricular ejection fraction in the intensive care unit. Crit Care 19:288
Weng L, Liu YT, Du B, Zhou JF, Guo XX, Peng JM, Hu XY, Zhang SY, Fang Q, Zhu WL (2012) The prognostic value of left ventricular systolic function measured by tissue Doppler imaging in septic shock. Crit Care 16:R71
Guarracino F, Ferro B, Morelli A, Bertini P, Baldassarri R, Pinsky MR (2014) Ventriculoarterial decoupling in human septic shock. Crit Care 18:R80
Kumar A, Schupp E, Bunnell E, Ali A, Milcarek B, Parrillo JE (2008) Cardiovascular response to dobutamine stress predicts outcome in severe sepsis and septic shock. Crit Care 12:R35
De Backer D, Creteur J, Dubois MJ, Sakr Y, Koch M, Verdant C, Vincent JL (2006) The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med 34:403–408
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Take-home message
Our study supports the hypothesis of a widespread alteration of LV contractility during human septic shock and suggests that knowledge of afterload, a major prognostic factor, is crucial for adequate interpretation of LV systolic function in this setting.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Boissier, F., Razazi, K., Seemann, A. et al. Left ventricular systolic dysfunction during septic shock: the role of loading conditions. Intensive Care Med 43, 633–642 (2017). https://doi.org/10.1007/s00134-017-4698-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-017-4698-z