Abstract
Purpose
Survivors of acute respiratory distress syndrome (ARDS) are at high risk for new or ongoing physical declines after hospital discharge. The objective of our study was to evaluate the epidemiology of physical declines over 5-year follow-up and identify patients at risk for decline.
Methods
This multi-site prospective cohort study evaluated ARDS survivors who completed a physical status assessment at 3 or 6 months post-discharge. Three measures were evaluated: muscle strength (Medical Resource Council sumscore); exercise capacity [6-min walk test (6MWT)]; physical functioning [36-Item Short Form Health Survey (SF-36 survey)]. Patients were defined as “declined” if a comparison of their current and prior score showed a decrease that was greater than the Reliable Change Index—or if the patient died. Risk factors [pre-ARDS baseline status, intensive care unit (ICU) illness severity, and other intensive care variables] were evaluated using longitudinal, generalized linear regression models for each measure.
Results
During the follow-up of 193 ARDS survivors (55 % male; median age 49 years), 166 (86 %) experienced decline in ≥1 physical measure (including death) and 133 (69 %) experienced a physical decline (excluding death). For all measures, age was a significant risk factor [odds ratios (OR) 1.34–1.69 per decade; p < 0.001]. Pre-ARDS comorbidity (Charlson Index) was independently associated with declines in strength and exercise capacity (OR 1.10 and 1.18, respectively; p < 0.02), and organ failure [maximum daily Sequential Organ Failure Assessment (SOFA) score in ICU] was associated with declines in strength (OR 1.06 per 1 point of SOFA score; p = 0.02).
Conclusions
Over the follow-up period, the majority of ARDS survivors experienced a physical decline, with older age and pre-ICU comorbidity being important risk factors for this decline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Survivors of acute respiratory distress syndrome (ARDS) and other critical illnesses frequently experience long-lasting physical complications [1–6]. Although not fully characterized, survivors have varying trajectories of physical recovery post-ARDS, including being at risk for ongoing or intermittent/relapsing patterns of decline after hospital discharge [2, 5, 6]. This impaired physical status poses high burdens for patients, caregivers, healthcare systems, and society [7–9]. Early identification of ARDS survivors at the highest risk for physical decline in the years after hospital discharge is important for creating targeted interventions that can reduce morbidity, mortality, and healthcare utilization. The objectives of this study were to evaluate the epidemiology of physical decline after hospital discharge in ARDS survivors and to identify patient and intensive care unit (ICU) risk factors for such decline over a 5-year follow-up. We hypothesized that patient characteristics (e.g., age, pre-ICU comorbidities) would increase the risk of physical decline.
Methods
Study design and population
This 5-year longitudinal analysis is part of the Improving Care for ALI Patients (ICAP) study which recruited mechanically ventilated patients with acute lung injury, as determined by the American–European Consensus criteria [10] in effect at the time of study recruitment (2004–2007) [11] (ClinicalTrials.gov identifier NCT00300248). Consistent with the more recent Berlin criteria [12], we used the term ARDS to describe these patients, who were followed until late 2012. The ICAP study recruited patients from 13 ICUs in four hospitals in Baltimore, MD [5]. Exclusion criteria, evaluated based on patients’ status prior to ARDS, are described in the Electronic Supplementary Material (ESM). Written informed consent was obtained from patients, participants received financial compensation for research assessments at clinic visits, and all sites had Institutional Review Board approval for this research [11]. This research was performed in accordance with ethical standards established in the 1964 Declaration of Helsinki and later amendments.
To target patients who may be capable of engaging in post-discharge interventions, ICAP participants were included in this analysis if they completed ≥1 physical assessments at 3 or 6 months of follow-up. The characteristics of the 193 survivors included in this study are compared to the originally enrolled patients in ESM Table 1. Notably, the included population was younger and healthier (e.g., lower Charlson comorbidity score) than participants who died and were ineligible for this evaluation.
Physical status measures and outcomes
We evaluated three distinct measures of physical status spanning the World Health Organization’s International Classification of Functioning, Disability and Health [13], namely, muscle strength, exercise capacity, and physical functioning. Muscle strength [14] measures “structure and functional impairment” and was scored as the percentage of the maximum Medical Research Council (MRC) sumscore (range 0–60, with higher scores indicating greater strength, and a score of <48 designated as “ICU-acquired weakness”) [15]. Exercise capacity [evaluated using the 6-min walk test (6MWT)] [16, 17]) measured “activity limitation”. The 6MWT was performed based on American Thoracic Society guidelines [16], using a single test and the longest walking distance available, and the results are reported as percent predicted value based on established norms [18]. Physical functioning [evaluated using the self-reported 36-Item Short Form Health Survey Physical Function domain (SF-36 PF)] measures “participation restriction.” The SF-36 PF score was measured as the percentage of age-and sex-matched predicted value, with higher scores indicating better function.
Similar to previously published definitions [19, 20], each patient was defined as “declined” when a comparison of his/her current and prior score revealed a decrease that was greater than the Reliable Change Index (RCI) [21] for each physical measure at each follow-up. Patients who died were marked as “declined” in all measures. If a patient did not “decline”, then the outcome was defined as “stable/improved”. The RCI was calculated using previously published data [14, 22], and the RCI thresholds were 3.2, 13.9, and 26.5 for muscle strength (MRC percent of maximum score), exercise capacity (6MWT percent predicted), and physical functioning (SF-36 PF percent predicted), respectively. At the 1-year follow-up, a patient was determined to have declined versus being stable/improved using the measures of the earlier of the preceding 3- and 6-month assessments; thereafter decline was determined based on a comparison of consecutive annual visits (i.e., 12 vs. 24 months; 24 vs. 36 months, and so forth).
Prior to analysis, patients with a missing outcome had their data reviewed to determine if the data were missing due to a known decline in physical function (e.g., testing not done due to the patient being bed bound). If this were the case, the outcome for that time point was imputed as “declined” with 11 such imputations for strength (2 % of observations), 17 (3 %) for exercise capacity, and three (<1 %) for physical function. For the subsequent assessment, imputed values were counted as missing since there was no comparison value in the previous year. Missing data that could not be imputed remained missing.
Exposure variables
The exposure variables were selected a priori [23]. Patient baseline (pre-ARDS) variables included: age, sex, Charlson Comorbidity Index (CCI) [24], and Functional Comorbidity Index [25]. ICU severity of illness measures included: Acute Physiology and Chronic Health Evaluation II score at ICU admission [26], organ failure status [maximum daily Sequential Organ Failure Assessment (SOFA) score in ICU] [27], and acute renal failure requiring dialysis (ever vs. never). ICU variables included: mean daily blood glucose level (modeled as >150 vs. ≤150 mg/dl based on prior research [28], along with a separate indicator for pre-existing diabetes); mean daily doses of benzodiazepines (in midazolam-equivalents [29]), opioids (in intravenous morphine-equivalents [30]), and systemic corticosteroids (in prednisone-equivalents [31], and also modeled as ever vs. never); coma (proportion of ICU days with Richmond Agitation Sedation Scale score [32] −4 or −5); delirium (proportion of non-comatose ICU days with a positive Confusion Assessment Method score for ICU assessment [33]); durations of mechanical ventilation, bed rest (see ESM), and ICU stay. Multiple imputation with chained equations [34] was used to impute missing data for sedation and delirium assessments, similar to prior studies [35].
Statistical methods
Descriptive analysis, including lasagna and spaghetti plots [36] to longitudinally display each patients’ outcome, was conducted.
For each outcome, a separate generalized linear mixed model, with a random intercept for each patient and main effects for each follow-up time, was used to evaluate bivariable associations with each exposure variable. Linearity of the association of each continuous exposure variable with each outcome was confirmed via inspection of locally weighted scatterplot smoothing (LOWESS) plots.
To avoid overfitting the multivariable models, we created three multivariable sub-models to separately evaluate exposure variables within each of the three categories, i.e., patient characteristics, severity of illness, and ICU variables. Pre-existing diabetes was included as an ICU exposure given its relevance with the hyperglycemia ICU variable. Exposure variables were included in their respective sub-model if the variable had a bivariable association, at p < 0.15, with any of the three physical status measures. Exposure variables with an independent association (p < 0.05) in the multivariable sub-models with any of the three physical status measures were included in a final multivariable model.
Standard regression diagnostics, including testing for multicollinearity [5, 37], were assessed. Due to collinearity with bed rest, ICU length of stay and mechanical ventilation were excluded from the ICU sub-model. A single statistical interaction evaluated the CCI and bed rest variables, revealing no important effect across common values of these variables. Hence, the interaction term was not included in the final model. To test the sensitivity of our results to a potential floor effect (i.e., patient scoring lower than the RCI and not be designated as “declined” in next assessment), the regression analyses were re-run as multinomial regression models with three possible outcomes, namely, stable/improved, decline, or not assessable due to floor effect, for comparison with the primary results. We also conducted a second sensitivity analysis using a multinomial regression model that separated mortality from physical decline to evaluate three distinct categories for patient outcome, namely, stable/improved, decline, or death, allowing a comparison of stable/improved versus decline without death. Additionally, a post hoc analysis assessed the impact of variables measuring ARDS severity (SOFA respiratory score at enrollment) and physical therapy in the ICU on the ICU sub-model (ESM). Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC) and STATA 13.0 (StataCorp LP, College Station, TX), with a two-sided p < 0.05 used to indicate statistical significance.
Results
Study population and physical status over 5-year follow-up
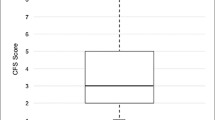
In total, 193 patients, with a median age of 49 (interquartile range 41–58) years, were eligible for the analysis, of whom 55 % (n = 107) were male (Table 1). Almost half of patients (n = 79, 41 %) were discharged home, with 31 % of all follow-up assessments conducted in the home setting (n = 309/1013) rather than at the research clinic. Summary data for the physical status measures over the 5-year follow-up period are given in Fig. 1 and ESM Table 2. The majority of patients (n = 166, 86 %) declined at least once in at least one outcome, with 133 (69 %) experiencing a physical decline ever (excluding death) and 64 (33 %) people eventually dying during the follow-up period (ESM Table 3; ESM Figure). Of the patients with any decline (including death), 153 (92 %) experienced decline(s) in muscle strength (MRC), 103 (62 %) had decline(s) in exercise capacity (6MWT), 109 (66 %) had decline(s) in physical functioning (SF-36 PF), and 78 (47 %) declined in all three physical status measures or died during follow-up. More than one-half of patients experienced decline(s) in strength and either exercise capacity or physical functioning (MRC and 6MWT n = 99, 60 %; MRC and SF-36 PF n = 98, 59 %), and 80 (48 %) patients experienced decline(s) in both exercise capacity and physical functioning (Fig. 2).
Individual patient physical health status at each assessment over the 5-year longitudinal follow-up period. Each black line illustrates an individual patient’s trajectory on the physical health outcome assessment between years 1 to 5. Red triangles Median outcome value at each time point. A total of 64 patients died during the study period
Number and percentage of survivors of acute respiratory distress syndrome (ARDS) with at least one decline in one of the three physical status measures (muscle strength, exercise capacity, and physical functioning) over the 5-year longitudinal follow-up. Among the entire sample of 193 ARDS survivors over the follow-up, 166 (86 %) patients had at least one decline in at least one physical status outcome measure, or died; 21 (11 %) patients never declined; six patients (3 %) were missing one or more of the physical status outcomes. Four patients who declined but were missing outcome variables prohibiting assignment to the above categories are not shown. MRC Medical Research Council sumscore, 6MWT 6-min walk test score, 36-SF PF 36-Item Short Form Health Survey Physical Function domain
Figure 3 illustrates each individual patient’s outcome over the follow-up period. The percentage of survivors with stable/improved status for all 5 years ranged from 9 to 36 % depending on the measure evaluated (n = 18 for strength, n = 54 for exercise capacity, n = 70 for physical functioning). More than 25 % of patients had a decline in physical status during their first year post-discharge, including 7 % who died during this time. Of the 36 patients who survived until their 12-month assessment and then died, 31 (86 %) experienced a decline in at least one outcome before death. A proportion of patients (11–23 %) who declined, but did not die in year 1, also declined at least once more in years 2–5 (n = 42/185 for muscle strength, n = 23/182 for exercise capacity, n = 21/189 for self-reported physical functioning). Within the cohort, 20–37 % were stable/improved in year 1 and then declined at least once in years 2–5 (n = 68/185 for muscle strength, n = 37/182 for exercise capacity, n = 54/189 for self-reported physical health).
Physical status outcome measure for each ARDS survivor over 5-year annual follow-up. a Muscle strength score, b exercise capacity, c physical functioning. Each row within each figure illustrates the outcome (e.g., decline, stable/improved) of one ARDS survivor at each assessment point between years 1 to 5. For each of the three physical status measures, the outcome for each individual patient was defined as “declined” if their current versus immediate prior score demonstrated a decrease greater than the Reliable Change Index [21] for the physical status measure or if the patient had died, as done in prior research [19, 20]. Cells shaded black represent a patient who died during the previous assessment and therefore no additional information is available. These graphs illustrate that, of the entire population, 153 patients (79 %) had ≥1 decline(s) in muscle strength (MRC sumscore), 103 (53 %) had ≥1 decline(s) in exercise capacity (6MWT), and 109 (56 %) had ≥1 decline(s) in self-reported physical functioning (SF-36 PF); declines in all outcomes include death as described in the “Methods” section
Unadjusted bivariable associations of exposures with decline
Patient age, functional comorbidity index, and CCI were significantly associated with decline for all three physical measures. Each severity of illness exposure variable was significantly associated with ≥1 measures, and the following ICU exposures associated with ≥1 measures: mean daily glucose >150 mg/dl, pre-existing diabetes, midazolam-equivalent dose, percentage of ICU days in coma, and durations of mechanical ventilation, bed rest and ICU stay (ESM Table 4).
Adjusted multivariable associations of exposures with decline
In the multivariable sub-models evaluating patient characteristics (Table 2), age and CCI were independently associated with decline (p < 0.05). In the severity of illness sub-model, maximum SOFA score and need for dialysis were independently associated with decline in strength and exercise capacity, respectively. In the ICU sub-model, pre-existing diabetes, midazolam-equivalent dose, and bed rest duration were independently associated with decline in ≥1 measures.
In the final multivariable model, the significant independent association between age and decline in each of the three physical measures remained, with a 34–69 % increase in the odds of decline for every decade increase in age (p < 0.001). The CCI was independently associated with decline in strength [odds ratio (OR) 1.10, 95 % confidence interval (CI) 1.02–1.18], and exercise capacity (OR 1.18, 95 % CI 1.05–1.32), but did not reach statistical significance for self-reported physical functioning (OR 1.08, 95 % CI 0.98–1.18). ICU organ dysfunction had a significant association with decline in strength (OR 1.06; 95 % CI 1.01–1.11) (Table 3).
Over 5-year follow-up, the odds of decline did not change significantly over time, with the exception of a reduced odds of decline for muscle strength and exercise capacity comparing year 5 to year 1 [OR (95 % CI): 0.47 (0.27–0.82), and 0.45 (0.22–0.91), respectively].
Sensitivity analysis
Sensitivity analysis to evaluate for a potential floor effect of the outcome measures demonstrated similar findings to the primary results. Moreover, the exclusion of death from the composite outcome measure produced results similar to those of the primary analysis, except that the association of CCI was attenuated in the sensitivity analysis versus the primary results [OR for strength 1.07 (p = 0.114) vs. 1.10 (p = 0.018); OR for exercise capacity: 1.09 (p = 0.159) vs. 1.18 (p = 0.006); see ESM Table 5a–c].
Discussion
In this multi-site, prospective cohort study of 193 survivors of ARDS, over the 5-year longitudinal follow-up period, 166 (86 %) experienced decline(s) in physical status from their post-discharge state (including death), with 133 (69 %) experiencing a physical decline (excluding death). The majority had a decline in ≥1 measures (i.e., strength, exercise capacity, and self-reported physical functioning). Patients who had stable or improved physical status during the first year after discharge commonly experienced a subsequent decline. Older age and greater comorbidity prior to ARDS onset were significantly associated with a decline in at least two of the three physical measures, while severity of illness and ICU variables were not consistently and significantly associated with decline.
Our findings compliment those reported in prior studies. A multi-site Canadian study evaluating ICU survivors aged ≥80 years reported that age and comorbidity were significantly associated with recovery (measured using SF-36 domain PF) at 1 year [20]. Our study extends these findings to younger ARDS survivors, given the similar findings in our cohort with a median age of <50 years. Moreover, among the adults aged ≥70 years admitted to the ICU, those with a greater disability prior to hospitalization had a worse disability 1 year after discharge [38], and only one-half recovered their pre-ICU function at 6 months post-discharge [39]. Our study furthers these findings by demonstrating that much younger survivors frequently have physical declines beyond 1 year of follow-up, even if they initially demonstrated improvement or stability.
Identifying survivors’ trajectories of physical outcomes after hospital discharge is important in terms designing interventions at the most beneficial time and in the appropriate population [40]. A multi-center Canadian study recently identified four disability-risk groups with differing recovery trajectories based on age, ICU length of stay, and functional dependency [41]. The oldest patients (>65 years) with the longest ICU stay (≥2 weeks) had the worst outcomes, with 40 % dying within the first year [41]. Our study provides additional data on the trajectories of recovery by reporting 5-year outcomes. Within our younger and healthier population, >25 % declined in ≥1 physical measures in the year after discharge, with a majority declining again during years 2–5 of follow-up.
Our study adds novel empirical data on patterns of long-term trajectories after critical illness. Using a proposed framework of prototypical trajectories after acute illness [6], we found that (depending on the physical outcome measured) 9–36 % of our cohort were stable/improved over the entire 5-year period, in accordance with the “big-hit” trajectory (i.e., acute functional decline during ICU, but subsequent recovery). Among survivors with available measures, 11–23 % declined in year 1 of follow-up and then declined again in years 2–5, similar to the proposed “slow-burn” trajectory (i.e., consistent decline over time), and 20–37 % were stable/improved in year 1 subsequently declining at least once in years 2–5, similar to the “relapsing recurrences” trajectory (i.e., repeated acute exacerbations and partial recoveries). Hence, ARDS survivors frequently experience declines during their post-discharge “recovery”, with approximately one-quarter to one-third fitting into each of three different proposed prototypical recovery trajectories.
This work emphasizes the importance of age and comorbidity [2, 8] in evaluating three distinct measures of physical status. Given the aging population [42, 43], more adults will be admitted to the ICU, survive, and be at risk of physical decline and increased healthcare utilization [8]. Our patient population was relatively young and still experienced substantial physical decline based on age- and sex-adjusted values. Identifying new interventions, such as rehabilitation and nutritional interventions, to keep vulnerable patients, such as older survivors with pre-existing comorbidity, from continued physical decline during recovery is important for improving healthcare quality and value. These findings are especially important in terms of targeting specific patient populations for future studies, given that existing research evaluating post-discharge interventions has proven challenging [44, 45].
In our prior research [5], the duration of bed rest in ICU was cross-sectionally associated with muscle weakness. In the current analysis, the ICU sub-model similarly demonstrated that bed rest was significantly associated with decline across strength, exercise capacity and physical functioning over the 5-year follow-up. However, in the final model, the association was attenuated, potentially because the current analysis is underpowered given differences in the prior versus current analysis (e.g., the prior analysis was cross-sectional evaluation in all patients versus the current analysis being a longitudinal analysis of a binary outcome of “decline” greater than the RCI for the measures).
The study strengths include multi-site enrollment across different types of ICUs, 5-year longitudinal follow-up, ascertainment of pre-ARDS comorbidities, and assessment of physical status using three distinct measures linked to clinically important outcomes [5, 17, 46, 47]. However, there are a number of limitations. First, this was an observational study, and we therefore cannot infer a cause–effect relationship between the risk factors and decline in physical outcome, nor can we determine the mechanisms for these findings. Second, because we focused on identifying risk factors available prior to hospital discharge, we did not account for any post-hospital events (e.g., repeat hospitalizations or rehabilitation/nutritional interventions) that may have affected patients’ physical status. Third, generalizability is limited because all patients were ARDS survivors recruited from four teaching hospitals in a single city and we only evaluated three physical health outcomes, excluding mental health outcomes. Fourth, while we included 21 exposure variables of interest, future studies should include additional risk factors (e.g., frailty) which have gained greater awareness since inception of this study. Finally, while we believe that these results do not solely represent normal aging-related changes occurring over the 5-year follow-up (e.g., due to the relatively young age of the cohort and because two of the three outcome measures were evaluated in comparison to age- and sex-adjusted predicted values), the study did not include a control group which would have allowed us to definitively understand if these findings are beyond those expected due to aging or hospitalization without critical illness.
Conclusions
This multi-site, prospective longitudinal cohort study of 193 ARDS survivors found that during a 5-year post hospital discharge recovery period, 166 (86 %) survivors experienced decline(s) (including death) in ≥1 physical measures and 133 (69 %) experienced a physical decline (excluding death). Older age and pre-ICU comorbidity, rather than severity of illness and other ICU factors, were most strongly and consistently associated with this physical decline, and should inform target populations when designing interventions to improve long-term physical health.
References
Jackson JC, Mitchell N, Hopkins RO (2009) Cognitive functioning, mental health, and quality of life in ICU survivors: an overview. Crit Care Clin 25:615–628. doi:10.1016/j.ccc.2009.04.005
Herridge MS, Tansey CM, Matté A et al (2011) Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 364:1293–1304. doi:10.1056/NEJMoa1011802
Dowdy DW, Eid MP, Dennison CR et al (2006) Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med 32:1115–1124. doi:10.1007/s00134-006-0217-3
Desai SV, Law TJ, Needham DM (2011) Long-term complications of critical care. Crit Care Med 39:371–379. doi:10.1097/CCM.0b013e3181fd66e5
Fan E, Dowdy DW, Colantuoni E et al (2014) Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med 42:849–859. doi:10.1097/CCM.0000000000000040
Iwashyna TJ (2012) Trajectories of recovery and dysfunction after acute illness, with implications for clinical trial design. Am J Respir Crit Care Med 186:302–304. doi:10.1164/rccm.201206-1138ED
Ruhl AP, Lord RK, Panek JA et al (2015) Health care resource use and costs of two-year survivors of acute lung injury. An observational cohort study. Ann Am Thorac Soc 12:392–401. doi:10.1513/AnnalsATS.201409-422OC
Lone NI, Gillies MA, Haddow C et al (2016) Five year mortality and hospital costs associated with surviving intensive care. Am J Respir Crit Care Med 194(2):198–208. doi:10.1164/rccm.201511-2234OC
van Beusekom I, Bakhshi-Raiez F, de Keizer NF et al (2016) Reported burden on informal caregivers of ICU survivors: a literature review. Crit Care 20:16. doi:10.1186/s13054-016-1185-9
Bernard GR, Artigas A, Brigham KL et al (1994) The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149:818–824
Needham DM, Dennison CR, Dowdy DW et al (2006) Study protocol: the improving care of acute lung injury patients (ICAP) study. Crit Care 10:R9. doi:10.1186/cc3948
ARDS Definition Task Force, Ranieri VM, Rubenfeld GD et al (2012) Acute respiratory distress syndrome: the Berlin definition. JAMA 307:2526–2533
Iwashyna TJ, Netzer G (2012) The burdens of survivorship: an approach to thinking about long-term outcomes after critical illness. Semin Respir Crit Care Med 33:327–338. doi:10.1055/s-0032-1321982
Fan E, Ciesla ND, Truong AD et al (2010) Inter-rater reliability of manual muscle strength testing in ICU survivors and simulated patients. Intensive Care Med 36:1038–1043. doi:10.1007/s00134-010-1796-6
De Jonghe B, Sharshar T, Lefaucheur J-P et al (2002) Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA 288:2859–2867
ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories (2002) ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 166:111–117. doi:10.1164/ajrccm.166.1.at1102
Chan KS, Pfoh ER, Denehy L et al (2015) Construct validity and minimal important difference of 6-minute walk distance in survivors of acute respiratory failure. Chest 147:1316–1326. doi:10.1378/chest.14-1808
Enright PL, Sherrill DL (1998) Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 158:1384–1387. doi:10.1164/ajrccm.158.5.9710086
Bienvenu OJ, Colantuoni E, Mendez-Tellez PA et al (2012) Depressive symptoms and impaired physical function after acute lung injury: a 2-year longitudinal study. Am J Respir Crit Care Med 185:517–524. doi:10.1164/rccm.201103-0503OC
Heyland DK, Garland A, Bagshaw SM et al (2015) Recovery after critical illness in patients aged 80 years or older: a multi-center prospective observational cohort study. Intensive Care Med 41:1911–1920. doi:10.1007/s00134-015-4028-2
Jacobson NS, Truax P (1991) Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol 59:12–19
Alison JA, Kenny P, King MT et al (2012) Repeatability of the six-minute walk test and relation to physical function in survivors of a critical illness. Phys Ther 92:1556–1563. doi:10.2522/ptj.20110410
Needham DM, Wang W, Desai SV et al (2007) Intensive care unit exposures for long-term outcomes research: development and description of exposures for 150 patients with acute lung injury. J Crit Care 22:275–284. doi:10.1016/j.jcrc.2007.02.001
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Groll DL, To T, Bombardier C, Wright JG (2005) The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol 58:595–602. doi:10.1016/j.jclinepi.2004.10.018
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Vincent JL, Moreno R, Takala J et al (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Hermans G, De Jonghe B, Bruyninckx F, Van den Berghe G (2009) Interventions for preventing critical illness polyneuropathy and critical illness myopathy. Cochrane Database Syst Rev (1):CD006832. doi:10.1002/14651858.CD006832.pub2
Wilson WC, Smedira NG, Fink C et al (1992) Ordering and administration of sedatives and analgesics during the withholding and withdrawal of life support from critically ill patients. JAMA 267:949–953
Jacobi J, Fraser GL, Coursin DB et al (2002) Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med 30:119–141
Brunton L, Chabner B, Knollman B (2011) Goodman and Gilman’s the pharmacological basis of therapeutics, 12th edn. McGraw Hill Educational, New York
Ely EW, Truman B, Shintani A et al (2003) Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA 289:2983–2991. doi:10.1001/jama.289.22.2983
Ely EW, Inouye SK, Bernard GR et al (2001) Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 286:2703–2710
Schafer JL, Graham JW (2002) Missing data: our view of the state of the art. Psychol Methods 7:147–177
Bienvenu OJ, Gellar J, Althouse BM et al (2013) Post-traumatic stress disorder symptoms after acute lung injury: a 2-year prospective longitudinal study. Psychol Med 43:2657–2671. doi:10.1017/S0033291713000214
Swihart BJ, Caffo B, James BD et al (2010) Lasagna plots: a saucy alternative to spaghetti plots. Epidemiology 21:621–625. doi:10.1097/EDE.0b013e3181e5b06a
Hardin JW, Hilbe JM, Hilbe J (2007) Generalized linear models and extensions, 2nd edn. STATA Press, College Station
Ferrante LE, Pisani MA, Murphy TE et al (2015) Functional trajectories among older persons before and after critical illness. JAMA Intern Med 175:523–529. doi:10.1001/jamainternmed.2014.7889
Ferrante LE, Pisani MA, Murphy TE et al (2016) Factors associated with functional recovery among older ICU survivors. Am J Respir Crit Care Med. doi:10.1164/rccm.201506-1256OC (rccm.201506–1256OC)
Herridge MS (2011) The challenge of designing a post-critical illness rehabilitation intervention. Crit Care 15:1002. doi:10.1186/cc10362
Herridge MS, Chu LM, Matté A et al (2016) The recover program: disability risk groups & 1 year outcome after ≥7 days of mechanical ventilation. Am J Respir Crit Care Med. doi:10.1164/rccm.201512-2343OC
Howden LM, Meyer JA (2010) Age and sex composition: 2010. 2010 census briefs. U.S. Department of Commerce Economics and Statistics Administration U.S. Census Bureau. doi: 10.1234/12345678. Available at: http://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf
Rechel B, Grundy E, Robine JM et al (2013) Ageing in the European Union. Lancet 381:1312–1322. doi:10.1016/S0140-6736(12)62087-X
Elliott D, McKinley S, Alison J et al (2011) Health-related quality of life and physical recovery after a critical illness: a multi-centre randomised controlled trial of a home-based physical rehabilitation program. Crit Care 15:R142. doi:10.1186/cc10265
Walsh TS, Salisbury LG, Merriweather JL et al (2015) Increased hospital-based physical rehabilitation and information provision after intensive care unit discharge: the recover randomized clinical trial. JAMA Intern Med 175:901–910. doi:10.1001/jamainternmed.2015.0822
Legrand D, Vaes B, Matheï C et al (2014) Muscle strength and physical performance as predictors of mortality, hospitalization, and disability in the oldest old. J Am Geriatr Soc 62:1030–1038. doi:10.1111/jgs.12840
Hermans G, Van Mechelen H, Clerckx B et al (2014) Acute outcomes and 1-year mortality of intensive care unit-acquired weakness. A cohort study and propensity-matched analysis. Am J Respir Crit Care Med 190:410–420. doi:10.1164/rccm.201312-2257OC
Acknowledgments
The authors thank all patients who participated in the study and the dedicated research staff who assisted with data collection and management for the study, including Kimberly Boucher, Abdulla Damluji, Kristin Sepulveda, Faisal Siddiqi, Jennifer McGrain, Lin Chen, Alexandra Chong, Laura Methvin, Jahnavi Chatterjee, and Mariela Pinedo.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare that they have no conflict of interest to disclosure.
Sources of funding
This research was supported by the National Institutes of Health (P050HL73994, R01HL088045, and K24HL088551) along with the Johns Hopkins Institute for Clinical and Translational Research (ICTR) (UL1 TR 000424-06). Dr. Pfoh’s time was supported by an Institutional National Research Service Award (T32HP10025B0) to Johns Hopkins School of Medicine. The funder played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Additional information
E. R. Pfoh and A. W. Wozniak contributed equally to the manuscript as co-first authors.
Take-home message: Over a 5-year longitudinal follow-up, 86 % of survivors experienced ≥1 episodes of physical decline (including death), and 69 % experienced a physical decline (excluding death). Age and pre-existing comorbidities were independently associated with declines in muscle strength and exercise capacity. Physical rehabilitation interventions should be specifically designed and evaluated for ARDS survivors who are older and have greater pre-ICU comorbidity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pfoh, E.R., Wozniak, A.W., Colantuoni, E. et al. Physical declines occurring after hospital discharge in ARDS survivors: a 5-year longitudinal study. Intensive Care Med 42, 1557–1566 (2016). https://doi.org/10.1007/s00134-016-4530-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4530-1