Abstract
Purpose
To determine whether protein C zymogen (protein C concentrates or human protein C) improves clinically relevant outcomes in adult patients with severe sepsis and septic shock.
Methods
This is a randomized, double-blind, placebo-controlled, parallel-group trial that from September 2012 to June 2014 enrolled adult patients with severe sepsis or septic shock and high risk of death and of bleeding (e.g., APACHE II greater than 25, extracorporeal membrane oxygenation or disseminated intravascular coagulopathy). All patients completed their follow-up 90 days after randomization and data were analyzed according to the intention-to-treat principle. Follow-up was performed at 30 and 90 days after randomization. The primary endpoint was a composite outcome of prolonged intensive care unit (ICU) stay and/or 30-day mortality. Secondary endpoints included mortality.
Results
The study was stopped early in a situation of futility for the composite outcome of prolonged ICU stay and/or 30-day mortality that was 79 % (15 patients) in the protein C zymogen group and 67 % (12 patients) in the placebo group (p = 0.40) and for a concomitant safety issue: ICU mortality was 79 % (15 patients) in the protein C zymogen group vs 39 % (7 patients) in the placebo group (p = 0.020), and 30-day mortality was 68 vs 39 % (p = 0.072).
Conclusion
Protein C zymogen did not improve clinically relevant outcomes in severe sepsis and septic shock adult patients. Given its high cost and the potential increase in mortality, the use of this drug in adult patients should be discouraged.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe sepsis and septic shock are life-threatening medical emergencies [1] and are among the most significant challenges in critical care [2–4]. Recombinant human activated protein C (Xigris®) has antithrombotic, anti-inflammatory, and profibrinolytic properties and produces a dose-dependent reduction in the markers of coagulation and inflammation in patients with severe sepsis [5–7]; however, its use is associated with an increased risk of bleeding [5]. Indeed the PROWESS-SHOCK trial [8] did not confirm any beneficial effect on survival [9], though its beneficial effects on mortality in high-risk patients had been confirmed [10].
The protein C (PC) pathway is a modulator of the coagulation system. Protein C plays an integral role in the host response to infection, modulating the inflammatory and immunomodulatory processes. Protein C is synthesized by the liver as a vitamin K-dependent zymogen (proenzyme) of a serine protease, and it is activated in the blood (activated PC) by the endothelial and platelet thrombin–thrombomodulin complexes and by an endothelial receptor (EPCR) [11, 12]. The anticoagulant effect of PC occurs only when it is becomes activated PC. Since it requires the presence of the thrombin–thrombomodulin complexes, the consumption of these compounds leads to physiological self-limitation of the process, avoiding the risk of having “too much” activated PC and of having an increased risk of bleeding [11, 12].
The US Food and Drug Administration approved plasma-derived protein C zymogen (protein C concentrates or human protein C) as replacement therapy in pediatric patients with congenital deficiency for the prevention and treatment of venous thrombosis and purpura fulminans.
In pediatric patients with life-threatening conditions associated with acquired protein C zymogen deficit (e.g., purpura fulminans and severe sepsis) the use of this plasma-derived drug is widespread with no report of toxicity or bleeding ever being described [13] and with its off-label use approved by the Italian Medicines Agency in this setting (27 August 2012 Official Gazette of the Italian Republic no. 199).
Several case reports and case series [12, 14, 15] recently suggested that protein C zymogen is safe in adult patients and is associated with a lower-than-predicted mortality in severe sepsis and septic shock settings [16].
We previously reported improved outcomes in terms of lower-than-expected mortality in a population of cardiac surgery patients with severe sepsis and septic shock treated with protein C zymogen [14]. Evidence has accumulated on the relevance of the PC pathway in modulating overwhelming inflammation and preventing coagulation derangements, two key mediators of organ damage in sepsis [17, 18]. At the clinical level, protein C zymogen has been administered to more than 340 patients with congenital protein C deficits without any bleeding or allergic complications, and with improvements in coagulation abnormalities [16]. Recent reports suggest that its use is expanding in other settings [19–21]. However, the absence of a randomized clinical trial (RCT) leaves clinicians uncertain as to whether protein C zymogen, an expensive drug, should be prescribed in adult patients with severe sepsis and septic shock to improve clinically relevant outcomes. Accordingly, we conducted an investigator-initiated, double-blind, randomized, placebo-controlled trial to test whether protein C zymogen infusion reduces the incidence of the composite endpoint of prolonged ICU stay and/or mortality in adult patients with severe sepsis or septic shock.
Materials and methods
Study design and participants
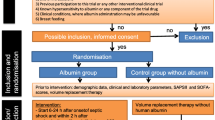
From September 2012 to June 2014 we conducted a randomized, double-blind, parallel-group trial in a 14-bed intensive care unit (ICU). Patients 18 years of age or older who met the eligibility criteria were assessed for enrollment. The study protocol was approved by the ethics committee at San Raffaele University Hospital, Milan, Italy [22]. The full protocol of the study (GR-2009-1607350) is available as Supplementary Material and received a grant from the Italian Ministry of Health.
Prior informed written consent or deferred consent was obtained from all patients or their legal surrogates. The trial planned to enroll 120 patients with severe sepsis or septic shock admitted in the ICU. The study drug protein C zymogen (50 IU/kg in 20 min followed by continuous infusion at 3 IU/kg/h) or equivalent volume normal saline as placebo was administered for 72 h, and the patients were followed up until hospital discharge. We previously described [14] that the dose of 50 IU/kg as a bolus followed by continuous infusion of 3 IU/kg/h for 72 h is effective in increasing protein C levels; indeed, even in patients who did not show a significant increase in circulating activated protein C levels, an early drop in interleukins and near-normalization of prothrombin time, activated partial thromboplastin time, antithrombin, and thrombin–antithrombin complex levels was observed. Schellongowski et al. showed that continuous infusion of protein C reduces the overall amount of administered drug and avoids overtreatment and its associated costs [23]. Telephone follow-up was performed at 30 and 90 days. The principal endpoint was a composite of 30-day mortality and/or prolonged ICU stay (defined as need for ICU 30 days after the randomization).
Adults (age at least 18 years) were eligible if they were at high risk of death as defined by at least one of the following three criteria: venous–venous extracorporeal membrane oxygenation (ECMO) for septic adult respiratory distress syndrome (ARDS); septic disseminated intravascular coagulopathy (DIC) [24] as defined in the Supplementary Material; sepsis-induced organ dysfunction associated with a clinical assessment of high risk of death (e.g., APACHE II score at least 25; or at least two organ dysfunction). Patients were admitted to the ICU mostly for cardiac pathology or acute lung failure and enrolled in the trial in an early phase of sepsis as soon as they met the inclusion criteria (time from meeting inclusion criteria to study drug administration is reported in Table 1).
Culture examinations were requested before starting large spectrum antibiotics. All the patients received the standard best available treatment for sepsis [4, 25] plus protein C zymogen or placebo, according to their allocation group. Our report accords with the CONSORT 2010 statement.
Exclusion criteria, randomization and masking details, together with procedural details and secondary outcomes are presented in the Supplementary Material. The study was stopped early by the Italian Medicines Agency (Italian Food and Drug Administration) with details available in the Supplemental Material.
This study was registered on ClinicalTrials.gov (NCT01705808).
Statistical analysis and sample size
Anticipating a rate of the composite endpoint (prolonged ICU stay and/or mortality) of 50 % in the treatment group and 75 % in the placebo group, we originally determined that enrollment of 116 patients (increased to 120 to take into account possible loss to follow-up or withdrawal of consent) would give the study 80 % power with the use of a two-sided Chi-square test with continuity correction, at a significance level of 0.05. No formal interim analysis was planned. The study was stopped early by the Italian Medicines Agency (Italian Food and Drug Administration) because of an overall high mortality rate (ICU mortality was 59 % at the moment of study interruption). Since there was no planned interim ad analysis, before unblinding, we did a simulation hypothesizing the proportion of events in the experimental and in the control group (p1 and p2) and using three different conditional power values (Supplementary Refs. 6–12 in Supplementary Material) for each simulation. The conditional power indicated that it was highly unlikely (percentage 0.00 %) to observe a beneficial effect of protein C on the primary composite endpoint at trial completion (Supplemental Table 1) thereby confirming that the study had been interrupted by the Italian Medicine Agency in a situation of clear futility (Supplementary Refs. 6–12 in Supplementary Material). The statistically significant (p = 0.02) increase in mortality in the treatment group raised a clear safety reason to interrupt the trial even if this statistically significant difference did not satisfy the stopping rules of the classic O’Brien Fleming approach since the p value should have been 0.00011.
Data were stored electronically and analyzed by STATA 13.0 (STATA). Missing data for baseline characteristics and secondary outcomes were less than 10 % if not otherwise stated in tables. We did not apply any imputation for missing data. All data analysis was carried out according to a pre-established intention-to-treat analysis plan (the only reason for exclusion was withdrawal of patient consent). Dichotomous data (including the primary outcome) were compared by two-tailed χ 2 test with the Yates correction or Fisher’s exact test when appropriate. Continuous measurements were compared using the Mann–Whitney U test. Two-sided significance tests were used throughout. Data are presented as medians (25th and 75th percentiles) or as means (±standard deviation, SD). Means and SDs were used when the variables were normally distributed, whereas medians and interquartile ranges were used with non-normally distributed variables. Differences between the treatment and control group were assessed using univariate and multivariate regression analysis. Risk difference was assessed for categorical variables. Mean or percentile differences were calculated for continuous variables where appropriate. Multivariate regression analyses were performed for mortality. A logistic regression model was used. The pre-randomization clinical data were entered into the model if they had a univariate p value of less than 0.2. Collinearity and overfitting were used. In the multivariate analyses, independent predictors of mortality were expressed as odds ratio with 95 % confidence interval (CI). All p values reported are two-sided.
Role of the funding source
The funder of the study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The corresponding author had full access to all the data in the study and had the final responsibility for the decision to submit for publication.
Results
Study patients
From September 2012 to June 2014 we enrolled 38 patients, and the analyses were conducted on 37 patients because of one consent withdrawal (Supplemental Fig. 1—flow chart). All patients completed their follow-up 30 and 90 days after randomization.
Patients baseline clinical characteristics are reported in Table 1 with no differences between groups. Overall, patients had severe baseline comorbidities such as myocardial infarction (30 %), congestive heart failure (35 %) or ongoing conditions at randomizations such as acute kidney injury (54 %), need for ECMO (27 %), need for vasopressors or inotropic therapy (95 %), need for mechanical ventilation (86 %), and recent complicated major surgery (54 %), with similar distribution between groups. The high risk condition of these patients was also documented by the risk scores (e.g. SAPS II = 61 that corresponds to an overall expected mortality risk of approximately 71 %).
Baseline laboratory findings (Table 2) were similar between groups with the exception of C-reactive protein values that were higher in the placebo group and of fibrinogen that tended to be higher in the placebo group (p = 0.062). Results of blood culture are reported in the Supplemental Table 2.
Patients were randomized when reaching the inclusion criteria at a median of 3 days after ICU admission.
Study treatment
Study drug (Table 3) was administered as a mean total dose of 19,149 IU over 63 h. Study drug interruption before completion occurred in two patients (11 %) in the placebo group who died during administration and in seven patients (37 %) in the protein C zymogen group (four died during administration, two were discharged to the main ward in good general conditions, and one was interrupted by mistake after 2 days of treatment).
Study outcomes
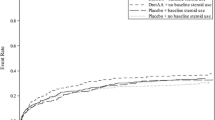
No difference in the primary endpoint (prolonged ICU stay and/or mortality) was noted, as primary endpoint occurred in 15 patients (79 %) in the study group vs 12 patients (67 %) in the placebo group (p = 0.4). However, ICU mortality was increased in the treatment group [15 patients (79 %) vs 7 patients (39 %) in the placebo group, p = 0.020], as was hospital mortality [16 (84 %) vs 8 (44 %) patients, p = 0.017], while it was not statistically different at 30 days [13 (68 %) vs 7 (39 %) patients, p = 0.072] (Table 4). Figure 1 shows the survival curves censored at day 90 for the two intervention groups. Kaplan–Meier analysis confirmed that survival differed significantly between the two groups (p = 0.035).
Univariate predictors of ICU mortality are described in Supplemental Table 3. The only multivariate predictor of ICU mortality was protein C zymogen administration (odds ratio = 5.00, 95 % CI 1.45–17.3, p = 0.011).
The clear difference in mortality between groups and the clear futility for the principal composite endpoint (Supplemental Table 1) dictated that the study be interrupted with meaningful and powered results.
d-dimer, fibrinogen, platelet count, PT ratio, and aPTT ratio values after randomization did not differ between groups and are reported in Supplemental Fig.2.
Safety and toxicity
No adverse reactions were noted during study drug infusion (Table 3). No difference in bleeding, transfusion, or surgical revision was reported (Table 4). No pulmonary thromboembolism, bowel ischemia, amputation, or intracranial hemorrhage were reported.
Discussion
Key findings
In this double-blind RCT in adult patients with severe sepsis and septic shock we found that protein C zymogen was not effective in reducing the composite outcome of prolonged ICU stay and/or mortality at 30 days. Indeed, there was an unexpected statistically significant increase in ICU and hospital mortality in the treatment group, though not associated with an increase in bleeding and/or thrombosis.
This is the first RCT on the use of protein C zymogen performed in adult patients. We chose to perform it in the severe sepsis and septic shock setting and in patients at very high risk of death because this was the most promising setting according to published literature [6, 12, 14] and because the cost of the drug (more than US$30,000 per patient) represents a limit to the use in an early phase of the septic disease [26].
This study was extremely original and innovative since, in spite of the recent widening of the off-label indications in pediatric [20, 21, 27, 28] and adult [19] patients, no randomized, placebo-controlled trial in critically ill pediatric or adult patients has ever investigated clinically relevant endpoints. Another unique feature of our trial is that it enrolled patients admitted to the ICU for a primary disorder other than sepsis (mostly cardiac pathology or acute lung failure).
Our study confirmed previous findings [11, 12, 14, 16, 29] that the use of protein C zymogen is not associated with an increased risk for thrombosis or bleeding. As a matter of fact, incidence of bleeding complications in our study was lower than reported in other case series [30]. This might be explained by the mechanism of action of PC. Protein C can be activated by thrombin alone but more efficiently by the interaction with the thrombin/thrombomodulin complex bound to the endothelial membrane and to the surface of platelets. This process takes place where and when activated PC (APC) is needed, thus preventing clotting with little or no bleeding [11]. As described by other authors, no adverse reaction nor bleeding and/or thrombosis was observed [28]. Similarly, also those patients with impaired coagulation or at risk of hemorrhage did not suffer from adverse events [13, 23]. Nevertheless, the results of our study give a clear and definitive answer on the lack of beneficial effects of this drug on clinically relevant endpoints.
The study also showed an unexpected statistically significant increase in ICU and hospital mortality in patients receiving protein C, which was confirmed in the Kaplan–Meier analyses with 90-day follow-up. The sample size is low and does not permit one to draw definitive conclusions on this important point even if the multivariate analyses confirmed that protein C zymogen was the only independent predictor of mortality. The high costs of this drug and the potential increase in mortality suggest not to use this drug in adult septic patients even if the 30-day mortality, i.e., the follow-up commonly used to investigate drugs, was not statistically significant between protein C zymogen and placebo [26, 31]. It should be recognized that mortality was statistically different at ICU and hospital discharge but not at fixed time points (e.g., 30 or 90 days) raising the possibility of an immortal time bias/attrition bias. Nonetheless, the Kaplan–Meier and multivariate analyses confirmed that the difference in mortality was statistically significant.
Previous relevant studies
The first report on protein C zymogen administration in humans was published by Gerson et al. [32] in 1993 and reported on a case of purpura fulminans in a 13-year-old boy. Rintala et al. [33] were the first to report on the use of protein C zymogen in adult patients. The only randomized experience on protein C zymogen ever published is a dose-finding study in a pediatric population that was not powered to show an effect on mortality rate or clinically relevant endpoints (e.g., length of mechanical ventilation or death) but did show a positive effect on sepsis-induced coagulation disturbances.
Baratto was the first to describe the efficacy of protein C concentrate to restore physiological values in adult septic patients [12]. The largest case series ever published on protein C zymogen in adult patients [34] suggested safety and a reduction in mortality of 30 % vs the expected 53 %, and this difference was even more evident if only septic patients with a cardiac index of at least 2.5 L/min/m2 were considered.
Overall, over 340 patients with non-congenital protein C deficits received protein C zymogen and had the results published [16]. No bleeding complications related to the study drug were reported, and most studies underlined normalization of inflammatory markers and of coagulation abnormalities. Notably, recent case reports suggest that the use of protein C zymogen is widespread in in other clinical settings such as adult solid tumors, oncohematology, and pediatric amputations [19, 20, 27].
Interestingly, even if the activated drug (recombinant human APC) was withdrawn from the market after the large multicenter RCT PROWESS-SHOCK trial concluded that there was no effect on survival [8], meta-analytic evidence is still in favor of a beneficial effect of recombinant human APC on mortality in the highest risk septic population [6].
Strengths and limitations
This trial was randomized and double-blinded in design with allocation concealment, thus reducing the risk of selection bias [35]. It focused on patient-centered, objectively verifiable, and clinically relevant outcomes, thus reducing biases. The intervention had biological plausibility and was supported by a series of case series with promising results, thus justifying the initial trial hypothesis. Even if our population was different from that studied in other sepsis trials, was single centered, and included a mixed population of high-risk patients often taking anticoagulants and with a high transfusion rate, our results appear to have high reproducibility, as the trial protocol was simple, with routine practice maintained throughout, except for protein C zymogen or placebo infusion.
Our study has some limitations. The study was interrupted and fewer patients than planned were randomized. However, this is the only RCT of protein C zymogen ever performed in adult patients. In addition, the case for the futility of the intervention for the primary endpoint was clear. Mortality was more frequent in the protein C zymogen group and it is possible that protein C zymogen caused harm. Even if our study had insufficient power to draw definitive conclusions on survival, this effect was alarming and the multivariate analysis confirmed a causative association between protein C zymogen and mortality. Overall mortality was high in this trial, but the comorbidities and the ongoing acute conditions contributed to the high mortality. The patients included in this trial had an elevated predicted hospital mortality (71 %) and this confirmed that the overall high hospital mortality rate observed in this trial (65 %) was due to prerandomization conditions. Sepsis-related mortality has steadily decreased over time even after adjustments for illness severity, center effect, regional effects, hospital size, risk of being septic, and other key variables [36]. This is happening in our center as well, and we have an overall low hospital mortality [37]. Unfortunately, the patients included in this trial had refractory dysfunctions of the heart and lungs and many of them were treated with mechanical supports. Even if this also happened in other trials [38], we might speculate that patients died with sepsis and not because of sepsis, not allowing protein C zymogen to produce any benefit and maybe only adding harm. More patients experienced an interruption in protein C zymogen treatment than the placebo infusion and this could have limited efficacy or harm.
Our findings differ from several small previous studies. However, the limitations of case reports and case series are well known and may account, alone, for the difference in outcome between our study and previous.
The dose of protein C zymogen administered in this trial might be considered low, but it is similar to that of previous non-randomized case series [12, 14] that had apparently positive findings.
Study implications
Our findings discourage the use of protein C zymogen in adult patients even if we cannot completely rule out its benefit in less sick patients with community-acquired sepsis, without anticoagulants and without bleeding risk associated with invasive procedures.
Conclusions
Protein C zymogen did not improve clinically relevant outcomes in severe sepsis and septic shock adult patients. Given the high costs of this drug and the potential increase in mortality, the use of this drug in adult patients should be discouraged [26, 31]. Further studies are warranted to address new pharmacological targets for this devastating disorder.
References
Zhao H, Heard SO, Mullen MT et al (2012) An evaluation of the diagnostic accuracy of the 1991 American College of Chest Physicians/Society of Critical Care Medicine and the 2001 Society of Critical Care Medicine/European Society of Intensive Care Medicine/American College of Chest Physicians/American Thoracic Society/Surgical Infection Society sepsis definition. Crit Care Med 40:1700–1706. doi:10.1097/CCM.0b013e318246b83a
Gaieski DF, Edwards JM, Kallan MJ, Carr BG (2013) Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 41:1167–1174. doi:10.1097/CCM.0b013e31827c09f8
Brun-Buisson C, Meshaka P, Pinton P, Vallet B (2004) EPISEPSIS Study Group: EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Med 30:580–588. doi:10.1007/s00134-003-2121-4
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348:138–150. doi:10.1056/NEJMra021333
Bernard GR, Vincent JL, Laterre PF et al (2001) Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 344:699–709. doi:10.1056/NEJM200103083441001
Vincent JL, Bernard GR, Beale R et al (2005) Drotecogin alfa (activated) treatment in severe sepsis from the global-label trial ENHANCE: further evidence for survival and safety and implications for early treatment. Crit Care Med 33:2266–2277. doi:10.1097/01.CCM.0000181729.46010.83
Dellinger RP, Levy MM, Carlet JM et al (2008) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med 34:17–60. doi:10.1007/s00134-007-0934-2
Ranieri VM, Thompson BT, Barie PS et al (2012) Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med 366:2055–2064. doi:10.1056/NEJMoa1202290
Vincent JL (2012) The rise and fall of drotrecogin alfa (activated). Lancet Infect Dis 12:649–651. doi:10.1016/S1473-3099(12)70175-5
Greco M, Landoni G, Nobile L et al (2014) Decreasing mortality with drotrecogin alfa in high risk septic patients A meta-analysis of randomized trials in adult patients with multiple organ failure and mortality >40 %. Signa Vitae 9:16–21
Bruley DF, Jagannath SB, Streiff MB (2011) Zymogen Protein C to prevent clotting without bleeding during invasive medical procedures. Adv Exp Med Biol 701:91–97. doi:10.1007/978-1-4419-7756-4_13
Baratto F, Michielan F, Meroni M, Dal Palù A, Boscolo A, Ori C (2008) Protein C concentrate to restore physiological values in adult septic patients. Intensive Care Med 34:1707–1712. doi:10.1007/s00134-008-1140-6
Landoni G, Monti G, Facchini A et al (2010) Human protein C concentrate in pediatric septic patients. Signa Vitae 5:13–19
Crivellari M, Della Valle P, Landoni G et al (2009) Human protein C zymogen concentrate in patients with severe sepsis and multiple organ failure after adult cardiac surgery. Intensive Care Med 35:1959–1963. doi:10.1007/s00134-009-1584-3
Fourrier F, Leclerc F, Aidan K et al (2003) Combined antithrombin and protein C supplementation in meningococcal purpura fulminans: a pharmacokinetic study. Intensive Care Med 29:1081–1087. doi:10.1007/s00134-003-1784-1
Silvetti S, Crivellari M, Mucchetti M et al (2013) Administration of protein C concentrates in patients without congenital deficit: a systematic review of the literature. Signa Vitae 8:15–19
Esmon CT (2005) The interactions between inflammation and coagulation. Br J Haematol 131:417–430. doi:10.1111/j.1365-2141.2005.05753.x
Fisher CJ Jr, Yan SB (2000) Protein C levels as a prognostic indicator of outcome in sepsis and related diseases. Crit Care Med 28:S49–S56
Carobbio A, Finazzi G, Thiele J et al (2012) Blood tests may predict early primary myelofibrosis in patients presenting with essential thrombocythemia. Am J Hematol 87:203–204. doi:10.1002/ajh.22241
Piccin A, O’ Marcaigh A, Mc Mahon C et al (2014) Non-activated plasma-derived PC improves amputation rate of children undergoing sepsis. Thromb Res 134:63–67. doi:10.1016/j.thromres.2014.04.019
Salvo I, Landoni G, Mucchetti M, Cabrini L, Pani L (2014) Use and reimbursement of off-label drugs in pediatric anesthesia: the Italian experience. Pediatr Anaesth 24:625–631. doi:10.1111/pan.12403
(2001) ICH harmonised tripartite guideline: guideline for good clinical practice. 8. Essential documents for the conduct of a clinical trial. J Postgrad Med 47: 264–67
Schellongowski P, Bauer E, Holzinger U et al (2006) Treatment of adult patients with sepsis-induced coagulopathy and purpura fulminans using a plasma-derived protein C concentrate (Ceprotin). Vox Sang 90:294–301. doi:10.1111/j.1423-0410.2006.00760.x
Taylor FB Jr, Toh CH, Hoots WK, Wada H, Levi M, Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH) (2001) Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost 86:1327–1330
Dellinger RP, Levy MM, Rhodes A et al (2013) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39:165–228. doi:10.1007/s00134-012-2769-8
Guidet B, Beale R (2015) Should cost considerations be included in medical decisions? Yes. Intensive Care Med 41:1838–1840. doi:10.1007/s00134-015-3988-6
De Kleijn ED, de Groot R, Hack CE et al (2003) Activation of protein C following infusion of protein C concentrate in children with severe meningococcal sepsis and purpura fulminans: a randomized, double-blinded, placebo-controlled, dose-finding study. Crit Care Med 31:1839–1847. doi:10.1097/01.CCM.0000072121.61120.D8
De Leonardis F, Koronica R, Bruno SD, Santoro N (2014) Non-activated protein C rescue treatment in Wilms tumour associated hepatic sinusoidal obstructive syndrome. Pediatr Blood Cancer 61:940–941. doi:10.1002/pbc.24859
Decembrino L, D’Angelo A, Manzato F et al (2010) Protein C concentrate as adjuvant treatment in neonates with sepsis-induced coagulopathy: a pilot study. Shock 34:341–345. doi:10.1097/SHK.0b013e3181e7623e
Yeo HJ, Kim do H, Jeon D, Kim YS, Cho WH (2015) Low-dose heparin during extracorporeal membrane oxygenation treatment in adults. Intensive Care Med 41:2020–2021. doi:10.1007/s00134-015-4015-7
Hernu R, Cour M, de la Salle S, Robert D, Argaud L (2015) Cost awareness of physicians in intensive care units: a multicentric national study. Intensive Care Med 41:1402–1410. doi:10.1007/s00134-015-3859-1
Gerson WT, Dickerman JD, Bovill EG, Golden E (1993) Severe acquired protein C deficiency in purpura fulminans associated with disseminated intravascular coagulation: treatment with protein C concentrate. Pediatrics 91:418–422
Rintala E, Kauppila M, Seppälä OP et al (2000) Protein C substitution in sepsis-associated purpura fulminans. Crit Care Med 28:2372–2378
Crivellari M, Silvetti S, Gerli C et al (2014) Protein C zymogen in adults with severe sepsis or septic shock. Med Intensiva 38:278–282. doi:10.1016/j.medin.2013.04.005
Gattinoni L, Giomarelli P (2015) Acquiring knowledge in intensive care: merits and pitfalls of randomized controlled trials. Intensive Care Med 41:1460–1464. doi:10.1007/s00134-015-3837-7
Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R (2014) Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 311:1308–1316. doi:10.1001/jama.2014.2637
Landoni G, Pasin L, Monti G, Cabrini L, Beretta L, Zangrillo A (2013) Towards zero perioperative mortality. Heart Lung Vessel 5:133–136
Morelli A, Ertmer C, Westphal M et al (2013) Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA 310:1683–1691. doi:10.1001/jama.2013.278477
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The study received a grant from the Italian Ministry of Health (Grant-2009-1607350). NA and DT received part of their salary through the funder’s grant. GL received modest speaker fees from Octapharma. All other authors declare no competing interests.
Additional information
Take-home message: Protein C zymogen possibly increases mortality in severe sepsis and septic shock patients and its use should be discouraged.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pappalardo, F., Crivellari, M., Di Prima, A.L. et al. Protein C zymogen in severe sepsis: a double-blinded, placebo-controlled, randomized study. Intensive Care Med 42, 1706–1714 (2016). https://doi.org/10.1007/s00134-016-4405-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4405-5