Abstract
Purpose
The association between extracorporeal membrane oxygenation (ECMO) use and the development of thrombocytopenia is widely presumed yet weakly demonstrated. We hypothesized that longer duration of ECMO support would be independently associated with worsened thrombocytopenia.
Methods
We performed a single-center retrospective cohort study of 100 adults who received ECMO for acute respiratory failure. We used generalized estimating equations to test the association between days on ECMO and daily percentage of platelets compared to the first post-cannulation platelet count. We constructed a multivariable logistic regression model with backwards stepwise elimination to identify clinical predictors of severe thrombocytopenia (≤50,000/μL) while on ECMO.
Results
Days on ECMO was not associated with a decrease in platelet count in the unadjusted analysis (β −0.85, 95 % CI −2.05 to 0.36), nor after considering and controlling for days hospitalized prior to ECMO, APACHE II score, platelet transfusions, and potential thrombocytopenia-inducing medications (β −0.83, 95 % CI −1.9 to 0.25). Twenty-two subjects (22 %) developed severe thrombocytopenia. The APACHE II score and platelet count at the time of cannulation predicted the development of severe thrombocytopenia. The odds of developing severe thrombocytopenia increased 35 % for every 5-point increase in APACHE II score (OR 1.35, 95 % CI 0.94–1.94) and increased 35 % for every 25,000/μL platelets below a mean at cannulation of 188,000/μL (OR 1.35, 95 % CI 1.10–1.64).
Conclusions
Duration of ECMO is not associated with the development of thrombocytopenia. The severity of critical illness and platelet count at the time of cannulation predict the development of severe thrombocytopenia while receiving ECMO for respiratory failure. Future studies should validate these findings, especially in cohorts with more venoarterial ECMO patients, and should characterize the association between thrombocytopenia and bleeding events while on ECMO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extracorporeal membrane oxygenation (ECMO) supports gas exchange in cases of severe hypoxemic or hypercapnic respiratory failure refractory to conventional mechanical ventilation [1–7]. Extracorporeal circuits, which consist of drainage and reinfusion cannulae, centrifugal pumps, and membrane oxygenators, require the use of anticoagulation to minimize clotting within the circuit [8, 9]. Because of this need for anticoagulation, ECMO introduces the risk of serious, sometimes fatal, bleeding complications [10–14]. While the development of biocompatible ECMO circuit components in the past decade has allowed for the use of lower levels of anticoagulation, clinically significant hemorrhagic complications still occur, albeit less frequently than in the past [9, 15–18].
Several case series and expert-opinion articles claim that ECMO circuits cause thrombocytopenia by inducing platelet activation and aggregation [19–21]. The potential platelet-reducing effect of ECMO therapy is clinically important since ECMO patients who develop thrombocytopenia may have an increased risk of bleeding [10]. Two recent single-center cohort studies examined the association between ECMO use and the development of thrombocytopenia among adults with acute respiratory failure [13, 22]. While both studies report a significant decline in platelet count over days on ECMO, neither controlled for potential confounding factors including subjects’ severity of illness or the duration of ECMO therapy.
In light of the current ECMO technology now used and recent prior studies that have reported only unadjusted associations between ECMO use and the development of thrombocytopenia, we performed a single-center retrospective cohort study of adults with acute respiratory failure to test the hypothesis that longer time on ECMO is associated with a decrease in platelet counts independent of several important potential confounders. We also sought to determine what other clinical characteristics at the time of ECMO cannulation are associated with the development of severe (≤50,000/μL) thrombocytopenia while on ECMO.
Methods
Subjects, setting, and data sources
We conducted a single-center retrospective cohort study with adults (age ≥18 years) who received venovenous, venoarterial, or venoarterial-venous ECMO for severe acute respiratory failure and were cared for in the Medical Intensive Care Unit (MICU) at Columbia University Medical Center between May 2009 and March 2014. Study subjects were either admitted from our emergency room, or were first hospitalized elsewhere, referred for ECMO support, and transported to our medical center by our ECMO team who cannulated subjects either just prior to or after transportation. Venoarterial or venoarterial-venous ECMO was used if patients had a combination of severe acute respiratory failure and cardiogenic shock. We excluded those who received ECMO support for respiratory failure due to acute pulmonary thromboembolism or decompensated pulmonary arterial hypertension.
We selected ECMO cannula size [21–23 French (Fr) venous drainage, 18–22 Fr venous reinfusion, 14–17 Fr arterial reinfusion, and 20–31 Fr bicaval dual-lumen cannulae] based upon patients’ blood vessel diameter and estimated cardiac output. We used the Rotaflow centrifugal pump (Maquet, Rastatt, Germany) and Quadrox D or I oxygenator (Maquet, Rastatt, Germany) or a CARDIOHELP system (Maquet, Rastatt, Germany). We administered a bolus of unfractionated heparin (3000–5000 units) at the time of cannulation, followed by a continuous intravenous infusion of unfractionated heparin for a target activated partial thromboplastin time (aPTT) of 40–60 s, unless there was an indication for a higher level of anticoagulation. The transfusion thresholds for red blood cells and platelets were hemoglobin <7 g/dL and platelet count ≤20,000 or ≤50,000/µL with bleeding, respectively [9]. We replaced the oxygenator if the patient had inadequate blood oxygenation and there was evidence of low oxygen transfer across the membrane.
We prospectively assessed the Acute Physiology and Chronic Health Evaluation (APACHE) II score, the ratio of partial pressure of oxygen in arterial blood to fraction of inspired oxygen (PaO2/FIO2), the platelet count immediately prior to ECMO cannulation, days hospitalized before ECMO cannulation, and the indications for and duration of ECMO. We retrospectively ascertained comorbid conditions, laboratory data, transfusions received, medications given, use of renal replacement therapy, and oxygenator circuit changes while receiving ECMO.
Measurements
The key exposure variable of interest was days of ECMO support. We chose the daily percentage of platelets compared to the first post-cannulation platelet count to be the primary outcome measure in order to normalize for differences in baseline platelet counts between subjects. The daily percentage of platelets was calculated by dividing the daily mean platelet count (the mean value for all platelet counts available on a given calendar day) by the first post-cannulation platelet count. We used the first post-cannulation platelet count because 52 subjects were missing pre-cannulation platelet count data from the referring hospital. We performed a sensitivity analysis to evaluate whether using the first post-cannulation platelet count (rather than the last platelet count prior to ECMO cannulation) potentially affected our results (see supplementary methods).
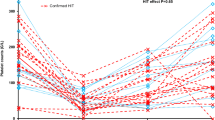
Platelet transfusions were measured in units of platelets. We counted the number of medications given that could potentially cause thrombocytopenia (see supplementary methods), but we did not include heparin because all subjects received heparin. We evaluated subjects for heparin-induced thrombocytopenia (HIT) when it was suspected (see supplementary methods) [23–25]. Comorbid conditions were quantified with the Charlson index [26]. While on ECMO, hepatic dysfunction was assessed by daily liver SOFA scores [27, 28], and renal dysfunction was assessed either as acute kidney injury that was defined as 1.5-fold increase in creatinine [29], or the by the use of renal replacement therapy.
Statistical analysis
Baseline characteristics were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR). We compared categorical variables using Chi square or Fischer exact tests, and compared continuous variables across quartiles of ECMO duration using analysis of variance or Kruskall–Wallis tests.
We used generalized estimating equations to examine the association between days on ECMO and the daily percentage of platelets compared to the first post-cannulation platelet count. We used an autoregressive structure of the correlation matrix and a robust (sandwich) variance estimation because we assumed that repeated measures of platelets would be most strongly correlated within subjects and when close together in time, and least correlated when furthest apart in time. We included covariables from Table 1 in the adjusted model that were either associated with the daily percentage of platelets compared to baseline while on ECMO at p < 0.2, or that changed the size of the effect estimate for days on ECMO by >10 % in bivariate analyses (see footnote to Table 2 for adjusted model covariables).
To determine what other clinical characteristics predicted the development of thrombocytopenia while receiving ECMO support, we created logistic regression models with the outcome defined as a platelet count of ≤50,000/µL. We first conducted univariable analyses for patient characteristics. Since we were interested in predicting the development of severe thrombocytopenia at the time of ECMO cannulation, we constructed a multivariable model using only patient characteristics known at the time of ECMO cannulation. We used backward stepwise elimination, initially including all variables for which p < 0.2 in univariable analyses. We assessed the model’s discrimination by the area under the receiver operating characteristic curve (AUC). We examined the model’s calibration by using the Hosmer–Lemeshow goodness of fit (GoF) Chi-squared test statistic for ten equally sized groups. To correct for optimism and internally validate the model, we repeatedly fit the model with 100 bootstrap samples to calculate the average AUC [30].
Statistical significance was defined as a two-tailed p < 0.05. Analyses were performed with Stata 13.0 (StataCorp LP, College Station, TX). This study was conducted in accordance with the amended Declaration of Helsinki. The Columbia University Institutional Review Board approved the study (Protocol: AAAQ6609).
Results
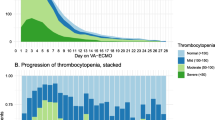
One hundred subjects had a median (IQR) age of 37 (28–53) years, 59 % were men, and few had any comorbidities [median (IQR) Charlson Index 0 (0–1)]. None had cirrhosis or HIV, three had hematologic malignancies, one was found to have hemophagocytic lymphohistiocytosis, and nine and two had undergone solid organ transplantation and bone marrow transplantation on prior hospitalizations, respectively. Ninety-one percent of subjects had severe acute respiratory distress syndrome (ARDS), 5 % had refractory status asthmaticus, and 4 % had acute exacerbations of chronic obstructive pulmonary disease (COPD). The mean (±SD) APACHE II score was 27 (±8), the mean (±SD) baseline platelet count after ECMO cannulation was 188,000/µL (±105,000), and the mean (±SD) platelet nadir while on ECMO support was 98,000/µL (±59,000). None of these characteristics differed significantly by duration of ECMO support. Twenty-two subjects (22 %) developed a platelet nadir ≤50,000/µL. No subjects developed HIT. Subjects in the fourth quartile of duration of ECMO support (12–21 days) tended to receive more thrombocytopenia-inducing medications than the other subjects (p < 0.01). Only 5 % of subjects had a single daily liver SOFA score ≥3, and liver SOFA scores and use of renal replacement therapy were similar across quartiles of days on ECMO. Seventy-three percent of subjects survived to hospital discharge (Table 1; supplementary Table 1).
Figure 1 shows that the daily percentage of platelets compared to the first post-cannulation platelet count varies widely across the cohort with many patients’ platelets increasing after ECMO initiation and others decreasing. Subjects who remain on ECMO longer appear to more often have declining platelet counts over time. When stratified by survival to hospital discharge, it appears that several survivors and decedents have increases and decreases in their percentage of platelets from baseline over time (see supplementary Figs. 1, 2). In the unadjusted analysis, for every 1 day of ECMO support, the percentage of platelets compared to the baseline post-cannulation count decreased 0.85 % on average, but the association was not statistically significant (β −0.85, 95 % CI −2.05 to 0.36, Table 2). The association remained non-significant in the adjusted model (β −0.83, 95 % CI −1.9 to 0.25). In the sensitivity analysis using the pre-ECMO cannulation platelet count of those with available data to determine the daily relative percentage of platelets, the number of days on ECMO was still not associated with a decrease in platelet count in both the unadjusted and adjusted analyses (see supplementary results and supplementary Tables 2, 3).
At the time of ECMO initiation, the APACHE II score and baseline platelet count were the only two clinical factors associated with the development of thrombocytopenia ≤50,000/μL during ECMO support in univariable logistic regression analyses (p < 0.2) (Table 3) and were therefore included in the multivariable logistic regression model. We attempted backward stepwise elimination with a likelihood ratio test and found that both these variables should be included in the final model. For every 5-point increase in APACHE II score, the odds of developing thrombocytopenia ≤50,000/μL increased by 35 % (OR 1.35, 95 % CI 0.94–1.94), and for every 25,000/μL platelets below a mean at cannulation of 188,000/μL, the odds of developing thrombocytopenia ≤50,000/μL also increased by 35 % (OR 1.35, 95 % CI 1.10–1.64) (Table 3). The AUC for the model was 0.78 (SE 0.005). The average AUC after bootstrap resampling decreased marginally to 0.77 (SE 0.01), suggesting the model is internally valid. The model was well calibrated on the basis of a GoF test p = 0.24. The associations of APACHE II score and platelet count at the time of cannulation with platelet count nadirs while on ECMO are shown in the box plots of Fig. 2a, b. Median platelet nadir while on ECMO declines over increasing quartiles of APACHE II scores (p for trend = 0.015) and decreasing quartiles of platelet counts at the time of ECMO cannulation (p for trend = 0.004).
While on ECMO, for every 1-point increase in median daily liver SOFA score the odds of developing severe thrombocytopenia increased 84 % (OR 1.84, 95 % CI 1.15–2.92), and the need for renal replacement therapy was associated with 3.71 times the odds (95 % CI 1.38–9.99) of developing severe thrombocytopenia.
Discussion
In this single-center cohort study, we were unable to confirm our hypothesis that the number of days on ECMO is associated with a decrease in daily platelet counts after considering and controlling for several important potential confounders. Since ECMO duration was not associated with a decrease in platelet count, we sought to determine which clinical variables predict severe thrombocytopenia at the time of ECMO cannulation. We found that only a lower initial post-cannulation platelet count was associated independently with developing severe thrombocytopenia and that a lower post-cannulation platelet count and higher APACHE II score together provide the best prediction of developing severe thrombocytopenia. The development of renal and hepatic failure while on ECMO was strongly associated with the development of thrombocytopenia ≤50,000/μL. This new knowledge suggests that the perceived association between ECMO and the development of thrombocytopenia is best explained by the initial severity of critical illness and the development of multi-organ failure while on ECMO. Our prediction model will help clinicians better evaluate the risk of thrombocytopenia, potential need for transfusions, and bleeding in adults with acute respiratory failure who are being considered for or are receiving ECMO support.
There are several reasons why our findings contradict two prior cohort studies that concluded that platelets counts decline with ECMO use [13, 22]. In a cohort study of 225 adults with severe ARDS who were supported with either venovenous ECMO or pumpless arteriovenous interventional lung assist (iLA), platelet counts decreased significantly in those who received ECMO but not in those who received iLA. ECMO subjects, however, were more critically ill than iLA subjects with higher SOFA scores and a higher prevalence of disseminated intravascular coagulation (DIC). Accordingly, the observed association between ECMO use and thrombocytopenia may be confounded by the subjects’ severity and duration of critical illness. Indeed, 22 % of subjects in our study developed severe thrombocytopenia while receiving ECMO, but we show that a higher initial severity of critical illness, lower baseline platelet count, and the development of hepatic or renal failure account for that association, not ECMO use. In a cohort study of 12 adults with acute respiratory failure who were supported with venovenous ECMO, investigators observed a significant decline in platelet count between the first day on ECMO and 1 week later, but they did not control for subjects’ severity of critical illness nor the duration of ECMO therapy (only 75 % of subjects remained on ECMO when the platelet measurement was made at 1 week) [22]. In our study, we controlled for the severity of critical illness and employed a repeated measures analysis using generalized estimating equations that accounted for the different durations of ECMO support between subjects.
Our finding of a lack of an association between duration of ECMO use and thrombocytopenia is biologically plausible for several reasons. Historically, thrombocytopenia occurred with conventional cardiopulmonary bypass circuitry as a result of shear forces, hypothermia, and exposure to artificial surfaces with resultant platelet aggregation [31]. However, more recent in vitro studies of platelet aggregation in the context of centrifugal pumps and hollow-fiber oxygenators, such as the ones used in our study, suggest that these phenomena may not necessarily occur with modern extracorporeal technology [29]. Many critically ill patients develop thrombocytopenia either as a result of inflammation-related bone marrow suppression or platelet consumption due to DIC [32–35]. Higher critical illness severity, organ dysfunction, and sepsis have been shown to be independent predictors of the development of thrombocytopenia in critically ill patients [36], and the component variables of APACHE II score capture direct and indirect measures of critical illness physiology, organ dysfunction, and inflammation reflecting sepsis. Our finding that a lower initial post-cannulation platelet count predicts the development of severe thrombocytopenia is likely an indirect measure of subjects’ platelet reserve, which is likely affected by the severity of critical illness both prior to and immediately after ECMO cannulation. The observation in Fig. 1 that subjects who remain on ECMO longer tend to have greater declines in their platelet counts may represent confounding by indication. That is, subjects who require a longer duration of ECMO support may also develop worse thrombocytopenia because they have a higher severity of critical illness, are more likely to develop multi-organ failure, and are exposed to more thrombocytopenia-inducing medications over a longer period of time [37].
Being able to identify patients at risk for the development of severe thrombocytopenia during ECMO support based on the initial severity of critical illness and baseline platelet count may help providers anticipate which patients are most likely to develop a transfusion need and have an increased risk of bleeding. Future studies are needed to determine what degree of thrombocytopenia in conjunction with other coagulation parameters will predict hemorrhagic complications while receiving ECMO support.
While we enrolled more participants and evaluated and controlled for more confounders than several prior studies that have examined ECMO use and hemostatic changes [22, 38], our study also has several weaknesses. There is the potential that unmeasured or poorly measured confounding and bias secondary to missing data limited our results. We considered and controlled for several baseline demographic and clinical characteristics including the APACHE II score as well as several measures of organ dysfunction while on ECMO, but we could not fully control for the severity of critical illness over time with complete daily SOFA scores since we did not prospectively collect the Glasgow coma scale data on a daily basis. Future studies should explore whether different types of patients treated with ECMO might still develop thrombocytopenia due to ECMO and assess potential associations between thrombocytopenia and the development of DIC in ECMO patients, which we did not systematically evaluate. Measurements of negative pressure gradients and microscopic measurement of platelet adherence to the ECMO membrane after ECMO weaning should be also considered in future studies. We used the first post-cannulation platelet count as our reference because approximately half of the cohort was missing pre-ECMO platelet count data. Given that a prior study of ECMO in neonates showed a large decrease in platelet counts immediately following cannulation [39], we performed several analyses to show that using the post-cannulation platelet count in our study is unlikely to bias our results. We showed that subjects with and without pre-ECMO platelet count data did not appear to differ, that the percentage change in platelet count before and after ECMO cannulation did not differ, and that our results were robust to a sensitivity analysis using pre-ECMO platelet counts rather than the first post-ECMO cannulation platelet counts. While use of venoarterial ECMO was not associated with development of thrombocytopenia in our study, there are too few subjects to make any conclusions from this observation [40]. The findings of this retrospective single-center study may not be generalizable to centers that employ different ECMO technologies and anticoagulation strategies. Future studies should validate these findings, especially in cohorts with more venoarterial ECMO patients, and should characterize the association between thrombocytopenia and bleeding events while on ECMO.
Conclusion
In a single-center retrospective cohort study of patients receiving ECMO for acute respiratory failure, ECMO duration was not associated with a decline in platelet counts. Clinicians should consider the severity of critical illness and platelet counts at the time of cannulation when considering a potential ECMO patient’s likelihood of developing thrombocytopenia that may increase risk of bleeding or necessitate transfusion of platelets.
References
Brodie D, Bacchetta M (2011) Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med 365:1905–1914
Abrams D, Brodie D (2013) Emerging indications for extracorporeal membrane oxygenation in adults with respiratory failure. Ann Am Thorac Soc 10:371–377
Brogan TV, Thiagarajan RR, Rycus PT, Bartlett RH, Bratton SL (2009) Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi-center database. Intensive Care Med 35:2105–2114
Roch A, Lepaul-Ercole R, Grisoli D, Bessereau J, Brissy O, Castanier M, Dizier S, Forel JM, Guervilly C, Gariboldi V, Collart F, Michelet P, Perrin G, Charrel R, Papazian L (2010) Extracorporeal membrane oxygenation for severe influenza A (H1N1) acute respiratory distress syndrome: a prospective observational comparative study. Intensive Care Med 36:1899–1905
Schmidt M, Tachon G, Devilliers C, Muller G, Hekimian G, Brechot N, Merceron S, Luyt CE, Trouillet JL, Chastre J, Leprince P, Combes A (2013) Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensive Care Med 39:838–846
Abrams D, Brodie D, Combes A (2013) What is new in extracorporeal membrane oxygenation for ARDS in adults? Intensive Care Med 39:2028–2030
Azoulay E, Citerio G, Bakker J, Bassetti M, Benoit D, Cecconi M, Curtis JR, Hernandez G, Herridge M, Jaber S, Joannidis M, Papazian L, Peters M, Singer P, Smith M, Soares M, Torres A, Vieillard-Baron A, Timsit JF (2014) Year in review in Intensive Care Medicine 2013: II. Sedation, invasive and noninvasive ventilation, airways, ARDS, ECMO, family satisfaction, end-of-life care, organ donation, informed consent, safety, hematological issues in critically ill patients. Intensive Care Med 40:305–319
Abrams D, Combes A, Brodie D (2014) Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol 63:2769–2778
Agerstrand CL, Burkart KM, Abrams DC, Bacchetta MD, Brodie D (2015) Blood conservation in extracorporeal membrane oxygenation for acute respiratory distress syndrome. Ann Thorac Surg 99:590–595
Paden ML, Rycus PT, Thiagarajan RR, Registry E (2014) Update and outcomes in extracorporeal life support. Semin Perinatol 38:65–70
Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, Forrest P, Gattas D, Granger E, Herkes R, Jackson A, McGuinness S, Nair P, Pellegrino V, Pettila V, Plunkett B, Pye R, Torzillo P, Webb S, Wilson M, Ziegenfuss M (2009) Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA 302:1888–1895
Pham T, Combes A, Roze H, Chevret S, Mercat A, Roch A, Mourvillier B, Ara-Somohano C, Bastien O, Zogheib E, Clavel M, Constan A, Marie Richard JC, Brun-Buisson C, Brochard L (2013) Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 187:276–285
Weingart C, Lubnow M, Philipp A, Bein T, Camboni D, Muller T (2015) Comparison of coagulation parameters, anticoagulation, and need for transfusion in patients on interventional lung assist or veno-venous extracorporeal membrane oxygenation. Artif Organs 39:765–773
Nair P, Davies AR, Beca J, Bellomo R, Ellwood D, Forrest P, Jackson A, Pye R, Seppelt I, Sullivan E, Webb S (2011) Extracorporeal membrane oxygenation for severe ARDS in pregnant and postpartum women during the 2009 H1N1 pandemic. Intensive Care Med 37:648–654
Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, Morris AH, Peirce EC 2nd, Thomas AN, Proctor HJ, Drinker PA, Pratt PC, Bagniewski A, Miller RG Jr (1979) Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 242:2193–2196
Morris AH, Wallace CJ, Menlove RL, Clemmer TP, Orme JF Jr, Weaver LK, Dean NC, Thomas F, East TD, Pace NL, Suchyta MR, Beck E, Bombino M, Sittig DF, Bohm S, Hoffmann B, Becks H, Butler S, Pearl J, Rasmusson B (1994) Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 149:295–305
Combes A, Bacchetta M, Brodie D, Muller T, Pellegrino V (2012) Extracorporeal membrane oxygenation for respiratory failure in adults. Curr Opin Crit Care 18:99–104
Yeo HJ, Kim do H, Jeon D, Kim YS, Cho WH (2015) Low-dose heparin during extracorporeal membrane oxygenation treatment in adults. Intensive Care Med 41:2020–2021
Haneya A, Philipp A, Diez C, Ried M, Puehler T, Camboni D, Zausig Y, Lehle K, Schmid C (2009) Comparison of two different minimized extracorporeal circulation systems: hematological effects after coronary surgery. ASAIO J 55:592–597
Oliver WC (2009) Anticoagulation and coagulation management for ECMO. Semin Cardiothorac Vasc Anesth 13:154–175
Peek GJ, Firmin RK (1999) The inflammatory and coagulative response to prolonged extracorporeal membrane oxygenation. ASAIO J 45:250–263
Panigada M, Artoni A, Passamonti SM, Maino A, Mietto C, L’Acqua C, Cressoni M, Boscolo M, Tripodi A, Bucciarelli P, Gattinoni L, Martinelli I (2016) Hemostasis changes during veno-venous extracorporeal membrane oxygenation for respiratory support in adults. Minerva Anestesiol 82:170–179
Glick D, Dzierba AL, Abrams D, Muir J, Eisenberger A, Diuguid D, Abel E, Agerstrand C, Bacchetta M, Brodie D (2015) Clinically suspected heparin-induced thrombocytopenia during extracorporeal membrane oxygenation. J Crit Care 30:1190–1194
Cuker A, Gimotty PA, Crowther MA, Warkentin TE (2012) Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood 120:4160–4167
Laverdure F, Louvain-Quintard V, Kortchinsky T, Rezaiguia-Delclaux S, Imbert A, Stephan F (2016) PF4-heparin antibodies during ECMO: incidence, course, and outcomes. Intensive Care Med. doi:10.1007/s00134-016-4262-2
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S (1998) Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 26:1793–1800
Schmidt M, Zogheib E, Roze H, Repesse X, Lebreton G, Luyt CE, Trouillet JL, Brechot N, Nieszkowska A, Dupont H, Ouattara A, Leprince P, Chastre J, Combes A (2013) The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med 39:1704–1713
Meyer AD, Wiles AA, Rivera O, Wong EC, Freishtat RJ, Rais-Bahrami K, Dalton HJ (2012) Hemolytic and thrombocytopathic characteristics of extracorporeal membrane oxygenation systems at simulated flow rate for neonates. Pediatr Crit Care Med 13:e255–e261
Steyerberg EW, Harrell FE Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD (2001) Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol 54:774–781
Weerasinghe A, Taylor KM (1998) The platelet in cardiopulmonary bypass. Ann Thorac Surg 66:2145–2152
Bonfiglio MF, Traeger SM, Kier KL, Martin BR, Hulisz DT, Verbeck SR (1995) Thrombocytopenia in intensive care patients: a comprehensive analysis of risk factors in 314 patients. Ann Pharmacother 29:835–842
Crowther MA, Cook DJ, Meade MO, Griffith LE, Guyatt GH, Arnold DM, Rabbat CG, Geerts WH, Warkentin TE (2005) Thrombocytopenia in medical-surgical critically ill patients: prevalence, incidence, and risk factors. J Crit Care 20:348–353
Lee KH, Hui KP, Tan WC (1993) Thrombocytopenia in sepsis: a predictor of mortality in the intensive care unit. Singap Med J 34:245–246
Strauss R, Wehler M, Mehler K, Kreutzer D, Koebnick C, Hahn EG (2002) Thrombocytopenia in patients in the medical intensive care unit: bleeding prevalence, transfusion requirements, and outcome. Crit Care Med 30:1765–1771
Hui P, Cook DJ, Lim W, Fraser GA, Arnold DM (2011) The frequency and clinical significance of thrombocytopenia complicating critical illness: a systematic review. Chest 139:271–278
Schmidt M, Brechot N, Combes A (2015) Ten situations in which ECMO is unlikely to be successful. Intensive Care Med. doi:10.1007/s00134-015-4013-9
Lubnow M, Philipp A, Dornia C, Schroll S, Bein T, Creutzenberg M, Diez C, Schmid C, Pfeifer M, Riegger G, Muller T, Lehle K (2014) D-dimers as an early marker for oxygenator exchange in extracorporeal membrane oxygenation. J Crit Care 29(473):e471–e475
Cheung PY, Sawicki G, Salas E, Etches PC, Schulz R, Radomski MW (2000) The mechanisms of platelet dysfunction during extracorporeal membrane oxygenation in critically ill neonates. Crit Care Med 28:2584–2590
Muller G, Flecher E, Lebreton G, Luyt CE, Trouillet JL, Brechot N, Schmidt M, Mastroianni C, Chastre J, Leprince P, Anselmi A, Combes A (2016) The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med 42:370–378
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Brodie is currently on the medical advisory boards of ALung Technologies and Kadence. All compensation for these activities is paid to Columbia University. All other authors have no conflicts of interest to report.
Additional information
M. Bacchetta and D. Brodie are co-senior authors.
D. Abrams and M. R. Baldwin contributed equally to this work.
Take-home message: This study demonstrates that in a large single-center cohort, duration of ECMO support was not associated with a decrease in platelet count, which is contrary to the generally held belief that thrombocytopenia is an inevitable consequence of ECMO use. Instead, the development of thrombocytopenia was associated with severity of illness and lower baseline platelet count, suggesting that the current paradigm, attributing thrombocytopenia to the use of ECMO over time, needs to be revisited.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abrams, D., Baldwin, M.R., Champion, M. et al. Thrombocytopenia and extracorporeal membrane oxygenation in adults with acute respiratory failure: a cohort study. Intensive Care Med 42, 844–852 (2016). https://doi.org/10.1007/s00134-016-4312-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4312-9