Abstract
Purpose
To study the impact of pre-morbid glycemic control on the association between acute hypoglycemia in intensive care unit (ICU) patients and subsequent hospital mortality in critically ill patients.
Methods
We performed a multicenter, multinational, retrospective observational study of patients with available HbA1c levels within the 3-month period preceding ICU admission. We separated patients into three cohorts according to pre-admission HbA1c levels (<6.5, 6.5–7.9, ≥8.0 %, respectively). Based on published data, we defined a glucose concentration of 40–69 mg/dL (2.2–3.8 mmol/L) as moderate hypoglycemia and <40 mg/dL (<2.2 mmol/L) as severe hypoglycemia. We applied logistic regression analysis to study the impact of pre-morbid glycemic control on the relationship between acute hypoglycemia and mortality.
Results
A total of 3084 critically ill patients were enrolled in the study. Among these patients, with increasing HbA1c levels from <6.5, to 6.5–7.9, and to ≥8.0 %, the incidence of both moderate (3.8, 11.1, and 16.4 %, respectively; p < 0.001) and severe (0.9, 2.5, and 4.3 %, respectively; p < 0.001) hypoglycemia progressively and significantly increased. The relationship between the occurrence of hypoglycemic episodes in the ICU and in-hospital mortality was independently and significantly affected by pre-morbid glucose control, as assessed by adjusted odds ratio (OR) and 95 % confidence interval (CI) for hospital mortality: (1) moderate hypoglycemia: in patients with <6.5, 6.5–7.9, and ≥8.0 % of HbA1c level—OR 0.54, 95 % CI 0.25–1.16; OR 0.82, 95 % CI 0.33–2.05; OR 3.42, 95 % CI 1.29–9.06, respectively; (2) severe hypoglycemia: OR 1.50, 95 % CI 0.42–5.33; OR 1.59, 95 % CI 0.36–7.10; OR 23.46, 95 % CI 5.13–107.28, respectively (interaction with pre-morbid glucose control, p = 0.009). We found that the higher the glucose level before admission to the ICU, the higher the mortality risk when patients experienced hypoglycemia.
Conclusions
In critically ill patients, chronic pre-morbid hyperglycemia increases the risk of hypoglycemia and modifies the association between acute hypoglycemia and mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent guidelines for the management of blood glucose levels in critically ill patients recommend the use of a protocolized approach with a target level of blood glucose of <150 mg/dL (<8.3 mmol/L) and the absolute avoidance of levels above 180 mg/dL (10 mmol/L) [1]. This glycemic target is recommended irrespective of pre-morbid glycemic control. However, stricter glycemic control exposes patients to a greater risk of experiencing hypoglycemia.
The incidence of moderate and severe hypoglycemia has been shown to be independently associated with increased mortality in critically ill patients [2–4]. This association has been reported not to be influenced by the presence of diabetes mellitus [3]. However, the glycemic thresholds at which patients with chronic hyperglycemia experience an increase in adrenergic symptoms and release of epinephrine, norepinephrine, cortisol, and growth hormone are significantly higher than those in patients with well-controlled glycemia [5, 6]. Thus, both the incidence and impact of even a mild acute hypoglycemic event should be greater in such patients, and if such events did have greater effects on the patient, the influence of hypoglycemia on outcomes might be expected to differ according to pre-morbid glycemic control.
Accordingly, to test the independent impact of pre-morbid glycemic control on the interaction between hypoglycemic episodes in intensive care unit (ICU) patients and subsequent mortality, we conducted a multicenter, multinational retrospective observational study. We hypothesized that the degree of pre-admission hyperglycemia would affect the interaction between acute hypoglycemia in ICU patients and hospital mortality in critically ill patients.
Methods
Study design
This was a multicenter, international retrospective observational study. The data collection and data analysis parts of this study were part of a pre-existing quality assurance activity which had been approved by local Institutional Ethics Committees of the participating hospitals. These committees waived the need for informed consent for studies involving the use of the database. The Okayama University Hospital Ethics Committee approved this investigation.
Patients
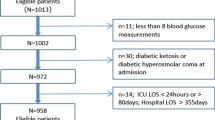
The hospitals participating in this study were located in Japan, USA, and Australia. Table 1 provides an overview of the organizational structure of the ICUs and the glycemic control practices of the different centers. All adult ICU patients admitted to these ICUs during the study period who had their glycated hemoglobin (HbA1c) level measured during the 3 months before or at ICU admission were included in the study in order to assess a wide range of pre-admission glycemic control [7]. We excluded patients without HbA1c measurements at these time-points, including those whose latest HbA1c level had been measured >3 months prior to admission to the ICU.
Primary outcome
The primary endpoint for this analysis was all-cause hospital mortality, defined as death before hospital discharge.
Data source
The blood glucose data collected during each patient’s stay in the ICU and used for this study were retrieved electronically from the relevant data storage unit. Age, sex, requirement of mechanical ventilation, reason for ICU admission (medical or surgical), presence of co-morbidities, including diabetes mellitus, cirrhosis, cardiac dysfunction, and sepsis, and Acute Physiology and Chronic Health Evaluation (APACHE) II score [8] were obtained from the clinical data repositories of each ICU, using data which had been collected prospectively by trained data collectors. Collection of mortality and unit-based outcomes is ongoing for all institutions.
Definition of each cohort
We separated patients into three cohorts according to pre-admission HbA1c levels. The first cohort included patients whose HbA1c level was <6.5 % (normal pre-morbid glycemia). The second cohort included patients whose HbA1c level was between 6.5 and 7.9 % (moderate pre-morbid hyperglycemia). The third cohort included patients with poor pre-admission glycemic control whose HbA1c level was ≥8.0 % (severe pre-morbid hyperglycemia).
The lower threshold of HbA1c level (6.5 %) was selected based on a recent international recommendation for the diagnosis of diabetes [9], and the higher threshold of HbA1c (7.9 %) was selected according to median value of conventional glucose control arm in the United Kingdom Prospective Diabetes Study (UKPDS) study [10].
Statistical analysis
Data are presented as percentages and number (n) or as the median and interquartile range (25 % quartile, 75 % quartile). Comparisons among groups were conducting using the Chi-square test for equal proportions or Kruskal–Wallis tests.
For the analysis, we obtained the lowest blood glucose value during ICU stay (Glumin) in each patient. We applied the hypoglycemic threshold used in the NICE-SUGAR trial [11, 12] and defined a Glumin of 40–69 mg/dL (2.2–3.8 mmol/L) as moderate hypoglycemia and that of <40 mg/dL (<2.2 mmol/L) as severe hypoglycemia. Odds ratios (ORs) are reported relative to patients without hypoglycemia [Glumin > 69 mg/dL (>3.8 mmol/L]. To determine if the relationship between glycemic control and mortality differed according to the three cohorts, logistic regression analysis was used, fitting main effects for pre-morbid HbA1c, the incidence of hypoglycemia, and an interaction between the two in relation to mortality.
We performed multivariate logistic regression analysis adjusting for a priori defined covariates: site, gender, APACHE II score, whether surgical or medical admission, co-morbidities, including diabetes mellitus, liver cirrhosis, and chronic cardiac failure, and the presence of sepsis at ICU admission, the reason for admission, use of mechanical ventilation, year of admission, and subgroup of Glumin. The dependent variable was hospital mortality. To determine whether the association between the incidence of hypoglycemia and the risk of death was biased by increased glycemic variability, we further developed a multivariable logistic analysis model adjusted for the coefficient of variability of glucose measurements [100 × “standard deviation of glucose measurement”/mean of glucose measurements” (%)] [13].
Results from the multivariable models are reported using ORs with 95 % confidence intervals (CIs). The analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). A two-sided p value of 0.05 was considered to be statistically significant.
Results
During the study period, there were 22,458 patients admitted to the ICUs of the participating hospitals. Among these, we identified 3084 critically ill patients with HbA1c levels measured within 3 months of ICU admission, of whom, 275 had died in hospital (8.9 %). The 3084 patients had 48,980 blood glucose measurements during their time in ICU with blood glucose levels measured every 4.4 h on average. We excluded 19,374 patients due to lack of data on HbA1c levels, among whom were 3814 patients with a pre-admission diagnosis of diabetes mellitus (Supplementary table 1).
We compared the clinical characteristics of the three cohorts according to their pre-admission HbA1c levels (see Table 2). Median pre-admission HbA1c levels were 5.5 % in patients with normal pre-admission glycemia, 7.0 % in those with moderate pre-morbid hyperglycemia, and 9.8 % in those with severe pre-morbid hyperglycemia. Patients with pre-morbid hyperglycemia were more likely to have a medical admission and sepsis at ICU admission, less likely to have cirrhosis before ICU admission, and significantly more severely ill than patients with normal chronic glycemic control.
Among patients with normal pre-admission glycemia, 43.2 % were diagnosed with diabetes before ICU admission. Among those patients diagnosed with moderate or severe pre-morbid hyperglycemia, 80.7 % were diagnosed with diabetes prior to ICU admission, and the remaining 19.3 % were newly diagnosed with diabetes at ICU admission.
The time-weighted mean within-ICU glycemia was significantly higher in patients with poor pre-morbid glycemic control than in those with moderate and severe pre-morbid hyperglycemia [median: 140 mg/dL (7.7 mmol/L) vs. 162 mg/dL (9.0 mmol/L) and vs. 172 mg/dL (9.6 mmol/L), respectively; p < 0.001]. In addition, the incidence of moderate and severe hypoglycemia was significantly higher in patients with pre-morbid hyperglycemia (p < 0.001) (Table 2; Fig. 1).
Incidence of moderate and severe hypoglycemia in the three patient cohorts defined according to pre-admission glycated hemoglobin (HbA1c) levels (HbA1c <6.5 %, 6.5–7.9 %, and >8.0 %, respectively). Black bar Incidence of severe hypoglycemia, defined as <40 mg/dL (<2.2 mmol/L), gray bar incidence of moderate hypoglycemia, defined as 40–69 mg/dL (2.2–3.8 mmol/L). The incidence of moderate and severe hypoglycemia was also significantly higher in patients with pre-morbid hyperglycemia (p < 0.0001)
Hypoglycemic patients had a mean of 1.66 episodes of hypoglycemia (<70 mg/dL) during their ICU stay. Among ICU non-survivors, the median time from last hypoglycemic episode to death was 18 h. Overall, 57.5 % of patients had one episode of hypoglycemia, while 42.5 % experienced two or more episodes. The incidence of two or more episodes of hypoglycemia increased according to the level of pre-morbid hyperglycemia (Table 1).
The in-hospital mortality among patients with hypoglycemia was 21.6 %, which was significantly higher than the 7.7 % mortality seen among patients without hypoglycemia (p < 0.001). The mortality in patients with two or more episodes of hypoglycemia was 28.4 %, which was significantly higher than the 16.5 % mortality seen among patients with one episode of hypoglycemia (p = 0.03).
Figure 2 shows the association of hypoglycemic episodes in ICU patients and the risk of hospital death. Hospital mortality was significantly higher according to the severity of hypoglycemia in each cohort of pre-morbid glycemia (p < 0.001, respectively). Moreover, the unadjusted association of hypoglycemia with increased hospital mortality grew progressively stronger with increasing pre-morbid HbA1c levels (non-adjusted trend p = 0.096; Fig. 2). Finally, after adjustment for key confounders on multivariate logistic regression analysis, pre-morbid hyperglycemia had a significant impact on the relationship between acute hypoglycemia and mortality (p = 0.009), such that the greater the degree of pre-morbid hyperglycemia, the greater the risk of death in patients experiencing any hypoglycemic episode while in the ICU (Table 3; Fig. 2, lower panel). There was a significant association between the occurrence of hypoglycemia and an increased risk of death, even after adjustment for the co-efficient of variation of glucose measurements (Supplementary Table 2).
Interaction of pre-morbid glycemic control with the association between acute hypoglycemia and hospital mortality. Filled circles/bars for each category indicate the un-adjusted (upper) or adjusted (lower) odds ratio between patients with hypoglycemia and those without hypoglycemia for hospital mortality according to pre-morbid glycemic control. The unadjusted association of hypoglycemia with increased hospital mortality became progressively stronger with increasing pre-morbid HbA1c levels (non-adjusted trend p = 0.096). After adjustment for relevant confounders on multivariate logistic regression analysis, pre-morbid hyperglycemia had a significant impact on the relationship between acute hypoglycemia and mortality (p = 0.006)
Discussion
Statement of main findings
We conducted a multicenter retrospective international study to test the hypothesis that there may be an interaction between pre-morbid glycemic control and the association between the occurrence of acute in-ICU hypoglycemia and subsequent hospital mortality. We found that, in a cohort of several thousand patients from ICUs in three different countries and continents, those with higher pre-admission HbA1c levels were at significantly greater risk of in-ICU hypoglycemia. More importantly, we also found that pre-morbid glycemic control (assessed by pre-admission HbA1c levels) had a significant impact on the association between acute in-ICU hypoglycemia and subsequent mortality. As a consequence, the higher the degree of chronic hyperglycemia before ICU admission, the higher the hospital mortality among those patients who experienced hypoglycemia while in ICU.
Comparison to previous studies
To our knowledge, this study is the first to assess the impact of pre-morbid glycemic control on the relationship between in-ICU hypoglycemia and mortality in critically ill patients. A small retrospective study (n = 415) using information obtained before the NICE-SUGAR trial (2000–2004), had previously reported that the association between the time weighted average of glycemia in ICU and mortality differed according to pre-admission HbA1c levels in critically ill patients with DM [14]. Specifically, it showed that a lower mean time-weighted glucose level during ICU stay was associated with higher mortality in patients with diabetes and higher pre-admission HbA1c levels, a finding consistent with our observations. Another prospective observational study after NICE-SUGAR trial also showed that maximum glucose level during ICU stay were associated with increased mortality in patients with adequate pre-morbid glycemic control, but not in patients with pre-morbid hyperglycemia in 1000 ICU patients [15].
The only other potentially relevant data come from the ambulatory setting. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study was a randomized controlled trial of 10,251 patients with type 2 diabetes which aimed to compare the effect of intensive reduction of HbA1c level (targeting a level below 6.0 %) with standard therapy (targeting a level from 7.0 to 7.9 %) [16]. In this trial, intensive reduction in blood glucose levels significantly increased mortality among patients with poor chronic glycemic control (adjusted hazard ratio 1.41; p = 0.02). Furthermore, the effect of reducing blood glucose levels on the incidence of fatal or non-fatal cardiovascular events was significantly affected by baseline glycemic control (p = 0.009), such that the higher the glucose level before trial enrollment, the greater the trend toward morbidity with intensive HbA1c reduction [17].
Our additional findings are that patients with poor chronic glycemic control are at a higher risk of hypoglycemia. Although the presence of diabetes is known to be a significant risk factor for hypoglycemia in critically ill patients [18, 19], to our knowledge, this is first study to show a linear increase in the risk of acute hypoglycemia among critically ill patients according to their pre-morbid glycemic control. This finding is consisted with the finding of ACCORD that patients with worse glycemic control have a greater risk of hypoglycemia [17, 20].
Implications of study findings
Our findings support the hypothesis that patients with chronic pre-admission hyperglycemia differ from those critically ill patients without pre-admission hyperglycemia and that they should be considered separately. Patients with chronic hyperglycemia are at higher risk of acute hypoglycemic episodes and, additionally, in such patients, the relationship between acute hypoglycemia and mortality is adversely affected by poor pre-morbid glycemic control. Hypoglycemia may be harmful in these patients by increasing the systemic inflammatory response [21], inducing neuroglycopenia [22], impairing sympathetic system responsiveness [23], and causing cerebral vasodilatation [24]. It is also possible that the treatment of hypoglycemia with high-dose glucose administration may be hazardous.
The notion that lowering glucose levels too rapidly in patients with chronic hyperglycemia is harmful has biological plausibility. For example, in other conditions of chronic physiological derangement, such as hyponatremia [25], hypernatremia [26], or chronic CO2 retention [27], rapid correction is also associated with increased risk. In patients with poor chronic glycemic control, such risk might be due to an increased systemic inflammatory response [21] or to relative neuroglycopenia [22] induced by the rapid glycemic correction—or perhaps to other as yet unidentified mechanisms. Additionally, it is possible that our findings may be related to a difference in the anti-inflammatory effect of insulin among patients with different pre-morbid glucose control [28], as the administration of insulin is a major risk factor for hypoglycemia [29]. To understand the complex relationship between the use and dose of insulin, chronic pre-admission hyperglycemia, acute hypoglycemia, and mortality, detailed data on all of these variables will be required in future studies. Given that patients with chronic hyperglycemia represent 15–20 % of all ICU admissions, our findings might be relevant to approximately 1 million ICU patients each year in developed countries.
In our cohort, the incidence of hypoglycemia increased significantly according to the presence of pre-morbid hyperglycemia. The glucose counter-regulatory responses may be different in patients with poorly controlled diabetes [30] who have a weakened hormonal response to falling blood glucose levels and lower glucose level thresholds needed to trigger autonomic responses to hypoglycemia [31, 32]. Our study also provides a plausible explanation for the findings of previous observational studies which reported that the association between acute glycemia and mortality was different among the patients with and without diabetes in their respective studies [33–36]. The authors of these studies suggest that the observed differences may have been predominantly affected by pre-admission hyperglycemia, rather than by diabetic status per se. Moreover, they also provide a possible biological explanation for the observed differential association between acute glycemia and outcome in diabetic patients compared with non-diabetic patients. Finally, they suggest the need for trials of tailor-made acute glycemic targets versus standard care in patients with chronic hyperglycemia.
Strengths and limitations of the study
Our study has several strengths. We assessed approximately 50,000 blood glucose measurements, and all values were available for analysis, lending robustness to our observations. The study was conducted in hospitals from three different countries and continents, lending greater external validity to its findings. This is the first study to compare the association between the incidence of hypoglycemia in ICU patients and mortality in critically ill patients with different pre-morbid glucose control. As the incidence of diabetes is known to be 20–30 % worldwide [11], our findings are potentially relevant to many patients over the globe.
Our study does, however, have several limitations. First, it is retrospective in design and thus potentially subject to systematic error and bias. However, the clinical and electronic data were collected prospectively, are numerical in nature, and were measured independently; as such the data were not amenable to unintended manipulation.
Second, although we collected information on the presence of cirrhosis, chronic cardiac failure, and diabetes mellitus before ICU admission, we did not have any other information on relevant co-morbidities, including type of diabetes mellitus and acute liver failure [37, 38], or data on the duration and treatment of glycemia and the adequacy of glycemic control during the non-ICU component of the hospital stay. Such information might have influenced and altered our findings. Therefore, further studies, especially prospective ones, where the above information is collected and included in an assessment are important.
Third, we did not have information on nutritional support and insulin administration and lacked information on how hypoglycemia was treated. This limits our ability to understand other possible interactions that might explain the impact of HbA1c on the relationship between chronic hyperglycemia, acute hypoglycemia in ICU, and mortality.
Fourth, the method of blood glucose measurements was not standardized among study sites. One site used the Accu-Check® Inform system (Roche Diagnostics, Risch-Rotkreuz, Switzerland), and the rest used arterial blood gas analyzers. The Accu-Check® Inform appears to have sufficient analytic accuracy for use in critically ill patients [39], but our results might be skewed by this variability in measurement technology. As the blood glucose measurements were standardized within each site, we included “site” as an independent variable to adjust for its effect in our multivariate analysis. Nonetheless, future studies should be conducted using standardized and more reliable devices for blood glucose measurements.
Fifth, in our multivariable model, hospital sites were independently associated with the risk of mortality. We believe this effect was related to differences in case-mix, policy of ICU admission, health insurance, and the medical system in each hospital and country. We should note that our findings, after adjustment for these factors, might carry more generalizability compared with single-center or single-nation studies.
Sixth, in our study, the glucose measurements in the general wards were not available. Future study should collect data on the presence of hypoglycemia outside the ICU and assess its impact on outcomes.
Seventh, we included critically ill patients with HbA1c levels measured within 3 months of ICU admission. In this regard, our study patients did not represent all patients with diabetes. Thus, our findings could not be generalized to such patients. Future studies with a prospective design should be conducted that measure HbA1c levels at ICU admission in all critically ill patients [15, 40].
Eighth, in our analysis, the interaction of pre-morbid hyperglycemia with the association between hypoglycemia and hospital mortality was predominantly seen in patients with severe pre-morbid hyperglycemia (pre-admission HbA1c ≥ 8.0 %), but not in those with moderate hyperglycemia (pre-admission HbA1c 6.5–7.9 %). It is unclear whether such an interaction would present only in severe pre-morbid hyperglycemia or whether it exists in a dose-dependent manner. To understand this issue, further studies with a larger number of patients are necessary.
Finally, our study was an observational study and not a controlled trial. Accordingly, any association observed cannot be taken to indicate causality. Thus, the direct contribution of hypoglycemia to mortality in our patients remains unknown. Nonetheless, the coherence of the association in acute and ambulatory patients, its biological plausibility, its analogy with other medical conditions where the rapid modification of a chronic physiological abnormality also results in unfavorable outcomes, its close temporality, its specificity to glycemic control, the presence of an effect gradient, and its permanence after correction for confounding all fulfill the classic criteria which define a functional relationship between one variable and another, as seminally described by Sir Bradford Hill [41].
Future studies
To confirm or refute our findings, similar studies should be performed in other hospitals and healthcare settings using a prospective design. Furthermore, our findings suggest the need for a prospective clinical trial to explore the safety and feasibility of aiming for tailor-made acute glycemic control in patients with higher HbA1c levels.
Conclusions
We have studied the impact of pre-admission glycemia on the association between the occurrence of hypoglycemic episodes in patients admitted to the ICU and subsequent hospital mortality. We found that this association was adversely modified by pre-morbid hyperglycemia, such that patients with poor glucose control before ICU admission had a higher risk of hypoglycemic episodes in the ICU and a stronger association between acute hypoglycemia and mortality. This study might be used to generate the hypothesis that the optimal acute glycemic target in patients with poor pre-admission glycemic control may differ from that of patients with good pre-admission glycemic control. Given the number of patients potentially affected, controlled studies are now necessary to further test this hypothesis.
References
Jacobi J, Bircher N, Krinsley J, Agus M, Braithwaite S, Deutschman C, Freire A, Geehan D, Kohl B, Nasraway S, Rigby M, Sands K, Schallom L, Taylor B, Umpierrez G, Mazuski J, Schunemann H (2012) Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med 40:3251–3276
Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Taori G, Hegarty C, Bailey M (2010) Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc 85:217–224
Finfer S, Liu B, Chittock DR, Norton R, Myburgh JA, McArthur C, Mitchell I, Foster D, Dhingra V, Henderson WR, Ronco JJ, Bellomo R, Cook D, McDonald E, Dodek P, Hebert PC, Heyland DK, Robinson BG (2012) Hypoglycemia and risk of death in critically ill patients. N Engl J Med 367:1108–1118
Sonneville R, Vanhorebeek I, den Hertog HM, Chretien F, Annane D, Sharshar T, Van den Berghe G (2015) Critical illness-induced dysglycemia and the brain. Intensive Care Med 41:192–202
Widom B, Simonson DC (1990) Glycemic control and neuropsychologic function during hypoglycemia in patients with insulin-dependent diabetes mellitus. Ann Intern Med 112:904–912
Marik PE, Egi M (2014) Treatment thresholds for hyperglycemia in critically ill patients with and without diabetes. Intensive Care Med 40:1049–1051
Saudek CD, Derr RL, Kalyani RR (2006) Assessing glycemia in diabetes using self-monitoring blood glucose and hemoglobin A1c. JAMA 295:1688–1697
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
International Expert Committee (2009) International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 32:1327–1334
UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352: 837–853
Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hebert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ (2009) Intensive versus conventional glucose control in critically ill patients. N Engl J Med 360:1283–1297
Finfer S, Chittock D, Li Y, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Hebert P, Henderson W, Heyland D, Higgins A, McArthur C, Mitchell I, Myburgh J, Robinson B, Ronco J (2015) Intensive versus conventional glucose control in critically ill patients with traumatic brain injury: long-term follow-up of a subgroup of patients from the NICE-SUGAR study. Intensive Care Med 41:1037–1047
Egi M, Bellomo R, Stachowski E, French CJ, Hart G (2006) Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology 105:244–252
Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Taori G, Hegarty C, Bailey M (2011) The interaction of chronic and acute glycemia with mortality in critically ill patients with diabetes. Crit Care Med 39:105–111
Plummer MP, Bellomo R, Cousins CE, Annink CE, Sundararajan K, Reddi BA, Raj JP, Chapman MJ, Horowitz M, Deane AM (2014) Dysglycaemia in the critically ill and the interaction of chronic and acute glycaemia with mortality. Intensive Care Med 40:973–980
Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358:2545–2559
Hempe JM, Liu S, Myers L, McCarter RJ, Buse JB, Fonseca V (2015) The hemoglobin glycation index identifies subpopulations with harms or benefits from intensive treatment in the ACCORD trial. Diabetes Care 38:1067–1074
Krinsley JS, Grover A (2007) Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med 35:2262–2267
Vriesendorp TM, van Santen S, DeVries JH, de Jonge E, Rosendaal FR, Schultz MJ, Hoekstra JB (2006) Predisposing factors for hypoglycemia in the intensive care unit. Crit Care Med 34:96–101
Miller ME, Bonds DE, Gerstein HC, Seaquist ER, Bergenstal RM, Calles-Escandon J, Childress RD, Craven TE, Cuddihy RM, Dailey G, Feinglos MN, Ismail-Beigi F, Largay JF, O’Connor PJ, Paul T, Savage PJ, Schubart UK, Sood A, Genuth S (2010) The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ 340:b5444
Dotson S, Freeman R, Failing HJ, Adler GK (2008) Hypoglycemia increases serum interleukin-6 levels in healthy men and women. Diabetes Care 31:1222–1223
Schlenk F, Graetz D, Nagel A, Schmidt M, Sarrafzadeh AS (2008) Insulin-related decrease in cerebral glucose despite normoglycemia in aneurysmal subarachnoid hemorrhage. Crit Care 12:R9
Herlein JA, Morgan DA, Phillips BG, Haynes WG, Sivitz WI (2006) Antecedent hypoglycemia, catecholamine depletion, and subsequent sympathetic neural responses. Endocrinology 147:2781–2788
Dieguez G, Fernandez N, Garcia JL, Garcia-Villalon AL, Monge L, Gomez B (1997) Role of nitric oxide in the effects of hypoglycemia on the cerebral circulation in awake goats. Eur J Pharmacol 330:185–193
Lin SH, Chau T, Wu CC, Yang SS (2002) Osmotic demyelination syndrome after correction of chronic hyponatremia with normal saline. Am J Med Sci 323:259–262
Pokaharel M, Block CA Dysnatremia in the ICU. Curr Opin Crit Care 17:581–593
Webster NR, Kulkarni V (1999) Metabolic alkalosis in the critically ill. Crit Rev Clin Lab Sci 36:497–510
Sun Q, Li J, Gao F (2014) New insights into insulin: the anti-inflammatory effect and its clinical relevance. World J Diabetes 5:89–96
Arabi YM, Tamim HM, Rishu AH (2009) Hypoglycemia with intensive insulin therapy in critically ill patients: predisposing factors and association with mortality. Crit Care Med 37:2536–2544
Sprague JE, Arbelaez AM (2011) Glucose counter regulatory responses to hypoglycemia. Pediatr Endocrinol Rev 9:463–473 (Quiz 474–465)
Cryer PE (2005) Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes 54:3592–3601
Segel SA, Paramore DS, Cryer PE (2002) Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes 51:724–733
Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Hegarty C, Bailey M (2008) Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med 36:2249–2255
Krinsley JS, Meyfroidt G, van den Berghe G, Egi M, Bellomo R (2012) The impact of premorbid diabetic status on the relationship between the three domains of glycemic control and mortality in critically ill patients. Curr Opin Clin Nutr Metab Care 15:151–160
Lanspa MJ, Hirshberg EL, Phillips GD, Holmen J, Stoddard G, Orme J (2013) Moderate glucose control is associated with increased mortality compared to tight glucose control in critically ill patients without diabetics. Chest 143(5):1226–1234
Krinsley JS, Egi M, Kiss A, Amin DN, Schuetz P, Maurer PM, Schultz MJ, van Hooijdonk RT, Kiyoshi M, Mackenzie IM, Annane D, Stow P, Nasraway SA, Holewinski S, Holzinger U, Preiser JC, Vincent JL, Bellomo R (2013) Diabetic status and the relationship of the 3 domains of glycemic control to mortality in critically ill patients: an international multi-center cohort study. Crit Care 17:R37
Greenfield S, Billimek J, Pellegrini F, Franciosi M, De Berardis G, Nicolucci A, Kaplan SH (2009) Comorbidity affects the relationship between glycemic control and cardiovascular outcomes in diabetes: a cohort study. Ann Intern Med 151:854–860
Falciglia M, Freyberg RW, Almenoff PL, D’Alessio DA, Render ML (2009) Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med 37:3001–3009
Mitsios JV, Ashby LA, Haverstick DM, Bruns DE, Scott MG (2013) Analytic evaluation of a new glucose meter system in 15 different critical care settings. J Diabetes Sci Technol 7:1282–1287
Nanayakkara N, Nguyen H, Churilov L, Kong A, Pang N, Hart GK, Owen-Jones E, White J, Ross J, Stevenson V, Bellomo R, Lam Q, Crinis N, Robbins R, Johnson D, Baker ST, Zajac JD, Ekinci EI (2015) Inpatient HbA1c testing: a prospective observational study. BMJ Open Diabetes Res Care 3:e000113
Hill AB (1965) The environment and disease: association or causation? Proc R Soc Med 58:295–300
Acknowledgments
This study was supported by the grants-in-aid for scientific research from the Ministry of Education, Science, and Culture of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Drs. Egi, Kanazawa, Morita, Bailey, Amin, Bellomo report no relevant disclosures. Dr. Krinsley reports receiving consultant fees from Medtronic Inc., Edwards Life Sciences, Roche Diagnostics, OptiScan Biomedical, and Alere and research support from OptiScan Biomedical. He also received royalty payments for sales of the ICU Tracker. Ms. Maurer works as a consultant for Alere, the distributor of ICU Tracker.
Additional information
Take-home message: Critically ill patients with higher pre-admission HbA1c levels were at significantly greater risk of hypoglycemia while in ICU. More importantly, the higher the degree of chronic hyperglycemia before ICU admission, the higher the hospital mortality among those patients who experienced hypoglycemia while in ICU.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Egi, M., Krinsley, J.S., Maurer, P. et al. Pre-morbid glycemic control modifies the interaction between acute hypoglycemia and mortality. Intensive Care Med 42, 562–571 (2016). https://doi.org/10.1007/s00134-016-4216-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4216-8